The small micromeres are multipotent cells of the sea urchin embryo that populate the coelomic pouches and contribute to various tissues of the adult (Juliano and Wessel 2010). This lineage forms by two sequential unequal cleavages, divides only once prior to gastrulation, and accumulates several mRNAs and proteins selectively, including vasa, nanos, and piwi. In manipulating this embryo for experimentation, investigators often inject mRNAs to over-express proteins, thought to occur uniformly throughout the embryo and to thereby influence cells equally. We show here a new character of the small micromeres, which is a selective retention of injected synthetic mRNA.
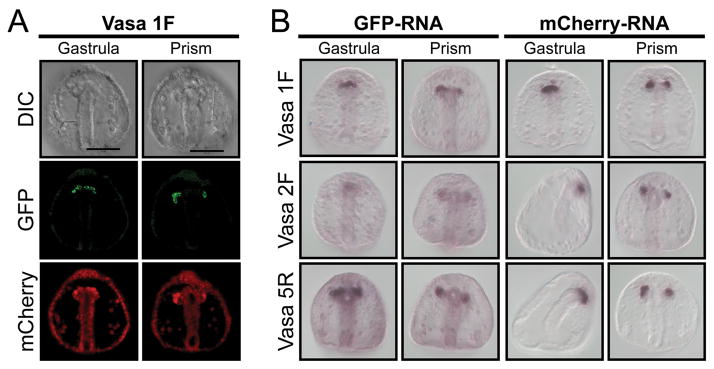
Vasa mRNA is present uniformly throughout the early embryo, but the Vasa protein accumulates selectively in the small micromeres (Voronina et al. 2008) by differential protein turnover (Gustafson et al., submitted). Here we test the ability of protein derived from exogenous mRNA to phenocopy endogenous Vasa protein by microinjecting mRNA from a vasa-GFP construct containing Xenopus β-globin UTRs. These reporter constructs were generated from the transcriptional vector pSP6T4, commonly used in sea urchin overexpression analyses (Lepage and Gache 2004). The Vasa 1F construct contains the sequence encoding the full length vasa open reading frame, and indeed, the protein from this mRNA injection accumulates selectively in the small micromeres, and eventually more in the small micromeres in the left coelomic pouch, just as the endogenous protein (Voronina et al. 2008). Co-injecting mRNA (using the same UTRs and transcriptional vector) that encodes mCherry shows fluorescence uniformly throughout the early embryo (data not shown) but later also selectively in the small micromeres. To test if either of these selective protein accumulations results from differential retention of the mRNA in small micromeres, we performed in situ hybridization on the injected embryos using sequences specific to the mRNA encoding the fluorescent proteins. Indeed, by gastrulation both the Vasa 1F-GFP and the mCherry mRNAs were retained selectively by the small micromeres.
Vasa is an RNA helicase that is thought to be involved in translational regulation. In testing whether it is involved in the mRNA retention phenomenon, we manipulated the vasa sequence such that it lacks its N-terminus or its catalytic domains; both regions are required for Vasa function. Following microinjection of these mRNAs and culture of the embryos, each of the vasa mRNAs and the mCherry mRNA showed persistent and specific retention in the small micromeres. The mechanism of this selective mRNA retention is unknown, but is unlikely due to a lack of mRNA turnover mechanisms in this relatively quiescent cell since several endogenous mRNAs are turned over during development in this same cell. Since the small micromeres appear to be a multipotent cell similar to those found in other organisms, especially in the lophotrochozoans (Juliano and Wessel, 2010), this phenomenon of selective mRNA retention may reflect a shared character of multipotency.
Figure 1. Small micromere retention of microinjected synthetic RNA.
(A) GFP (green) fluorescence from a Vasa- GFP mRNA construct assayed for protein expression in gastrula and prism-stage embryos along with mCherry (red) control mRNA. (B) In situ RNA hybridization for GFP or mCherry synthetic mRNA in gastrula and prism-stage embryos. Vasa 1F is a full-length ORF fused to Xenopus β-globin 5′ and 3′ UTRs. Vasa 2F-GFP is the same construct lacking the Vasa region N-terminal to the Zn-fingers, and Vasa 5R-GFP RNA instead lacks the catalytic domain and the helicase domain. GFP and mCherry RNA probes detect the spatial distribution of the exogenous RNA. Note that the Vasa protein accumulates selectively in the small micromeres on the left of the embryo, whereas the mRNA is retained equally in the small micromeres of both sides. Scale bar = 50 μm.
References
- Juliano CE, Wessel GM. Versatile Germline Genes. Science. 2010;329:640–641. doi: 10.1126/science.1194037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepage T, Gache C. Expression of Exogenous mRNAs to Study Gene Function in the Sea Urchin Embryo. Methods in Cell Biology. 2004;74:677–711. doi: 10.1016/s0091-679x(04)74027-3. [DOI] [PubMed] [Google Scholar]
- Voronina E, Lopez M, Juliano CE, Gustafson E, Song JL, Extavour C, George S, Oliveri P, McClay D, Wessel G. Vasa protein expression is restricted to the small micromeres of the sea urchin, but is inducible in other lineages early in development. Dev Biol. 2008;314(2):276–286. doi: 10.1016/j.ydbio.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]