Zygotic microRNAs coordinate the clearance of maternal mRNA in animals to facilitate developmental transitions. In a recent issue of Genes and Development, Nodine and Bartel (2010) uncover a reciprocal function in plants, where miRNA-156 preemptively represses genes that function later in development to prevent premature developmental transitions.
microRNAs (miRNAs) are small ∼22-24 nt RNA molecules that regulate mRNA translation, deadenylation and decay. miRNAs have been implicated in a broad range of developmental and disease contexts in animals and plants. With tens to hundreds of predicted targets for each miRNA, one of the biggest challenges in the field resides in understanding the physiological functions of individual miRNA-target interactions in vivo. Gene expression analyses of mutants lacking components of the miRNA-processing machinery or individual miRNAs have revealed that miRNAs constitute an evolutionarily conserved tool to provide temporal and spatial control of gene expression post-transcriptionally (Takacs and Giraldez, 2010).
One prominent example of temporal regulation is related to the lin-4 and let-7 miRNAs, which facilitate transitions between developmental stages in C. elegans by suppressing a developmental program that was previously active (Figure 1A). Similarly, it has been shown that miR-430/miR-427 in zebrafish and Xenopus, and the miR-309 cluster in Drosophila, play a major role in the clearance of maternal mRNAs during the transition from maternal to zygotic gene expression (Giraldez, 2010).
Figure 1. miRNAs cause mRNA decay or prevent mRNA accumulation.
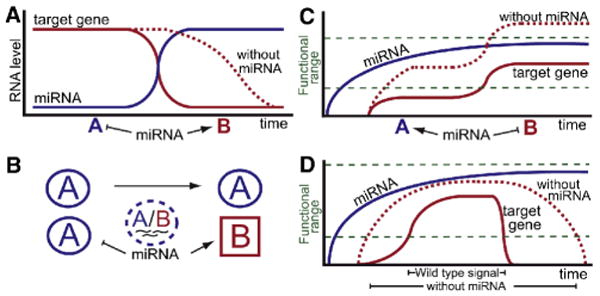
(A) miRNAs clear pre-existing target mRNAs. In the absence of miRNAs, target mRNA levels remain high for an extended period of time, or in a larger spatial domain. Blue A and red B are two different stages of development (A, C and D) or two cell types (B). (B) Restricted expression of miRNAs controls the expression of target genes in a subset of cells. (C) miRNAs prevent premature expression of de novo transcribed target mRNA. In the absence of miRNA, higher levels of mRNA are reached rapidly. (D) miRNAs that target transcripts for rapid degradation can shape signaling dynamics. In the absence of miRNA, the persistence of target transcripts will increase and prolong their activities as signaling effectors or agonists, blurring the spatiotemporal “shape” of signaling output and diminishing the resolution of dose-dependent regulation. In the presence of miRNA, the transcriptional dynamics of the target transcripts determine signaling output more precisely, allowing more accurate interpretation of concentration- and duration-dependent thresholds.
Alternatively, miRNAs with a restricted expression domain provide a fundamental means of controlling the expression of target genes in a subset of cells (Figure 1B). Examples of this type of regulation have been previously reported in animals and plants: In C. elegans, two morphologically symmetric and bilateral taste-receptor neurons ASEL (left) and ASER (right) express different sets of transcription factors and miRNAs (Johnston and Hobert, 2003). The miRNAs shape gene expression of the left and right ASE neurons by inhibiting the expression of the contralateral transcription factor, defining two terminally differentiated states. In Arabidopsis, miRNA-165/6 are expressed in the root endodermis and move to surrounding cells, producing a degradation gradient of their primary target mRNA, PHABULOSA (Carlsbecker et al., 2010), thereby inducing a radial pattern of different cell types in the vascular cylinder, in a dosage-dependent manner.
In a second scenario, a ubiquitously expressed miRNA can also provide spatial control of gene expression by accelerating the rate of decay of a dynamically expressed transcript. This regulation sharpens the expression domain of the target by inducing the rapid degradation of “old” mRNAs that linger in previous expression domains (Figure 1D). For example, miR-430 facilitates dynamic expression of the chemokine ligand Sdf1a in zebrafish to ensure accurate germ cell migration.
In these examples of spatial and temporal regulation, the miRNA tends to modulate the activity of a preexisting target mRNA within a functional range or to reduce the transcript to inconsequential levels. In a recent issue of Genes and Development, Nodine and Bartel (2010) (Nodine and Bartel, 2010) reported a novel mode of temporal miRNA-mediated regulation whereby miRNA-156 is expressed early in embryonic development to prevent premature expression of its targets (Figure 1C). In this scenario, miRNA-156 function contrasts with that of previous examples where miRNAs dispose of residual maternal transcripts (miR-430, miR-427 and miR-309) after they are no longer needed. Using Arabidopsis embryos lacking DICER-LIKE1 (DCL1), an enzyme required to process miRNAs, the authors show that DCL1 is necessary for cell differentiation and proper cell division in the eight-cell embryo.
Because many targets in plants are typically cleaved and degraded when regulated by miRNAs, Nodine and Bartel analyzed the changes in gene expression in the early dcl1 mutant embryos, as a means to identify candidate miRNA target transcripts. Using genome-wide transcript profiling, they found higher expression levels for approximately 50 putative miRNA targets in early dcl1 embryos when compared to wild type embryos. Those up-regulated targets encoded transcription factors required for proper cotyledon formation during embryo development, which could potentially trigger the expression of hundreds of secondary target genes. In particular the most up-regulated targets in dcl1 were two redundant transcription factors: SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE (SPL) (SPL10 and SPL11). Reporter analysis and mutagenesis of the target site revealed that these are bona fide miRNA-156 targets in vivo, as early as the 8-cell stage. Indeed, reducing the levels of SPL10 and SPL11 in the dcl1 background rescues early defects in dcl1 mutants but not later aspects of development. Conversely, disrupting miRNA-156 regulation of SPL10 and SPL11 caused defects during early embryonic morphogenesis, but these were insufficient to phenocopy dcl1 mutant embryos, suggesting that additional targets are likely to account for the full spectrum of the phenotype observed in dcl1 mutants.
Indeed, analyses of SPL10 and SPL11 loss of function mutants suggest that these two transcription factors are likely inactive during early development, but rather appear to function later. If that is the case, what is the role of miRNA-156-mediated regulation? Could miRNA-156-regulation be preventing premature transitions to later embryonic stages? To address these questions the authors undertook a broad analysis of gene expression revealing that genes up-regulated in dcl1 embryos tend to be expressed later in embryonic development (for example, OLEOSINS). Interestingly, this effect was in part suppressed by reducing the levels of SPL11, suggesting that mis-regulation of this target in dcl1 mutants is in part responsible for the premature accumulation of transcripts that are typically expressed in later embryonic stages. Conversely, genes down-regulated in dcl1 embryos tend to be expressed in the early embryonic stages. Together, these results suggest that miRNAs in the early plant embryo have a somewhat different function than the early miRNAs in animals such as miR-430, miR-309, lin-4 or let-7, where instead of preventing premature transitions to later stages, they tend to regulate transcripts expressed in the previous stages to facilitate developmental transitions.
Recent findings in animals suggest a similar miRNA function to that reported by Nodine and Bartel in plants. In mammalian embryos, miRNAs maintain extra-embryonic stem cell properties, blocking expression of apoptotic, Mapk inhibitors and other genes (Spruce et al., 2010). In fish, it was recently demonstrated that let-7 might inhibit expression of regeneration-associated genes, such as ascl1a, hspd1, lin-28, oct4, pax6b and c-myc, preventing premature Muller glia dedifferentiation (Ramachandran et al., 2010).
The findings reported by Nodine and Bartel, together with these recent reports in animals call for a common emerging theme where miRNAs not only clear the past transcriptional memories in the cell, but also prevent precocious activation of differentiation factors. Future studies will be needed to gain further insight into this novel aspect of miRNA regulation and to understand whether in this later context, misregulation of such miRNAs may prevent differentiation, locking the cell in a stem cell state or proliferative state leading to diseases such as cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Carlsbecker A, Lee JY, Roberts CJ, Dettmer J, Lehesranta S, Zhou J, Lindgren O, Moreno-Risueno MA, Vaten A, Thitamadee S, et al. Nature. 2010;465:316–321. doi: 10.1038/nature08977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ. Curr Opin Genet Dev. 2010;20:369–375. doi: 10.1016/j.gde.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Hobert O. Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- Nodine MD, Bartel DP. Genes Dev. 2010;24:2678–2692. doi: 10.1101/gad.1986710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Fausett BV, Goldman D. Nat Cell Biol. 2010;12:1101–1107. doi: 10.1038/ncb2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruce T, Pernaute B, Di-Gregorio A, Cobb BS, Merkenschlager M, Manzanares M, Rodriguez TA. Dev Cell. 2010;19:207–219. doi: 10.1016/j.devcel.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Takacs CM, Giraldez AJ. Semin Cell Dev Biol. 2010;21:760–767. doi: 10.1016/j.semcdb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


