Abstract
Immune-privileged Sertoli cells survive long term after allogeneic or xenogeneic transplantation without the use of immunosuppressive drugs, suggesting they could be used as a vehicle to deliver therapeutic proteins. As a model to test this, we engineered Sertoli cells to transiently produce basal levels of insulin and then examined their ability to lower blood glucose levels after transplantation into diabetic SCID mice. Mouse and porcine Sertoli cells transduced with a recombinant adenoviral vector containing furin-modified human proinsulin cDNA expressed insulin mRNA and secreted insulin protein. Transplantation of 5–20 million insulin-expressing porcine Sertoli cells into diabetic SCID mice significantly decreased blood glucose levels in a dose-dependent manner, with 20 million Sertoli cells decreasing blood glucose levels to 9.8 ± 2.7 mM. Similar results were obtained when 20 million insulin-positive, BALB/c mouse Sertoli cells were transplanted; blood glucose levels dropped to 6.3 ± 2.4 mM and remained significantly lower for 5 days. To our knowledge, this is the first study to demonstrate Sertoli cells can be engineered to produce and secrete a clinically relevant factor that has a therapeutic effect, thus supporting the concept of using immune-privileged Sertoli cells as a potential vehicle for gene therapy.
Keywords: Sertoli cell, Gene therapy, Transplantation, Insulin, Diabetes
INTRODUCTION
Sertoli cells (SC) are somatic cells that reside within the seminiferous epithelium of the testis, where they play an important role in creating the immune-privileged environment of the testis (11,13). In addition to their natural role in protecting the developing spermatozoa, SC are unique because they have the ability to survive long term without the use of immunosuppressive drugs when transplanted across immunologic barriers (20) such as in allogeneic (2,18) or xenogeneic (9,12,23) transplantation. For example, porcine SC have been found to survive transplantation as discordant xenografts in dogs and rodents (9,12).
The ability of SC to survive transplantation when most other cells are destroyed led to the hypothesis that SC can be engineered as a vehicle for gene therapy. Previously, we tested this hypothesis by transplanting green fluorescent protein (GFP)-positive SC, isolated from transgenic mice engineered to constitutively produce GFP, as allografts into BALB/c mice (7). Examination of the grafts 60 days posttransplantation demonstrated that GFP-positive SC survived for a prolonged period of time after allogeneic transplantation (7). This indicates genetically modified SC retain their immune-privileged abilities and therefore, could potentially be useful for gene therapy. However, these experiments were not designed to demonstrate whether SC could produce and secrete a therapeutic protein at a level relevant to treat a disease state.
One model to test the ability of SC to deliver a therapeutic product is transplantation of insulin-expressing SC into streptozotocin-induced diabetic mice. This model was chosen because blood glucose levels allow for an easy measure of biologically functional insulin. Engineering a cell to produce insulin is not straightforward, because the ideal cell must be glucose responsive, process proinsulin correctly, and secrete functional insulin. Because our current objective was to determine whether SC can be engineered to produce a factor, in this case insulin, at a level to treat a disease, the more complex issue of glucose regulation was not addressed by the current study. However, the ability of SC to produce functional insulin is important and our goal was to accomplish this with the addition of furin cleavage sites to proinsulin cDNA. In islet β-cells, proinsulin is cleaved by the prohormone convertases (PC) 2 and 3 to generate mature insulin and C-peptide (3). Given that PC2 and 3 are not ubiquitously expressed, to allow proper cleavage and secretion of mature insulin in cells that lack these enzymes, PC2 and 3 cleavage sites are converted to furin cleavage sites. Furin is a ubiquitously expressed member of the convertase family that processes proteins in the constitutive secretory pathway (28). The addition of furin cleavage sites to proinsulin cDNA allows cells that contain the constitutive pathway of secretion to properly process proinsulin and leads to secretion of the mature product, functional insulin. Furin-modified proinsulin cDNA constructs have been used previously to create insulin-secreting hepatocytes, acinar cells, kidney cells, and muscle cells (14,19,24,29). To the best of our knowledge, the ability of SC to produce insulin has not been examined.
The objective of the current study was to determine whether SC could be altered to express a therapeutic protein at a level that could treat a disease. The model we used was basal insulin production as a method to lower blood glucose levels in streptozotocin-induced diabetic mice. In view of the fact that our future objective is to create transgenic animals with SC that produce therapeutic factors, we tested our current hypothesis with both mouse (a model for transgenic mice) and porcine (a model for a clinically relevant source of tissue) SC. Neonatal porcine Sertoli cells (NPSC) and pubertal mouse Sertoli cells (mSC) were transduced with a recombinant adenoviral vector engineered to express furin-modified human proinsulin cDNA (24). These transduced SC were characterized for insulin production and transplanted into streptozotocin-induced diabetic SCID mice. Blood glucose levels were measured as a therapeutic endpoint and achievement of normoglycemia was considered a success.
MATERIALS AND METHODS
Animals
Male neonatal pigs (Texas Tech University Research and Experimental Farm, aged 1–3 days) and male BALB/c mice (Charles River Laboratories, Wilmington, MA, aged 10–12 days) were used as SC donors. Male SCID mice (Taconic Farms, Germantown, NY or Charles River Laboratories), aged 6–8 weeks, were used as recipients. Mice were rendered diabetic by an intra-peritoneal injection of streptozotocin (275 mg/kg body weight, Sigma Chemical Co., St. Louis, MO) 1 week prior to transplantation. Only those animals exhibiting nonfasting blood glucose values >20 mM were used as recipients. After transplantation, blood samples were obtained from the tail vein of nonfasted animals for glucose assay (OneTouch Ultra; LifeScan, Milpitas, CA). Care and maintenance of all animals was in accordance with the ILAR Care and Use of Laboratory Animals and Texas Tech University Institutional Animal Care and Use Committee approved protocols and the guidelines of the NIH.
Cell Isolation and Adenoviral Transduction
SC were isolated from neonatal pigs or pubertal mice by collagenase and trypsin digestion as described previously (8,9). A typical NPSC preparation after 2 days of culture consists of 92% SC and 2% myoid cells (9) and a typical preparation of mSC at the time of isolation contains 74% SC, 6% myoid cells, and 20% germ cells (25). The germ cells in the mSC preparation are not expected to contribute significantly as they do not survive when cultured at 37°C (17). Cells were cultured overnight as a monolayer on tissue culture treated plates with Dulbecco's modified Eagle medium (DMEM) plus 10% fetal bovine serum (FBS) at 37°C. The next day these cells were transduced with a recombinant adenoviral vector containing furin-modified human proinsulin cDNA (AdCMVhInsM) under the control of the cytomegalovirus (CMV) promoter (24). SC were transduced at a multiplicity of infection (MOI) of 50, 75, 100, or 200 and cultured for 2–20 days in DMEM plus 2% FBS. Controls included cells that were nontransduced (MOI 0) and cells that were transduced with a recombinant adenoviral vector containing wild-type human proinsulin cDNA (AdCMVhInsWT; i.e., without the furin modification) under the control of the CMV promoter (24) or an irrelevant recombinant adenoviral vector containing green fluorescent protein (GFP) cDNA (AdRSVGFP) under the control of the Rous sarcoma virus (RSV) promoter at a MOI of 100.
RNA Isolation and RT-PCR
Freshly isolated SC were cultured overnight as a monolayer on six-well plates with DMEM plus 10% FBS. The next day, cells were transduced with 0 or 100 MOI of AdCMVhInsM, AdCMVhInsWT, or AdRSV GFP. Two days after transduction, cells were lysed in 1 ml of Trizol reagent (Invitrogen, Carlsbad, CA) and total RNA extracted according to the manufacturer's protocol. The RNA was DNase treated (Roche, Indianapolis, IN) and used as template for oligo dT primed cDNA synthesis using MultiScribe Reverse Transcriptase (Applied BioSystems, Foster City, CA) and TaqMan Reverse Transcription Reagents (Applied Biosystems). PCR was performed for insulin and the housekeeping gene β-actin using Fast Start© PCR Master Mix (Roche). Insulin was amplified from 50 ng of cDNA under the following conditions: 94°C for 2 min; 26 cycles of 94°C for 30 s, 61°C for 30 s, 72°C for 30 s, and a final extension of 72°C for 10 min. β-Actin was amplified from 50 ng of cDNA under the following conditions: 94°C for 2 min; 38 cycles of 94°C for 30 s, 52°C for 30 s, 72°C for 30 s, and a final extension of 72°C for 10 min. PCR fragments were separated on a 2% TAE (1× TAE = 40 mM Tris-acetate, 1 mM EDTA) agarose gel at 120 V for 30 min and fragments were visualized by ethidium bromide staining. All amplicons obtained were of the expected size (180 bp for insulin and 500 bp for β-actin). Additionally, the furin-modified insulin PCR product was extracted from the agarose gel using the QiaexII kit (Qiagen, Valencia, CA), ligated into the pCRII-TOPO vector (Invitrogen), and sequenced. Unknown sequences were analyzed using BLAST (NCBI) and compared with known GenBank sequences. The sequenced PCR product was identical to human insulin except for the furin modifications. Primers used were: human insulin forward 5′-GGG ACC TGA CCC AGC CGC A and reverse 5′-CAG GCT GCC TGC ACC AGG G, and β-actin forward 5′-TGT ATT CCC CTC CAT CGT G and reverse 5′-GGA TCT TCA TGA GGT AGT CTG TC.
Immunohistochemistry
SC (2.5 × 105 cells/well) were cultured overnight on chamber slides in DMEM plus 10% FBS (n > 3). The next morning, cells were transduced with 0–200 MOI of AdCMVhInsM or 100 MOI of AdCMVhInsWT or AdRSVGFP and further cultured in DMEM plus 2% FBS. Slides were collected after 2–20 days for the AdCMVhInsM or at day 2 posttransduction for the AdCMVhInsWT or AdRSVGFP and fixed with 1% paraformaldehyde for 30 min, permeabilized with 0.1% Triton X-100, and immunostained for insulin or C-peptide. The C-peptide antibody recognizes both C-peptide and the proinsulin molecule (15). Slides were incubated with 10% hydrogen peroxide, blocked with 20% normal goat serum, and incubated with guinea pig polyclonal anti-swine insulin (1:1000; DAKO, Carpinteria, CA) or mouse anti-human C-peptide (1:500; Cedarlane, Burlington, NC) primary antibodies for 30 min. This was followed by incubation with the appropriate biotinylated secondary antibody (1:200; Vector Laboratories, Burlingame, CA). Sections were then incubated with the ABC-enzyme complex (Vector Laboratories) followed by diaminobenzadine (DAB; Biogenex, San Ramon, CA) as chromagen and counterstained with hematoxylin. Negative controls included cells from each treatment group that were put through the same procedure without primary antibody. All negative controls lacked a positive reaction. The percentage insulin-positive cells at day 17 were determined after immunostaining for insulin. For each slide a minimum of 400 cells were counted. Images were acquired with a Zeiss Axiostar plus microscope and AxioCam MRc digital camera. Images were combined into figures with Adobe Photoshop 7.0.
Human Insulin and Proinsulin ELISAs
SC (2.5 × 105 cells/well) were cultured overnight on chamber slides in DMEM plus 10% FBS (n > 3). The next morning, cells were transduced with 0–200 MOI of AdCMVhInsM or 100 MOI of AdCMVhInsWT or AdRSVGFP and further cultured in DMEM plus 2% FBS. Medium was changed every 2 days and supernatant was collected to measure insulin secretion 2, 6, 12, 16, and 20 days posttransduction for the AdCMVhInsM or at day 2 posttransduction for the AdCMVhInsWT or AdRSVGFP and stored at −80°C. The amount of human insulin secreted by the SC was quantified using a human insulin ELISA kit (Linco Research, Inc., St. Charles, MO) as described by the manufacturer. This kit detects human insulin at 100% specificity, des(64,65) human proinsulin at 117% specificity and des(31,32) human proinsulin at 0.3% specificity. Human proinsulin and human C-peptide are not detectable at concentrations up to 120 nM with this kit. The amount of human proinsulin secreted by the SC was quantified using a human proinsulin ELISA kit (Linco Research, Inc.) as described by the manufacturer. This kit detects intact human proinsulin at 100% specificity and des(64,65) human proinsulin at 36% specificity. Human insulin and des(31,32) human proinsulin are not detectable with this kit. DMEM plus 2% FBS was used as the control.
Transplantation and Graft Characterization
For transplantation, cells that had been transduced with AdCMVhInsM or AdRSVGFP at a MOI of 100 and cultured for 24 h were transferred to nontreated petri dishes and cultured in Ham's F10 media (supplemented with 10 mM d-glucose, 2 mM l-glutamine, 50 μM isobutylmethylxanthine, 0.5% bovine serum albumin, 10 mM nicotinamide, 100 U/ml penicillin, 100 μg/ml streptomycin) and 10% FBS for 24 h at 37°C to allow the formation of SC aggregates (50–300 mm diameter) (6,7,9) prior to transplantation under the kidney capsule. The number of SC was calculated as described (9) based on 6.6 pg of DNA/cell and using a PicoGreen dsDNA quantitation assay (Invitrogen). Aliquots consisting of 5,10, or 20 × 106 cells were placed in polypropylene microcentrifuge tubes, aspirated into polyethylene tubing (PE-50), pelleted by centrifugation, and gently placed within the left renal subcapsular space of isofluorane-anesthetized diabetic SCID mice (5).
Grafts were removed for histological analysis between 1 and 30 days posttransplantation. The graft-bearing kidneys were immersed in Z-fix (Anatech LTD, Battle Creek, MI) and embedded in paraffin. Tissue sections were immunostained for insulin, vimentin (to identify NPSC), GATA-4 (to identify mSC), or proliferating cell nuclear antigen (PCNA) as described previously (8,9). The vimentin antibody does not cross-react with mouse tissue and is, therefore, specific to transplanted porcine tissue. Primary antibodies included mouse monoclonal anti-vimentin (1:100; Dako), mouse monoclonal anti-PCNA (1:50; Dako), mouse monoclonal anti-human GATA-4 (1:50; Santa Cruz Biotechnology, Santa Cruz, CA), and guinea pig polyclonal anti-swine insulin (1: 1000; DAKO). After incubation with primary antibody, sections were incubated with biotinylated secondary antibodies followed by the ABC-enzyme complex with DAB as chromagen. All sections were counterstained with hematoxylin. Negative controls included tissue sections that were put through the same procedure without primary antibody. All negative controls lacked a positive reaction.
Statistical Analysis
Data are expressed as the mean ± SEM of n independent experiments. The significance of differences between two groups was calculated by paired or unpaired Student t-test. For multiple comparisons, a one-way analysis of variance (ANOVA) was used. When significance was observed, comparisons between groups were made using the Fisher's PLSD test. A value of p < 0.05 was considered significant.
RESULTS
Insulin Production by Adenovirus-Transduced Porcine Sertoli Cells
In order to determine the optimal MOI for insulin protein production, NPSC were transduced with a recombinant adenoviral vector engineered to express furin-modified human proinsulin cDNA (AdCMV hInsM) at a MOI of 0, 50, 75, 100, or 200; 2 days posttransduction insulin immunohistochemistry and ELISA were performed on the cultured cells and supernatent, respectively. By immunohistochemistry there was an apparent dose-dependent increase in the amount of insulin detected within the NPSC (Fig. 1A, a–e) with the highest amount at 200 MOI. However, at 200 MOI the cells appeared unhealthy (Fig. 1A, e), while at 100 MOI the cells were both healthy and produced insulin (Fig. 1A, d). Additionally, the amount of insulin secreted by the NPSC into the culture media as measured by ELISA was higher at 100 MOI (Table 1) than at 200 MOI (7.3 ± 3.6 × 10−7 μg of insulin/cell). Therefore, the amount of AdCMVhInsM needed for optimal insulin protein production by NPSC was 100 MOI and all further experiments were performed at a MOI of 100.
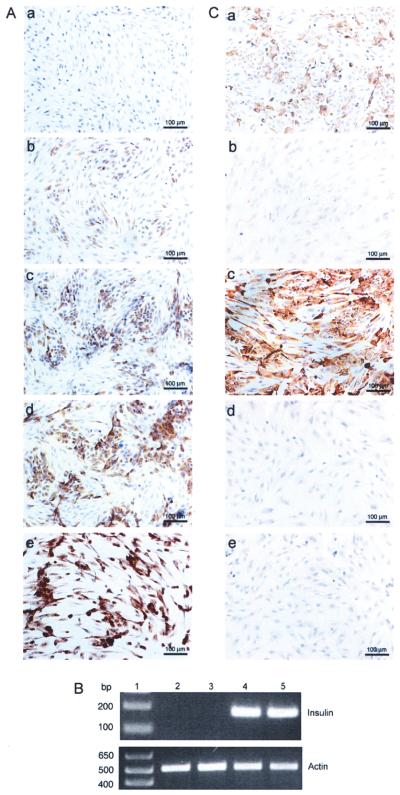
Figure 1.
Production of insulin by adenovirus transduced neonatal porcine Sertoli cells. (A, C) NPSC were cultured overnight as a monolayer on chamber slides, transduced with AdCMVhInsM at a MOI of 0 (A, a), 50 (A, b), 75 (A, c), 100 (A, d and C, a), or 200 (A, e), AdCMVhInsWT (C, b and c) at a MOI of 100 or AdRSVGFP (C, d and e) at a MOI of 100. Slides were collected after 2 days, fixed with 1% paraformaldehyde, and immunostained for insulin (A, a–e and C, b and d) or C-peptide (C, a, c, and e). All sections were counterstained with hematoxylin. (B) NPSC cultured overnight as a monolayer on six-well plates with DMEM plus 10% FBS were either nontransduced (MOI of 0; lane 2) or transduced with AdRSVGFP (lane 3), AdCMVhInsWT (lane 4), or AdCMVhInsM (lane 5) adenoviral vectors at a MOI of 100. Cells were collected after 2 days and RT-PCR was performed for insulin or β-actin. Lane 1 is the 1 kb Plus DNA Ladder (Invitrogen).
Table 1.
Insulin and Proinsulin Levels Secreted by Neonatal Porcine Sertoli Cells Cultured for 2 Days After Transduction
| Group | Insulin (10−7 μg/Cell) |
Proinsulin (10−5 μg/Cell) |
|---|---|---|
| MOI 0 | 0 | 0 |
| GFP MOI 100 | 0 | 0 |
| Wild-type MOI 100 | 2.2 ± 0.3 | 7.1 ± 1.6 |
| Mutant MOI 100 | 39 ± 27* | 0 |
Values are means ± SEM.
Insulin levels were not significantly higher (p = 0.086) than wild-type levels. Statistical significance of difference was calculated by unpaired t-test.
The production of insulin mRNA and protein by NPSC transduced with AdCMVhInsM at a MOI of 100 was analyzed by RT-PCR (Fig. 1B), immunohistochemistry (Fig. 1A, C), and ELISA. Controls included NPSC nontransduced, transduced with a recombinant adenoviral vector containing wild-type human proinsulin cDNA (AdCMVhInsWT), or transduced with an irrelevant recombinant adenoviral vector containing GFP cDNA (AdRSVGFP) at a MOI of 100. Insulin mRNA and protein were undetectable in nontransduced NPSC (Fig. 1A, a and B, lane 2) and NPSC transduced with AdRSVGFP (Fig. 1C, d and B, lane 3). The amount of insulin secreted into the culture media, as measure by insulin ELISA, was also below the limit of detection in these cells (Table 1). Cells transduced with AdCMVhInsWT expressed insulin mRNA (Fig. 1B, lane 4) but inefficiently processed proinsulin and thus demonstrated very low insulin protein expression (Fig. 1C, b). In contrast, NPSC transduced with the AdCMVhInsM produced insulin mRNA (Fig. 1B, lane 5) and protein (Fig. 1A, d) and secreted 18-fold higher levels of insulin than NPSC transduced with AdCMVhInsWT (Table 1).
In addition, the amount of proinsulin secreted into the culture media was measured by proinsulin ELISA and was not detected in cells nontransduced or transduced with AdRSVGFP or AdCMVhInsM (Table 1). This indicates the addition of furin cleavage sites to proinsulin allowed NPSC to efficiently process and secrete insulin. Proinsulin was present in the culture media of cells transduced with AdCMVhInsWT (Table 1), indicating the PC2 and PC3 cleavage sites in wild-type human proinsulin were inefficiently processed. Additional confirmation that NPSC transduced with AdCMVhInsM synthesize insulin protein was obtained by visualization of a positive immunohistochemical reaction for human specific C-peptide (Fig. 1C, a). Nontransduced NPSC (data not shown) and NPSC transduced with AdRSVGFP (Fig. 1C, e) were negative for C-peptide. NPSC transduced with AdCMVhInsWT were positive when immunostained with the C-peptide antibody (Fig. 1C, c), which is expected because C-peptide is also present in proinsulin.
Transplantation of Insulin-Expressing Porcine Sertoli Cells
NPSC transduced with AdCMVhInsM at the optimal MOI of 100 were cultured for 2 days and then transplanted underneath the kidney capsule of diabetic SCID mice. SCID mice were used as recipients in order to test the concept of engineering SC without interference from the immune system. Transplanted insulin-expressing NPSC significantly decreased blood glucose levels within 1 day after transplantation in a dose-dependent manner with 5, 10, and 20 million NPSC leading to an average decrease of 8.0, 10.3, and 16.2 mM glucose, respectively (Fig. 2). When 20 million NPSC were transplanted, the average blood glucose levels dropped to 9.8 ± 2.7 mM and remained significantly decreased for 5 days. In contrast, transplantation of 20 million NPSC transduced with AdRSVGFP at a MOI of 100 had no effect on blood glucose levels (Fig. 2A, n = 5; four of five mice had no change in blood glucose levels and the fifth mouse was found dead after transplantation due to hyperglycemia).
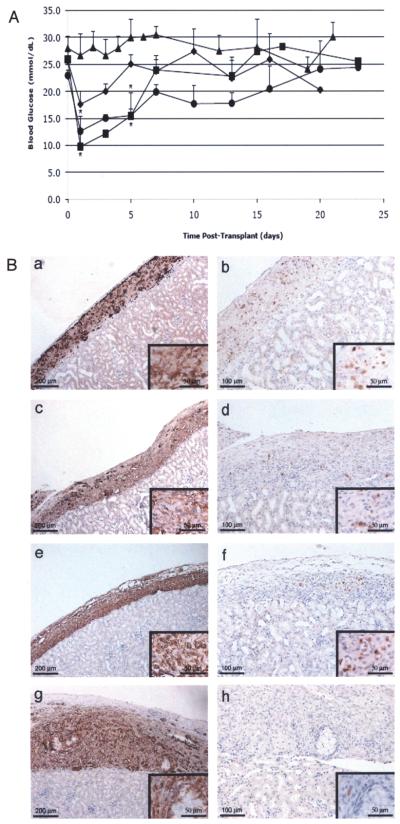
Figure 2.
Transplantation of insulin-expressing neonatal porcine Sertoli cells into diabetic SCID mice. (A) Average blood glucose values ± SEM of SCID mice after transplantation with 5 (filled diamonds; n = 5), 10 (filled circles; n = 4), or 20 (filled squares; n = 3) million insulin-expressing NPSC or 20 million (filled triangles; n = 4) GFP-expressing NPSC. NPSC were transduced with the AdCMVhInsM or AdRSVGFP adenoviral vector at a MOI of 100. *Mean blood glucose values were significantly decreased compared to the AdRSVGFP group values (p < 0.05). (B) Grafts were removed from SCID mice at 3 (a and b), 8 (c and d), 13 (e and f), and 30 (g and h) days after transplantation with NPSC and immunostained for the SC marker vimentin (column 1; a, c, e, and g) or insulin (column 2; b, d, f, and h). Higher magnification photomicrographs are included as insets (a–h). All sections were counterstained with hematoxylin.
The adenoviral vector is ideal for efficient high expression of the gene of interest. However, the epichromosomal nature of the adenoviral vector does not allow for stable integration of the insulin construct into the SC genome. Additionally, the NPSC were proliferating, as demonstrated by a positive signal when immunostained for PCNA (data not shown). Together, these factors probably led to a loss of insulin expression, which was the reason for the transient decrease in blood glucose levels and subsequent return to the diabetic state (>20 mM) within 15 days (Fig. 2A). To confirm the loss of insulin expression, nephrectomies were performed between 3 and 30 days after transplantation and tissue sections were immunostained for detection of NPSC (vimentin) and insulin (Fig. 2B). Consistent with a significant decrease in blood glucose levels prior to 5 days posttransplant, at 3 days the majority of the cells present within the NPSC grafts were insulin positive (Fig. 2B, a and b). As the blood glucose levels rose, the number of insulin-positive NPSC also appeared to decrease (Fig. 2B, column 2) with fewer insulin-positive NPSC present at 8 and 13 days (Fig. 2B, c–f), and only 2 to 10 insulin-positive cells detected within the tissue sections after 30 days (Fig. 2B, h). The loss of insulin expression was most likely due to loss of the adenoviral vector and not NPSC death as the majority of the cells within the grafts were vimentin-positive from 3 to 30 days (Fig. 2B, column 1). As expected, immune cell infiltrate was not detected in the grafts (Fig. 2B).
In Vitro Culture of Insulin-Positive Porcine Sertoli Cells
NPSC transduced with AdCMVhInsM at a MOI of 100 were cultured for 2 to 20 days posttransduction, media was collected for human insulin ELISA, and cells were fixed for insulin immunohistochemistry (Fig. 3). NPSC secreted detectable levels of insulin (>11 × 10−7 μg/cell/2 days) from days 2 to 12 posttransduction. The amount of insulin secreted by the NPSC significantly decreased (p < 0.0011) and was barely detectable by 16 and 20 days (<0.6 × 10−7 μg/cell/2 days). Correspondingly, insulin, as measured by immunostaining, was present in the NPSC from 2 to 12 days after transduction (Fig. 1A, d, and Fig. 3A and B), and then the number of insulin-positive NPSC decreased (Fig. 3C) with less than 2% insulin-positive NPSC detected at 17 days (Fig. 3D). Viable insulin-negative NPSC were still present on the slides for up to 20 days (data not shown).
Figure 3.
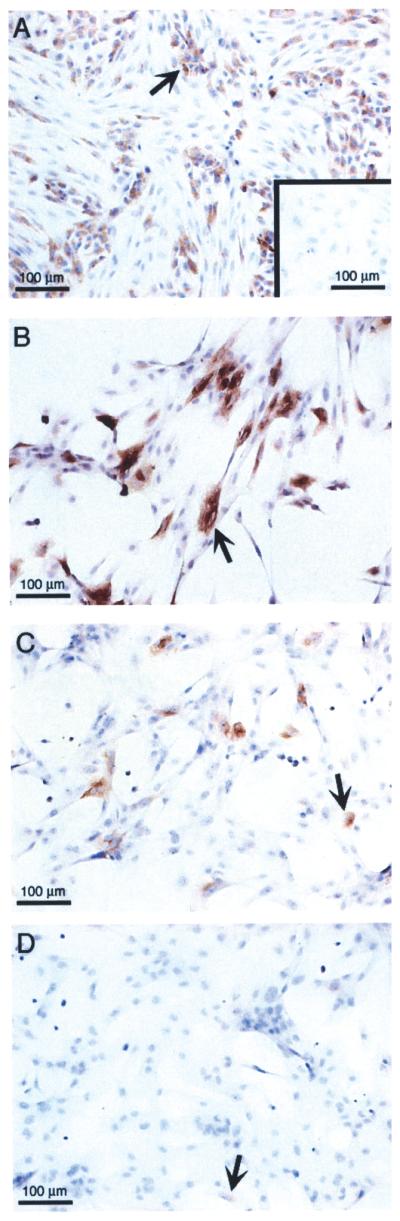
Long-term culture of neonatal porcine Sertoli cells transduced with the furin-modified adenoviral vector. NPSC were cultured overnight as a monolayer on chamber slides, transduced with AdCMVhInsM at a MOI of 0 (A, inset) or 100 (A–D) and collected after 5 (A), 12 (B), 15 (C), or 17 (D) days. Slides were fixed with 1% paraformaldehyde and immunostained for insulin. All sections were counterstained with hematoxylin. Arrow shows insulin-positive SC.
Characterization and Transplantation of Insulin-Expressing Mouse Sertoli Cells
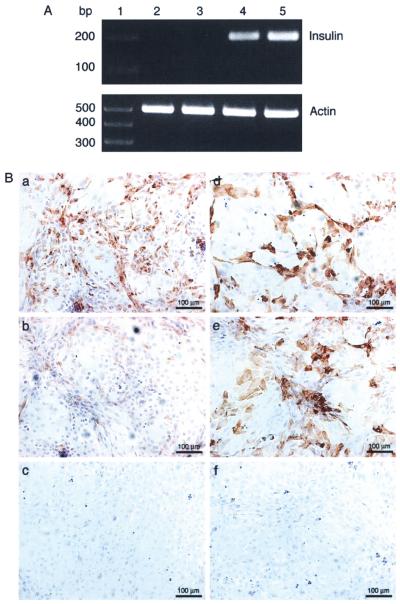
The ability of NPSC to produce biologically relevant levels of insulin was examined because porcine cells have potential as a relevant source of tissue for clinical xenotransplantation. However, because our ultimate goal is to create a transgenic animal with modified SC that can be used as an unlimited source of SC for clinical xenotransplantation, the first step will be to create transgenic mice with genetically modified SC. Therefore, it was important to examine the ability of mSC to produce biologically relevant levels of insulin. SC isolated from 10–12-day-old mice were transduced with AdCMV hInsM at a MOI of 100 and results comparable to those reported for NPSC were obtained. mSC engineered to express furin-modified human proinsulin expressed insulin mRNA (Fig. 4A, lane 5), produced insulin protein (Fig. 4B, a), and secreted insulin into the culture media (Table 2). In contrast, nontransduced mSC and mSC transduced with AdRSVGFP were negative for insulin mRNA (Fig. 4A, lanes 2 and 3) and protein (Fig. 4B, c and data not shown) and insulin was not detectable in the culture media (Table 2). mSC transduced with AdC MVhInsWT expressed insulin mRNA (Fig. 4A, lane 4) but very little protein was detected by imunohisto-chemistry (Fig. 4B, b) and significantly less insulin was secreted from these cells (Table 2) than cells transduced with AdCMVhInsM. Proinsulin was not detected in the culture media of mSC nontransduced, transduced with AdRSVGFP, or transduced with AdCMVhInsM, while cells transduced with AdCMVhInsWT secreted proinsulin (Table 2). C-peptide was detected in mSC transduced with AdCMVhInsM (Fig. 4B, d), while nontransduced mSC (data not shown) and mSC transduced with AdRS VGFP (Fig. 4B, f) were negative. As expected, mSC transduced with AdCMVhInsWT were positive for proinsulin (Fig. 4B, e).
Figure 4.
In vitro expression of insulin and C-peptide or proinsulin by adenovirus transduced mouse Sertoli cells. (A) mSC cultured overnight as a monolayer on six-well plates with DMEM plus 10% FBS were either nontransduced (MOI of 0; lane 2) or transduced with AdRSVGFP (lane 3), AdCMVhInsWT (lane 4), or AdCMVhInsM (lane 5) adenoviral vectors at a MOI of 100. Cells were collected after 2 days and RT-PCR was performed for insulin or β-actin. Lane 1 is the 1kb Plus DNA Ladder (Invitrogen). (B) mSC cultured overnight as a monolayer on chamber slides were transduced with AdCMVhInsM (a and d), AdCMVhInsWT (b and e), or AdRSVGFP (c and f) adenoviral vectors at a MOI of 100. Slides were collected after 2 days, fixed with 1% paraformaldehyde and immunostained for insulin (a–c) or C-peptide (d–f). All sections were counterstained with hematoxylin.
Table 2.
Insulin and Proinsulin Levels Secreted by Mouse Sertoli Cells Cultured for 2 Days Posttransduction
| Group | Insulin (10−7 μ/Cell) |
Proinsulin (10−5 μ/Cell) |
|---|---|---|
| MOI 0 | 0 | 0 |
| GFP MOI 100 | 0 | 0 |
| Wild-type MOI 100 | 2.3 ± 0.5 | 1.1 ± 0.1 |
| Mutant MOI 100 | 15 ± 0.2* | 0 |
Values are means ± SEM.
Insulin levels were significantly higher (p = 0.004) than wild-type levels. Statistical significance of difference was calculated by unpaired t-test.
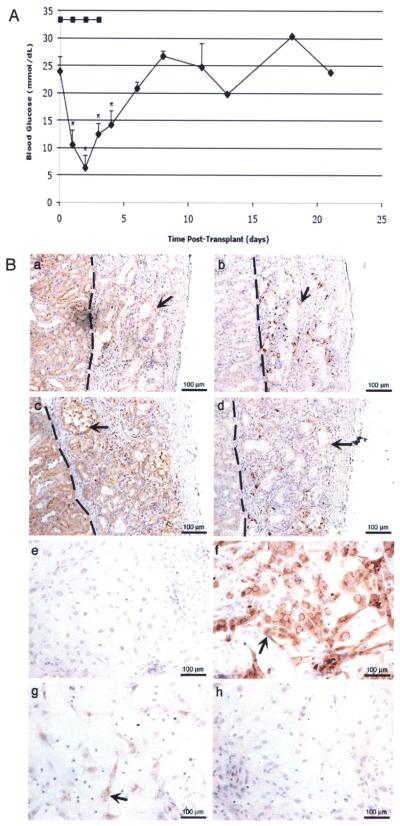
Moreover, when 20 million insulin-expressing mSC were transplanted under the kidney capsule of diabetic SCID mice, blood glucose levels dropped significantly within 1 day, were as low as 6.3 ± 2.4 mM within 2 days and remained significantly decreased for 4 days (Fig. 5A). Transplantation of mSC transduced with the AdRSVGFP had no effect on blood glucose levels (Fig. 5A, n = 1; two mice were initially transplanted but the second mouse died due to the diabetic state). Immunohistochemical analysis of AdCMVhInsM transduced mSC grafts harvested between 8 and 30 days posttransplantation confirmed the proliferation (by PCNA immunohistochemistry; data not shown) and survival of GATA-4-positive mSC and no immune cell infiltrate throughout the study (Fig. 5B, a and c). Similar to the results with NPSC, there was an apparent decrease in the number of insulin-positive mSC over time. At 8 days, several insulin-positive mSC were detected within the grafts (Fig. 5B, b); the number of insulin-positive cells then appeared to decrease with far fewer insulin-positive mSC detectable after 30 days (Fig. 5B, d). Similarly, when mSC transduced with AdCMVhInsM were cultured in vitro for 5 to 20 days, the number of insulin-positive cells decreased dramatically between 5 and 12 days (Fig. 5B, f and g) with very few insulin-positive cells remaining at 20 days (Fig. 5B, h). Thus, insulin-positive NPSC and mSC are able to synthesize and secrete functional insulin protein at biologically relevant levels that lowered blood glucose levels in diabetic mice. This demonstrates the potential of genetically engineered SC to act as a vehicle for gene therapy.
Figure 5.
Transplantation of insulin-expressing mouse Sertoli cells and immunohistochemical analysis of Sertoli cells transduced with the furin-modified adenoviral vector. (A) Average blood glucose values ± SEM of SCID mice after transplantation with 20 million mSC. mSC were transduced with AdCMVhInsM (filled diamonds; n = 3) or AdRSVGFP (filled squares; n = 1) at a MOI of 100. *Mean blood glucose values were significantly decreased compared to pretransplant values (p < 0.05). (B) mSC grafts were removed from SCID mice at 8 (a and b) and 30 (c and d) days after transplantation with mSC and immunostained for the SC marker GATA-4 (a and c) or insulin (b and d). The graft and kidney are separated by a dotted line. mSC cultured overnight as a monolayer on chamber slides were transduced with AdCMVhInsM at a MOI of 0 (e) or 100 (f–h) and collected after 5 (e and f), 12 (g), or 20 (h) days. Slides were fixed with 1% paraformaldehyde and immunostained for insulin (e-h). All sections were counterstained with hematoxylin. Arrow shows SC.
DISCUSSION
Allogeneic transplantation as a replacement therapy for damaged or destroyed cells has potential in treatment of diseases such as type I diabetes or Parkinson's disease. Still, this procedure is limited by the need for chronic immunosuppressive therapy and the paucity of organ donors (16). To overcome the shortage of available organ donors, one could potentially use pigs as an unlimited supply of transplantable tissue. Even so, the benefits of transplantation of porcine tissue, such as pancreatic islets, remain threatened by immunological destruction (10). Engineering an immune-privileged cell, like the SC, that would survive transplantation without the need for immunosuppression to produce therapeutically relevant factor(s) is a novel approach to bypass these issues.
We previously explored the idea of genetically altering SC for transplantation by transplanting SC isolated from transgenic mice modified to stably express GFP (7). These SC survived allogeneic transplantation and expressed GFP throughout the duration of the study (60 days) (7). However, while this verified the immune-privileged status of genetically modified SC, it did not demonstrate their ability to either express a clinically relevant factor or to treat a disease. More recently, Triveldi et al. demonstrated short-term production of human neurotrophin-3 (hNT-3) by SC after allogeneic transplantation (30). In that article, Lewis rat SC were transduced with an adenoviral vector that expressed hNT-3 under the control of the CMV promoter and transplanted as allografts to the injured spinal cord of Sprague-Dawley rats. Cell survival and hNT-3 production were examined. Allogeneic SC survived for at least 42 days, but hNT-3 production by these cells was only detectable for 3 days after transplantation. While these results support the concept of genetically modified SC surviving transplantation and expressing a transgene, they were unable to demonstrate in vivo function of the transgene.
In the current study, we explored the possibility of engineering SC to produce a therapeutically relevant protein; specifically, we modified them to express basal levels (nonglucose regulated) of insulin. SC isolated from neonatal pigs and pubertal mice were transduced with AdCMVhInsM and examined for the ability to express and secrete biologically active insulin. As shown in our results, these SC produced insulin mRNA and protein and secreted functional insulin. In addition, insulin was secreted at levels able to normalize blood glucose levels after transplantation into immune-compromised diabetic SCID mice. To the best of our knowledge, this is the first study to show genetically modified SC can express levels of a factor that can treat a disease in vivo.
Even so, in this study, the expression of insulin was transient. Because our current objective was to test the concept that SC can be genetically engineered as a vehicle for gene therapy, an adenoviral vector was used to drive insulin production. Use of an adenoviral vector as a delivery system was advantageous because of ease of use and the possibility of high levels of gene expression in the majority of transduced cells (26,27). However, due to the epichromosomal location of the vector, combined with proliferation of the SC, we were only able to achieve transient gene expression (26,27). There are currently other viral vectors and transgenic technologies (26,27) that could be used to overcome the obstacle of transient gene expression and now that we have demonstrated that SC can express functional insulin, future studies will focus on creating stable gene expression in SC.
Another factor related to the transient decrease in blood glucose levels is that very large amounts of human insulin are needed to lower blood glucose levels in mice. If the amount of insulin secreted by the mSC or NPSC over 2 days of culture is converted to units of insulin and adjusted for the 20 million cells transplanted, this is equivalent to approximately 17 or 44 U/kg/day, respectively. This value seems excessive when compared to the amount of insulin typically used for treatment of humans with diabetes (0.5–1.0 U/kg). However, it is important to point out that human insulin has been shown to be less effective in rodents than in humans, where 20–40 U/kg was needed to normalize blood glucose levels in rats (21,31). Taking this into consideration the amount of insulin secreted by the transduced mSC or NPSC and needed to lower blood glucose levels was similar to the previous reports.
The goal of gene therapy is to restore wild-type function in disease states by replacing defective or deficient proteins. While viral vectors are the most commonly used gene therapy method, because they generate sufficient expression of the gene of interest (26,27), the use of these vectors raises additional concerns regarding stable gene expression, safety, toxicity, and immunogenicity (22,27). As an alternative, generation of transgenic animals that express therapeutic factors could overcome the issues of transient gene expression and safety, but would still be subject to immune rejection. The use of SC, isolated from transgenic animals, engineered to express clinically relevant factors specifically in SC (using a SC specific promoter) presents a novel method by which gene therapy can overcome several of these issues, by providing long-term gene expression and an immune-privileged environment. Combining the results of the current study with our previous experiments using GFP transgenic mice (7), our findings provide further support for this concept.
In theory, genetically modified SC could be designed to produce many different therapeutically relevant factors, such as insulin, glucagon-like peptide-1 (GLP-1), or factor VIII for the treatment of diabetes or hemophilia, respectively. The current study examined the production of insulin by SC after introduction of furin-modified human proinsulin cDNA. The addition of furin cleavage sites allowed SC to properly process and secrete functional insulin. While our goal was not to make this construct glucose responsive, but instead to provide proof of concept, this construct still has potential therapeutic value by providing continuous basal levels of insulin. According to a previous study, basal insulin production combined with conventional insulin treatment, results in near normal glucose metabolism without fasting hypoglycemia (4). Glucose-regulated insulin secretion is also an area of great interest and active research, but is beyond the scope of this study. Currently, various methods such as glucose-regulated promoters and glucose-regulated production of furin have been investigated, but to date, no solution has been found (1,32,33).
In this study, we demonstrate that both mouse and porcine SC can be altered to express a biologically active and therapeutically relevant factor, insulin, at levels applicable for the treatment of a disease. Combined with our previous results demonstrating long-term survival of allogeneic and xenogeneic SC (5,7-9) and survival of allogeneic GFP-positive transgenic SC (7), these data suggest that immune-privileged cells genetically modified to secrete clinically relevant proteins could be created as an unlimited source of tissue for transplantation. Future studies will examine the ability of these engineered cells to treat disease conditions after transplantation into immune-competent animals.
ACKNOWLEDGMENTS
This work was supported in part by the American Diabetes Association, Texas Advanced Research Program and Texas Tech University Health Sciences Center School of Medicine Seed Grant Program. We would like to thank Patricia Frisbie and Stanley Harris (Texas Tech University Research and Experimental Farm) for technical assistance, Dr. Susan SanFrancisco (Center for Biotechnology and Genomics, Texas Tech University) for sequencing, and the Vector Core, Gene Therapy Program, Division of Medical Genetics, Department of Medicine at the University of Pennsylvania, School of Medicine for providing the adenoviral constructs. J. M. Dufour is a member of the Scientific Advisory Board for Sernova Corp.
REFERENCES
- 1.Barry SC, Ramesh N, Lejnieks D, Simonson WT, Kemper L, Lernmark A, Osborne WR. Glucose-regulated insulin expression in diabetic rats. Hum. Gene Ther. 2001;12(2):131–139. doi: 10.1089/104303401750061195. [DOI] [PubMed] [Google Scholar]
- 2.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377(6550):630–632. doi: 10.1038/377630a0. [DOI] [PubMed] [Google Scholar]
- 3.Davidson HW. (Pro)Insulin processing: A historical perspective. Cell Biochem. Biophys. 2004;40(3 Suppl.):143–158. doi: 10.1385/cbb:40:3:143. [DOI] [PubMed] [Google Scholar]
- 4.Dong H, Altomonte J, Morral N, Meseck M, Thung SN, Woo SL. Basal insulin gene expression significantly improves conventional insulin therapy in type 1 diabetic rats. Diabetes. 2002;51(1):130–138. doi: 10.2337/diabetes.51.1.130. [DOI] [PubMed] [Google Scholar]
- 5.Dufour JM, Dass B, Halley KR, Korbutt GS, Dixon DE, Rajotte RV. Sertoli cell line lacks the immunoprotective properties associated with primary Sertoli cells. Cell Transplant. 2008;17(5):525–534. doi: 10.3727/096368908785096033. [DOI] [PubMed] [Google Scholar]
- 6.Dufour JM, Hamilton M, Rajotte RV, Korbutt GS. Neonatal porcine Sertoli cells inhibit human natural antibody-mediated lysis. Biol. Reprod. 2005;72(5):1224–1231. doi: 10.1095/biolreprod.104.038315. [DOI] [PubMed] [Google Scholar]
- 7.Dufour JM, Hemendinger R, Halberstadt CR, Gores P, Emerich DF, Korbutt GS, Rajotte RV. Genetically engineered Sertoli cells are able to survive allogeneic transplantation. Gene Ther. 2004;11(8):694–700. doi: 10.1038/sj.gt.3302218. [DOI] [PubMed] [Google Scholar]
- 8.Dufour JM, Lord SJ, Kin T, Rayat GR, Dixon DE, Bleackley RC, Korbutt GS, Rajotte RV. Comparison of successful and unsuccessful islet/Sertoli cell cotransplant grafts in streptozotocin-induced diabetic mice. Cell Transplant. 2008;16(10):1029–1038. [PubMed] [Google Scholar]
- 9.Dufour JM, Rajotte RV, Seeberger K, Kin T, Korbutt GS. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation. 2003;10(6):577–586. doi: 10.1034/j.1399-3089.2003.00059.x. [DOI] [PubMed] [Google Scholar]
- 10.Dufrane D, Gianello P. Pig islet xenotransplantation into non-human primate model. Transplantation. 2008;86(6):753–760. doi: 10.1097/TP.0b013e3181840f55. [DOI] [PubMed] [Google Scholar]
- 11.Fijak M, Meinhardt A. The testis in immune privilege. Immunol. Rev. 2006;213:66–81. doi: 10.1111/j.1600-065X.2006.00438.x. [DOI] [PubMed] [Google Scholar]
- 12.Gores PF, Hayes DH, Copeland MJ, Korbutt GS, Halberstadt C, Kirkpatrick SA, Rajotte RV. Long-term survival of intratesticular porcine islets in nonimmunosuppressed beagles. Transplantation. 2003;75(5):613–618. doi: 10.1097/01.TP.0000052376.89400.8D. [DOI] [PubMed] [Google Scholar]
- 13.Griswold MD. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998;9(4):411–416. doi: 10.1006/scdb.1998.0203. [DOI] [PubMed] [Google Scholar]
- 14.Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J. Biol. Chem. 1994;269(8):6241–6245. [PubMed] [Google Scholar]
- 15.Hilgert I, Stolba P, Kristofova H, Stefanova I, Bendlova B, Lebl M, Horejsi V. A monoclonal antibody applicable for determination of C-peptide of human proinsulin by RIA. Hybridoma. 1991;10(3):379–386. doi: 10.1089/hyb.1991.10.379. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Moore DJ, Ketchum RJ, Nunemaker CS, Kovatchev B, McCall AL, Brayman KL. Resolving the conundrum of islet transplantation by linking metabolic dysregulation, inflammation, and immune regulation. Endocr. Rev. 2008;29(5):603–630. doi: 10.1210/er.2008-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ikeda M, Kodama H, Fukuda J, Shimizu Y, Murata M, Kumagai J, Tanaka T. Role of radical oxygen species in rat testicular germ cell apoptosis induced by heat stress. Biol. Reprod. 1999;61(2):393–399. doi: 10.1095/biolreprod61.2.393. [DOI] [PubMed] [Google Scholar]
- 18.Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46(2):317–322. doi: 10.2337/diab.46.2.317. [DOI] [PubMed] [Google Scholar]
- 19.Martinenghi S, Cusella De Angelis G, Biressi S, Amadio S, Bifari F, Roncarolo MG, Bordignon C, Falqui L. Human insulin production and amelioration of diabetes in mice by electrotransfer-enhanced plasmid DNA gene transfer to the skeletal muscle. Gene Ther. 2002;9(21):1429–1437. doi: 10.1038/sj.gt.3301804. [DOI] [PubMed] [Google Scholar]
- 20.Mital P, Kaur G, Dufour JM. Immunoprotective sertoli cells: making allogeneic and xenogeneic transplantation feasible. Reproduction. 2010;139(3):495–504. doi: 10.1530/REP-09-0384. [DOI] [PubMed] [Google Scholar]
- 21.Pepper AR, Gall C, Mazzuca DM, Melling CW, White DJ. Diabetic rats and mice are resistant to porcine and human insulin: Flawed experimental models for testing islet xenografts. Xenotransplantation. 2009;16(6):502–510. doi: 10.1111/j.1399-3089.2009.00548.x. [DOI] [PubMed] [Google Scholar]
- 22.Romano G, Michell P, Pacilio C, Giordano A. Latest developments in gene transfer technology: Achievements, perspectives, and controversies over therapeutic applications. Stem Cells. 2000;18(1):19–39. doi: 10.1634/stemcells.18-1-19. [DOI] [PubMed] [Google Scholar]
- 23.Saporta S, Cameron DF, Borlongan CV, Sanberg PR. Survival of rat and porcine Sertoli cell transplants in the rat striatum without cyclosporine-A immunosuppression. Exp. Neurol. 1997;146(2):299–304. doi: 10.1006/exnr.1997.6493. [DOI] [PubMed] [Google Scholar]
- 24.Shifrin AL, Auricchio A, Yu QC, Wilson J, Raper SE. Adenoviral vector-mediated insulin gene transfer in the mouse pancreas corrects streptozotocin-induced hyper-glycemia. Gene Ther. 2001;8(19):1480–1489. doi: 10.1038/sj.gt.3301544. [DOI] [PubMed] [Google Scholar]
- 25.Sipione S, Simmen KC, Lord SJ, Motyka B, Ewen C, Shostak I, Rayat GR, Dufour JM, Korbutt GS, Rajotte RV, Bleackley RC. Identification of a novel human granzyme B inhibitor secreted by cultured Sertoli cells. J. Immunol. 2006;177(8):5051–5058. doi: 10.4049/jimmunol.177.8.5051. [DOI] [PubMed] [Google Scholar]
- 26.Somia N, Verma IM. Gene therapy: Trials and tribulations. Nat. Rev. Genet. 2000;1(2):91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 27.Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat. Rev. Genet. 2003;4(5):346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- 28.Thomas G. Furin at the cutting edge: From protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3(10):753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thule PM, Liu J, Phillips LS. Glucose regulated production of human insulin in rat hepatocytes. Gene Ther. 2000;7(3):205–214. doi: 10.1038/sj.gt.3301076. [DOI] [PubMed] [Google Scholar]
- 30.Trivedi AA, Igarashi T, Compagnone N, Fan X, Hsu JY, Hall DE, John CM, Noble-Haeusslein LJ. Suitability of allogeneic sertoli cells for ex vivo gene delivery in the injured spinal cord. Exp. Neurol. 2006;198(1):88–100. doi: 10.1016/j.expneurol.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Wennberg L, Song Z, Bennet W, Zhang J, Nava S, Sundberg B, Bari S, Groth CG, Korsgren O. Diabetic rats transplanted with adult porcine islets and immunosuppressed with cyclosporine A, mycophenolate mofetil, and leflunomide remain normoglycemic for up to 100 days. Transplantation. 2001;71(8):1024–1033. doi: 10.1097/00007890-200104270-00002. [DOI] [PubMed] [Google Scholar]
- 32.Won JC, Rhee BD, Ko KS. Glucose-responsive gene expression system for gene therapy. Adv. Drug Deliv. Rev. 2009;61(7–8):633–640. doi: 10.1016/j.addr.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Xu R, Li H, Tse LY, Kung HF, Lu H, Lam KS. Diabetes gene therapy: Potential and challenges. Curr. Gene Ther. 2003;3(1):65–82. doi: 10.2174/1566523033347444. [DOI] [PubMed] [Google Scholar]