Abstract
Thirteen bovine leukemia virus- (BLV-) negative and 22 BLV-positive Holstein cows were immunized with J5 Escherichia coli bacterin at dry off, three weeks before calving, during the second week after calving, and three weeks after the third immunization. Serum was collected before the initial immunization, immediately before the third and fourth immunizations, and 21 days after the fourth immunization. Anti-J5 E. coli IgM, IgG1, and IgG2 titers were determined by ELISA. Anti-J5 E. coli IgM titers did not differ significantly (P = .98) between groups. Increases in anti-J5 E. coli IgG1 titers were higher in the BLV-negative cows (P = .057). Geometric mean anti-J5 E. coli IgG2 titers increased fourfold in the BLV-negative cows, which was significantly higher (P = .007) than the twofold increase in the BLV-positive cows. Cattle infected with BLV may have impaired serologic responses following immunization with J5 bacterin, and response may differ according to antibody isotype.
1. Introduction
Enzootic bovine leukosis is a contagious disease of cattle induced by an exogenous retrovirus, bovine leukemia virus (BLV). The disease complex is characterized by a persistent lymphocytosis which can culminate in B cell lymphoma [1]. The National Animal Health Monitoring System Dairy (NAHMS) 1996 study revealed that BLV is present in 89% of US dairy operations [2]. Most infected cows do not display outward signs of disease, and these animals are referred to as asymptomatic or aleukemic. Approximately 30–40% of BLV carriers will develop a persistent lymphocytosis, while fewer than 5% develop malignant lymphosarcoma [1].
The progression of BLV is known to affect host defense mechanisms. Although BLV is associated mainly with infections of B lymphocytes, BLV provirus has been detected in the DNA of immunoaffinity purified T lymphocytes from BLV-infected cattle [3]. There is a dramatic increase in B lymphocyte populations with decreases in the percentages of both CD4+ and CD8+ T lymphocyte populations [4]. Certain type 1 cytokines from CD4+ T lymphocytes, including interleukin-2 (IL2), IL12, and interferon gamma (IFNγ), are reduced during BLV infections, and this altered cytokine production was suggested to be responsible for suppressed mitogen-induced T lymphocyte proliferation [4–6]. BLV infection may suppress T-cell apoptosis and gene expression of proteins important in the apoptotic pathway [7]. Considering the critical role that T- and B-cell populations play in humoral immunity, the purpose of this study was to investigate the effect of BLV on the serologic response to an Escherichia coli bacterin that is commonly administered in dairy cattle.
2. Materials and Methods
2.1. Animal Selection and Care
Forty-four Holstein cows completing their first or greater lactation and starting their dry period were enrolled if the herd manager determined that the cows were healthy on the basis of a physical examination (appetite, attitude, milk production, and lack of clinical mastitis). The mean length of the dry period for enrolled cows was 61 days, with a range of 53 to 68 days. A sample of blood was collected from each cow, immediately before immunization with J5 bacterin. Subsequent to the results of ELISA serology (Diagnostic Center for Population and Animal Health, Michigan State University), 24 BLV-positive (BLV POS) and 20 BLV-negative (BLV NEG) cows were identified for continuing in the study. However, 2 cows from both groups were prematurely culled before the end of the trial and were, therefore, excluded from our study. Additionally, 2 BLV NEG cows seroconverted from negative to positive BLV status during the course of the trial, 1 BLV NEG cow acquired a case of clinical coliform mastitis, which was considered to likely affect her anti-J5 antibody response, and 2 additional BLV NEG cows were withdrawn from the immunization schedule after parturition because of complications associated with twinning. Thus, after exclusions, 35 animals remained on the trial, 22 BLV POS and 13 BLV NEG. All cows enrolled in the study were housed at the Kellogg Biological Station Dairy at Michigan State University, were milked 3 times/d, and had unlimited access to water and a total mixed ration. The study protocol was approved by the Institutional Animal Care and Use Committee.
2.2. Vaccination Schedule
Cows were administered a 5-mL dose of a commercial J5 Escherichia coli bacterin (J5 E. coli Bacterin, Pfizer Animal Health, Kalamazoo, Michigan, USA) by subcutaneous injection on the last day of lactation (approximately 7 weeks before parturition) and, subsequently, 3 weeks before parturition, the second week after parturition (between 8 and 14 days after parturition), and between 29 and 35 days after parturition. The first and third doses were administered subcutaneously in the left side of the neck, and the second and fourth doses were given on the right side of the neck.
2.3. Blood Collection Schedule
Blood samples were collected from the coccygeal vein into 15-mL sterile glass tubes, allowed to clot overnight at 4°C, and then centrifuged at 1,500 × g for 15 minutes at 4°C. Serum was harvested and stored in 3-mL aliquots at −20°C until assayed by ELISA for anti-J5 E. coli antibody titers. Blood samples were collected immediately before the first immunization and before the third, fourth, and 21 days after the fourth immunization.
2.4. Preparation of J5 E. coli Whole-Cell Antigen for ELISA
Isolated colonies from pure cultures of J5 E. coli were used to inoculate trypticase soy broth, which was incubated with shaking at 120 rounds/min for 18 hours at 37°C. The bacterial culture was checked for purity; then, 99% phenol was added and the solution was shaken for 1 hour at 120 rpm at 37°C. The phenol-killed whole-cell bacteria were centrifuged at 1,000 × g for 12 minutes at 4°C; the pellet was washed twice and centrifuged in 500 mL of sterile 0.9% NaCl solution. After the second centrifugation, the pellet was suspended in sterile PBS solution to attain a concentration of approximately (1 × 1011 CFU/mL) as determined from quantitative count of bacterial growth from serial dilutions. The whole-cell J5 antigen solution was stored in 5-mL aliquots at −80°C until used in the ELISA. ELISA plates were prepared by diluting the antigen 1 : 250 in sodium carbonate/bicarbonate buffer (pH 9.6) and adding 100 μL to each well of a 96-well flat bottom plate. Prepared plates were then incubated overnight in an ambient air incubator at 37°C.
2.5. ELISA to Determine Anti-J5 E. coli Antibody Titers
Serum anti-J5 E. coli titers were determined by modification of an ELISA protocol as described by Tyler et al. [8]. The antigen was phenol-killed whole-cell J5 E. coli, the detection antibody was horseradish peroxidase-conjugated sheep antibovine IgG1, IgG2, or IgM (Bethyl Laboratories, Montgomery, Texas, USA), each diluted 1 : 25,000 in sample diluent, and the substrate was hydrogen peroxide-azino-bis-3-ethylbenzthiazoline sulfonic acid. The assay positive control sample was pooled sera collected from steers hyperimmunized by administration of J5 E. coli bacterin [9], and the negative control sample was fetal bovine serum (Life Technologies, Rockville, Maryland, USA). Following antigen incubation, plates were washed twice with 250 μL of a commercial wash solution (KPL, Inc., Gaithersburg, Maryland, USA) and 200 μL of a blocking-diluent solution (0.2 M Tris, 0.3% Tween-20, and 1% ovalbumin) was added. Plates were then incubated for one hour in an ambient air incubator at 37°C. Wells were again washed twice and 200 μL of test sera were diluted serially, in duplicate, by three fold dilutions for each descending row, and plates incubated for one hour in an ambient air incubator at 37°C. For IgG2 and IgM ELISA, the initial dilution of sera was 1 : 67, with dilutions of subsequent rows of 1 : 200; 1 : 600; 1 : 1,800; 1 : 5,400; 1 : 16,200; and 1 : 48,600. Because of higher concentrations of IgG1 in serum relative to the other isotypes, the initial dilution of sera for IgG1 ELISA was 1 : 600, with dilutions of subsequent rows of 1 : 1,800; 1 : 5,400; 1 : 16,200; and 1 : 48,600; 1 : 145,800; and 1 : 437,400. Plates were washed five times after sera incubation, 100 μL of diluted conjugate antibody was added and again incubated for one hour. Plates were washed once more for five times, 100 μL of substrate was added, and after 25 minutes of room temperature incubation in the dark, the reaction was stopped with 100 μL of 1% SDS. Optical density was determined by dual-wavelength spectrometric analysis (405 nm for test and control samples, after normalization of the entire plate at 450 nm on the basis of blank wells containing only diluent) by use of an ELISA plate reader (SpectraMax 190, Molecular Devices, Sunnyvale, California, USA). Thus, normalized optical densities at 405 nm were the final data set for the statistical analysis. In order to reduce variation associated with daily changes in laboratory environment, titers for each immunoglobulin isotype (IgM, IgG1, and IgG2) were determined for the entire collection of serum samples (all cows, all serial samples per cow) on one day. Repetitions of each serum sample titer, as determined by ELISA, that had a coefficient of variation of ≥15% were repeated.
2.6. ELISA to Determine BLV Antibody
Antibodies to BLV- glycoprotein 51 (gp51) in bovine sera were detected by a commercial ELISA (VMRD Inc., Pullman, Washington, USA) performed at the Diagnostic Center for Population and Animal Health, Michigan State University. Briefly, sample serum antibodies bind to BLV gp51 molecules attached to the plastic wells of the microtiter plate. Binding of these serum antibodies is detected by reaction with horseradish peroxidase- (HRP-) labeled affinity-purified goat antibodies to bovine immunoglobulins. Attached HRP-labeled antibodies are detected by addition of enzyme substrate and quantitated by subsequent blue color product development. Strong color development indicates the presence of antibody to BLV gp51 in the sample serum. Very weak or no color development indicates the absence of antibody to BLV gp51 in the sample serum.
2.7. Statistical Analyses
The baseline pre-vaccination IgG1, IgG2 and IgM titers for the J5 antigen were subtracted from each of the corresponding repeated postvaccination measurements to determine the change in titer for each of the three repeated post vaccination measurements. The increases in serum titers (log10) between each serum collection time period were used as the repeated dependent variable in repeated measures ANOVA models (SAS Proc GLM (SAS-Institute, Cary, North Carolina, USA)). The sphericity test for orthogonal components was used to indicate if any assumptions of the statistical model had been severely violated [10].
3. Results
3.1. IgM
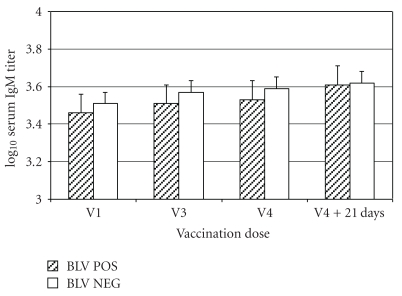
Geometric mean IgM titers increased from 3,250 at the time of the first immunization to 4,100 at 21 days after the fourth immunization in the BLV NEG cows and from 2,900 to 4,100 for the BLV POS cows (Figure 1). The repeated measures ANOVA indicated no overall significant difference in titer change between the BLV NEG and BLV POS cows (P = .90). The effect of BLV infection was not significant at P > .66 at all three of the repeated time points. The sphericity test for orthogonal components was P = .12, suggesting that the model was valid.
Figure 1.
Mean ± SEM serum IgM titers in 22 BLV-positive cows (BLV POS) and 13 BLV-negative cows (BLV NEG) administered a J5 E. coli bacterin with 4 doses of bacterin administered SC in the left side of the neck (odd numbered doses) or right side of the neck (even numbered doses). Blood samples were collected from all cows at the time of the first (V1: approx 7 weeks before parturition), third (V3: second week after parturition (between days 8 and 14 after parturition)), fourth (V4: 5 weeks (between 29 and 35 days) after parturition) vaccinations, and 21 days after the fourth vaccination.
3.2. IgG1
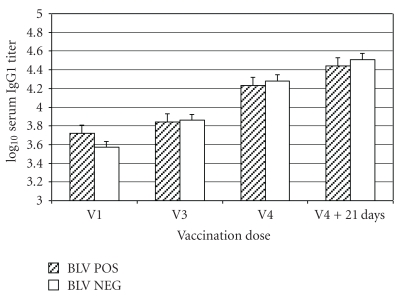
Anti-J5 E. coli IgG1 titers increased in all 13 BLV NEG cows at 21 days after the third and fourth immunization and 19/22 (86.4%) and 20/22 (90.9%) BLV POS cows 21 days after the third and fourth immunization, respectively. Geometric mean IgG1 titers increased from 3,700 at the time of the first immunization to 32,500 at 21 days after the fourth immunization in the BLV NEG cows, and from 5,300 to 27,500 for the BLV POS cows (Figure 2). The repeated measures ANOVA indicated a nearly significant difference in overall titer change between the BLV NEG and BLV POS cows (P = .057). The effect of BLV infection was at P = .22 at the first repeated measure, P = .085 at the second time point, and P = .14 at the time of the last repeated measure. However, the sphericity test for orthogonal components was P < .0001, suggesting that the model assumptions may have been violated.
Figure 2.
Mean ± SEM serum IgG1 titers in 22 BLV-positive cows (BLV POS) and 13 BLV-negative cows (BLV NEG) administered a J5 E. coli bacterin with 4 doses of bacterin administered SC in the left side of the neck (odd-numbered doses) or right side of the neck (even-numbered doses). Blood samples were collected from all cows at the time of the first (V1: approx 7 weeks before parturition), third (V3: second week after parturition (between days 8 and 14 after parturition)), fourth (V4: 5 weeks (between 29 and 35 days) after parturition) vaccinations, and 21 days after the fourth vaccination.
3.3. IgG2
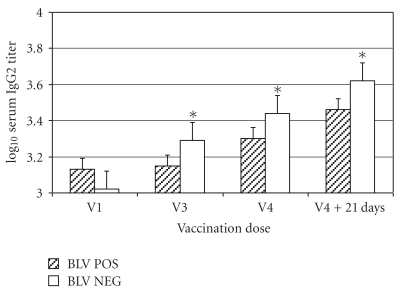
Anti-J5 E. coli IgG2 titers increased in 12/13 (92.3%) and 13/13 (100%) BLV NEG cows at 21 days after the third and fourth immunization, respectively, and 13/22 (59.1%) and 15/22 (68.2%) BLV POS cows 21 days after the third and fourth immunizations. Geometric mean IgG2 titers increased from 1,045 at the time of the first immunization to 4,200 at 21 days after the fourth immunization in the BLV NEG cows, and from 1,450 to 2,900 for the BLV POS cows (Figure 3). The repeated measures ANOVA indicated an overall significant difference in titer change between the BLV NEG and BLV POS cows at P = .0072. The effect of BLV infection was significant at P = .0045 at the first repeated measure, P = .034 at the second repeated measure, and P = .024 at the time of the last repeated measure. The sphericity test for orthogonal components was P < .025, indicating that the model fit was acceptable.
Figure 3.
Mean ± SEM serum IgG2 titers in 22 BLV-positive cows (BLV POS) and 13 BLV-negative cows (BLV NEG) administered a J5 E. coli bacterin with 4 doses of bacterin administered SC in the left side of the neck (odd-numbered doses) or right side of the neck (even-numbered doses). Blood samples were collected from all cows at the time of the first (V1: approx 7 weeks before parturition), third (V3: second week after parturition (between days 8 and 14 after parturition)), fourth (V4: 5 weeks (between 29 and 35 days) after parturition) vaccinations, and 21 days after the fourth vaccination. *Within a time point, mean values for groups differ significantly (P < .05).
4. Discussion
Numerous studies have determined that vaccination with J5 bacterin increases anti-J5 E. coli immunoglobulin, especially G1 and G2 isotypes, in serum and milk as compared to unvaccinated controls [11–14]. Additionally, the use of multiple doses of J5 bacterin (hyperimmunization), such as used in this study, increases the serum antibody response and has been associated with decreasing incidence of severe coliform mastitis compared to more traditional three-dose regimens [15]. In the present study, serum IgM titers only modestly increased over the course of the trial in both groups of cows. This is similar to results obtained in a recent New York study that determined serum IgM response did not increase following a two-dose J5 bacterin regimen, although IgG1 and IgG2 responses did increase [14]. Earlier studies suggested that serum IgM increases in response to J5 immunization [9, 12]. IgG2 and IgG1 have been identified as critical for mammary gland defense during infection although IgM and IgG2 promote phagocyte opsonization and ensuing phagocytosis of mastitis-causing pathogens [16]. Hyperimmunization with J5 bacterin induced recognition of proteins, derived from J5 E. coli whole cell lysates, by IgG2 that were not recognized by other isotypes [9].
We determined that anti-J5 IgG1 and IgG2 titers increased in both groups of cows following three and four doses of the bacterin. However, the increase in IgG1 titers tended to be higher in the NEG cows, and the increase in IgG2 titers, in particular, were significantly higher in the NEG cows. Thus, our data suggests that BLV infection in dairy cattle may decrease the antibody response to immunization. Whereas the presence of J5 E. coli antibodies in serum have been associated with protection against coliform mastitis, the effects of BLV on resistance to mastitis remain speculative. Therefore, broad extrapolations concerning BLV status, the efficacy of J5 vaccination, and mastitis resistance should not be made from this single descriptive study. Additionally, the immunization of the cattle in this trial occurred during late pregnancy and the periparturient period, a stage of lactation when the immune system is impaired [17]. What effects BLV may have on vaccine responsiveness in cattle in different physiological states is unknown.
BLV infection reduces expression of type 1 cytokines from CD4+ T lymphocytes, including interleukin-2 (IL2), IL12, and interferon gamma (IFNγ) [4–6]. Additionally, cytokine profiles from all peripheral blood mononuclear cell populations, including B lymphocytes, suggest that both type I and II cytokines are altered with increases in IL10 and IL4, and decreases in IL2, IL12, and IFNγ [5, 17]. The progression of BLV is also known to disrupt the homeostasis of lymphocyte proliferation and cell death, in both B-cells and T-cells [4, 6, 8]. These BLV-induced mechanisms may play a role on the homeostasis of lymphocyte populations, have a detrimental impact on the ability of cattle to resist the progression of infectious disease [5, 8, 18], or as in our study, response to an adjuvated vaccine. An issue raised by our results is whether BLV infection may interfere with the immunogenicity of other vaccine antigens. A previous report suggested a possible impairment of rotaviral immune responses in BLV-positive animals [19].
This study was limited in the number of enrolled animals and in that they were obtained from only one herd. Although the same standard of determining BLV infection status (ELISA) was employed for both treatment groups, the slow development of the disease, and the relatively long duration of animals in the trial, may have contributed to variability in our results if cows were falsely identified as to BLV status. Previous research determined that PCR assays for BLV detected new infections in 5 of 8 experimentally challenged calves more quickly than ELISA although the ELISA determined infection more readily in 2 of 8 calves, thus resulting in a nonsignificant difference [20]. A study incorporating a large population of cattle to determine the relative sensitivity and specificity of the ELISA to detect BLV antibodies relative to detecting BLV by PCR has not been reported. Thus, the BLV antibody ELISA remains a commonly used diagnostic tool to determine BLV status.
It is not known from this trial what effect BLV might have on immunization with other commonly used vaccines, nor on cell-mediated responses following immunization. Additionally, the stage of infection with regard to persistent lymphocytosis, and other pathological findings associated with BLV infection, were not determined in this study. Previous research indicated that changes in lymphocyte proliferation and/or apoptosis will depend on the stage of BLV infection [8].
5. Conclusions
Dairy cows that were infected with BLV had decreased antibody responses to J5 E. coli bacterin as compared to noninfected cows. This is consistent with reports that BLV induces changes in the complex balance of cytokine expression, cell proliferation, and programmed death in both T- and B-lymphocytes, which is critical for immune competence and effective response to infectious challenge. Although lymphosarcoma occurs in only a small proportion of dairy cattle infected with BLV, the virus may have broader, if not more subtle effects, on the health of dairy cattle. This potential relationship, and the effect that control of this disease may have on vaccination programs, should be further evaluated.
Acknowledgments
The authors would like acknowledge funding by the Sterner Fund for Bovine Health and technical support from Pfizer Animal Health, Kalamazoo, Michigan.
References
- 1.Schwartz I, Lévy D. Pathobiology of bovine leukemia virus. Veterinary Research. 1994;25(6):521–536. [PubMed] [Google Scholar]
- 2. United States department of agriculture-animal plant health inspection service, veterinary service, national animal health monitoring service 1996 Dairy Info Sheet, pp. 1-2, 1997.
- 3.Kabeya H, Ohashi K, Onuma M. Host immune responses in the course of bovine leukemia virus infection. Journal of Veterinary Medical Science. 2001;63(7):703–708. doi: 10.1292/jvms.63.703. [DOI] [PubMed] [Google Scholar]
- 4.Sordillo LM, Hicks CR, Pighetti GM. Altered interleukin-2 production by lymphocyte populations from bovine leukemia virus-infected cattle. Proceedings of the Society for Experimental Biology and Medicine. 1994;207(3):268–273. doi: 10.3181/00379727-207-43815. [DOI] [PubMed] [Google Scholar]
- 5.Konnai S, Usui T, Ohashi K, Onuma M. The rapid quantitative analysis of bovine cytokine genes by real-time RT-PCR. Veterinary Microbiology. 2003;94(4):283–294. doi: 10.1016/s0378-1135(03)00119-6. [DOI] [PubMed] [Google Scholar]
- 6.Stone DM, Hof AJ, Davis WC. Up-regulation of IL-2 receptor α and MHC class II expression on lymphocyte subpopulations from bovine leukemia virus infected lymphocytotic cows. Veterinary Immunology and Immunopathology. 1995;48(1-2):65–76. doi: 10.1016/0165-2427(95)05423-4. [DOI] [PubMed] [Google Scholar]
- 7.Erskine RJ, Corl C, Gandy JC, et al. Bovine leukosis virus infection in dairy cattle: effect on lymphocyte proliferation and apoptosis. doi: 10.2460/ajvr.72.8.1059. accepted for American Journal Veterinary Research. [DOI] [PubMed] [Google Scholar]
- 8.Tyler JW, Cullor JS, Dellinger JD. Cross-reactive affinity purification of immunoglobulin recognizing common gram-negative bacterial core antigens. Journal of Immunological Methods. 1990;129(2):221–226. doi: 10.1016/0022-1759(90)90442-x. [DOI] [PubMed] [Google Scholar]
- 9.Chaiyotwittayakun A, Burton JL, Weber PSD, Kizilkaya K, Cardoso FF, Erskine RJ. Hyperimmunization of steers with J5 Escherichia coli bacterin: effects on isotype-specific serum antibody responses and cross reactivity with heterogeneous gram-negative bacteria. Journal of Dairy Science. 2004;87(10):3375–3385. doi: 10.3168/jds.S0022-0302(04)73473-6. [DOI] [PubMed] [Google Scholar]
- 10.Hypothesis testing in repeated measures analysis. SAS/STAT(R) 9.2 User’s Guide, Second Edition, http://support.sas.com/documentation/cdl/en/statug/63033/HTML/default/viewer.htm#statug_glm_sect036.htm.
- 11.Hogan JS, Weiss WP, Todhunter DA, Smith KL, Schoenberger PS. Efficacy of an Escherichia coli J5 mastitis vaccine in an experimental challenge trial. Journal of Dairy Science. 1992;75(2):415–422. doi: 10.3168/jds.S0022-0302(92)77777-7. [DOI] [PubMed] [Google Scholar]
- 12.Tomita GM, Ray CH, Nickerson SC, Owens WE, Gallo GF. A comparison of two commercially available Escherichia coli J5 vaccines against E. coli intramammary challenge. Journal of Dairy Science. 2000;83(10):2276–2281. doi: 10.3168/jds.S0022-0302(00)75112-5. [DOI] [PubMed] [Google Scholar]
- 13.Wilson DJ, Mallard BA, Burton JL, Schukken YH, Gröhn YT. Milk and serum J5-specific antibody responses, milk production change, and clinical effects following intramammary Escherichia coli challenge for J5 vaccinate and control cows. Clinical and Vaccine Immunology. 2007;14(6):693–699. doi: 10.1128/CVI.00104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson DJ, Mallard BA, Burton JL, Schukken YH, Grohn YT. Association of Escherichia coli J5-specific serum antibody responses with clinical mastitis outcome for J5 vaccinate and control dairy cattle. Clinical and Vaccine Immunology. 2009;16(2):209–217. doi: 10.1128/CVI.00324-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erskine RJ, VanDyk EJ, Bartlett PC, Burton JL, Boyle MC. Effect of hyperimmunization with an Escherichia coli J5 bacterin in adult lactating dairy cows. Journal of the American Veterinary Medical Association. 2007;231(7):1092–1097. doi: 10.2460/javma.231.7.1092. [DOI] [PubMed] [Google Scholar]
- 16.Guidry AJ, Berning LM, Hambleton CN. Opsonization of Staphylococcus aureus by bovine immunoglobulin isotypes. Journal of Dairy Science. 1993;76(5):1285–1289. doi: 10.3168/jds.S0022-0302(93)77458-5. [DOI] [PubMed] [Google Scholar]
- 17.Amills M, Norimine J, Olmstead CA, Lewin HA. Cytokine mRNA expression in B cells from bovine leukemia virus-infected cattle with persistent lymphocytosis. Cytokine. 2004;28(1):25–28. doi: 10.1016/j.cyto.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Florins A, Boxus M, Vandermeers F, et al. Emphasis on cell turnover in two hosts infected by bovine leukemia virus: a rationale for host susceptibility to disease. Veterinary Immunology and Immunopathology. 2008;125(1-2):1–7. doi: 10.1016/j.vetimm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Archambault D, Morin G, Elazhary MA. Possible impairment of rotavirus immune response in cattle infected with BLV. Veterinary Record. 1989;124(21):570–572. doi: 10.1136/vr.124.21.570-b. [DOI] [PubMed] [Google Scholar]
- 20.Nagy DW, Tyler JW, Kleiboeker SB. Timing of seroconversion and acquisition of positive polymerase chain reaction assay results in calves experimentally infected with bovine leukemia virus. American Journal of Veterinary Research. 2007;68(1):72–75. doi: 10.2460/ajvr.68.1.72. [DOI] [PubMed] [Google Scholar]