Abstract
Objectives
We investigated whether adrenal beta-arrestin 1 (βarr1)-mediated aldosterone production plays any role in post-myocardial infarction (MI) heart failure (HF) progression.
Background
Heart failure represents 1 of the most significant health problems worldwide, and new and innovative treatments are needed. Aldosterone contributes significantly to HF progression after MI by accelerating adverse cardiac remodeling and ventricular dysfunction. It is produced by the adrenal cortex after angiotensin II activation of angiotensin II type 1 receptors (AT1Rs), G protein-coupled receptors that also signal independently of G proteins. The G protein-independent signaling is mediated by βarr1 and βarr2. We recently reported that adrenal βarr1 promotes AT1R-dependent aldosterone production leading to elevated circulating aldosterone levels in vivo.
Methods
Adrenal-targeted, adenoviral-mediated gene delivery in vivo in 2-week post-MI rats, a time point around which circulating aldosterone significantly increases to accelerate HF progression, was performed to either increase the expression of adrenal βarr1 or inhibit its function via expression of a βarr1 C-terminal-derived peptide fragment.
Results
We found that adrenal βarr1 overexpression promotes aldosterone elevation after MI, resulting in accelerated cardiac adverse remodeling and deterioration of ventricular function. Importantly, these detrimental effects of aldosterone are prevented when adrenal βarr1 is inhibited in vivo, which markedly decreases circulating aldosterone after MI. Finally, the prototypic AT1R antagonist losartan seems unable to lower this adrenal βarr1-driven aldosterone elevation.
Conclusions
Adrenal βarr1 inhibition, either directly or with AT1R “biased” antagonists that prevent receptor-βarr1 coupling, might be of therapeutic value for curbing HF-exacerbating hyperaldosteronism.
Keywords: adrenal beta-arrestin 1, aldosterone, angiotensin II, heart failure, myocardial infarction
Death due to chronic heart failure (HF) continues to rise, despite recent advances in prevention and management of heart disease, and new treatments are needed (1,2). Aldosterone is 1 of a number of hormones with detrimental effects to the myocardium, where circulating levels are elevated in chronic HF (3). It can contribute significantly to HF progression after myocardial infarction (MI) and to the morbidity and mortality of the disease (3–5). Its main actions on the post-MI heart include but are not limited to cardiac hypertrophy, fibrosis, and increased inflammation and oxidative stress, all of which result in adverse cardiac remodeling and progressive loss of cardiac function and performance (5,6). Accordingly, plasma aldosterone levels are a marker of HF severity (7,8), and aldosterone antagonists—such as spironolactone and eplerenone—have well-documented beneficial effects in HF, constituting a significant segment of the chronic HF pharmacotherapeutic regimen (9,10).
Aldosterone is a mineralocorticoid produced and secreted by the cells of the zona glomerulosa of the adrenal cortex in response to either elevated serum potassium levels or to angiotensin II (AngII) acting through its type 1 receptors (AT1Rs), endogenously expressed in the adrenocortical zona glomerulosa (AZG) cells (11). The AT1Rs belong to the superfamily of G protein coupled receptors (GPCRs) and, upon agonist activation, couple to the Gq/11 family of G proteins (12). Over the past few years, a number of GPCRs, including the AT1Rs, have been shown to also signal through G protein-independent pathways. The protein scaffolding actions of β-arrestin-1 and -2 (βarr1 and βarr2, also known as arrestins-2 and -3, respectively), universal receptor adapter/ scaffolding proteins originally discovered as terminators of GPCR signaling, play a central role in mediating this G protein-independent signal transduction (13,14).
We recently reported that adrenal βarr1 promotes AngII-dependent aldosterone production in vitro in human AZG cells, independently of G-proteins (15). Additionally, adrenal-specific βarr1 overexpression in vivo resulted in a marked elevation of circulating aldosterone levels in otherwise normal animals (15). In the present study, we sought to investigate whether adrenal βarr1 plays any role in regulation of circulating aldosterone levels in post-MI HF progression as well. For this purpose, we used our previously developed methodology for adrenal-targeted gene transfer in vivo (16,17), in 2-week post-MI rats, of either wild-type βarr1 to induce adrenal βarr1 overexpression or of a βarr1 protein fragment comprising the βarr1 C-terminus, which inhibits βarr1 signaling activity. The 2-week post-MI time point was chosen, because at approximately this time point, circulating aldosterone levels increase dramatically to accelerate post-MI HF progression in rats (18,19).
Methods
In vivo adrenal gene delivery in post-MI rats
All animal procedures and experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committees of Thomas Jefferson and Nova Southeastern Universities. Myocardial infarction was performed with a cryo-infarct method we have previously described (16). Adrenal-specific in vivo gene delivery was done essentially as described (17), via direct delivery of adenovirus in the adrenal gland. Drug treatments were performed with 50 mg/kg/day of losartan potassium (in drinking water) and 100 or 5 mg/kg/day eplerenone (both drugs from Sigma-Aldrich, St. Louis, Missouri).
Construction and purification of adenoviruses
Recombinant adenoviruses that encode full-length wild-type βarr1 (Adβarr1) or a rat βarr1 C-terminal fragment (aa. 369–418, Adβarr1ct) (Online Fig. 1A) were constructed as described previously (15,16). Briefly, transgenes were cloned into shuttle vector pAdTrack-cytomegalovirus, which harbors a cytomegalovirus-driven green fluorescent protein (GFP), to form the viral constructs with standard cloning protocols. As control adenovirus, empty vector that expressed only GFP (AdGFP) was used. The resultant adenoviruses were purified, as described previously, with 2 sequential rounds of cesium chloride density gradient ultracentrifugation (15,16).
Plasma aldosterone measurements
Rat plasma aldosterone levels were determined by EIA (Aldosterone EIA kit, ALPCO Diagnostics, Salem, New Hampshire), as described (15,20).
Echocardiographic and hemodynamic measurements
Two-dimensional guided M-mode and Doppler echocardiography with a 14-MHz transducer (Vevo 770 Echograph, VisualSonic, Inc., Toronto, Canada) and closed chest cardiac catheterization were performed in rats, as described previously (16,21). Three independent echocardiographic measurements were taken in both modes.
Western blotting
Western blots to assess protein levels of steroidogenic acute regulatory (StAR) protein (sc-25806), cardiac levels of plasminogen activator inhibitor (PAI)-1 (sc-8979), transforming growth factor (TGF)- β (sc-1460), βarr1 transgenes (A1CT antibody, a generous gift from Dr. R.J. Lefkowitz, Duke University Medical Center, Durham, North Carolina), and glyceraldehyde-3-phosphate dehydrogenase (MAB374, Chemicon, Temecula, California) were done with protein extracts from rat adrenal glands or hearts, as described previously (15,16). Visualization of Western blot signals was performed with Alexa Fluor 680–(Molecular Probes, Eugene, Oregon) or IRDye 800CW–coupled (Rockland, Inc., Gilbertsville, Pennsylvania) secondary antibodies on a LI-COR infrared imager (Odyssey, Li-COR Biosciences, Lincoln, Nebraska).
Real-time polymerase chain reaction (PCR)
Total heart ribonucleic acid (RNA) isolation and reverse transcription (RT) and real-time PCR were performed as previously described (16,21). The following primer pairs were used: 5′-GTCCACGAGGTGACAAAGGT-3′ and 5′-CATCTTTTCCAGGAGGTCCA-3′ for Col3α1; 5′-CACCCCTTCTGCGTTGTATT-3′ and 5′-TTGACCCTAACCAAGGATGC-3′ for Col1α1; 5′-TGCCTGCACCTTTGTGATATCG-3′ and 5′-CATGGCAGGACAATCGAACC-3′ for B-type natriuretic peptide (NPR-B); 5′-CATCCTGGACAACCTGC-3′ and 5′-TAGGTCCGAACCTTGCC-3′ for atrial natriuretic peptide (ANP) (NPR-A); and finally, 5′-TCAAGAACGAAAGTCGGAGG-3′ and 5′-GGACATCTAAGGGCATCAC-3′ for 18S ribosomal RNA. Real time RT-PCR was performed with SYBR Green Supermix (Bio-Rad, Hercules, California). Normalization was done with 18S ribosomal RNA levels. No bands were seen in the absence of RT.
Masson-trichrome staining
Masson-trichrome staining was performed as described (22).
Statistical analyses
Data are generally expressed as mean ± SEM. Unpaired 2-tailed Student t test and 1- or 2-way analysis of variance with Bonferroni test were generally performed for statistical comparisons, unless otherwise indicated. For most 3-group statistical comparisons, Dunnett’s test with SAS version 8.2 software (SAS, Cary, North Carolina) was used as well. For all tests, a p value of <0.05 was generally considered to be significant.
Results
Adrenal βarr1 and post-MI aldosterone levels
In the present study, we set out to investigate the potential role played by adrenal βarr1 in modulation of in vivo post-MI HF aldosterone levels. To this end, we overexpressed—specifically in the adrenal glands of 2-week post-MI rats—wild-type βarr1 or a βarr1 C-terminal fragment (βarr1ct), which is unable to bind receptor substrates, thus acting as an inhibitor of βarr1 scaffolding/signaling activity (Online Fig. 1A). To confirm the inhibitory effects of βarr1ct on βarr1 activity in vitro, we performed an extensive molecular characterization of its effects on AngII-induced signaling to aldosterone production in the human AZG cell line H295R (Online Fig. 1B). The βarr1ct was indeed found to abrogate βarr1- and G protein-mediated signaling from AT1R to extracellular signal-regulated kinase activation and StAR protein up-regulation; both signaling events are absolutely necessary for AngII-driven aldosterone production and secretion from these adrenocortical cells (15) (Online Fig. 1B). Thus, after confirming that βarr1ct acts as an inhibitor of adrenal βarr1-mediated aldosterone production in vitro, we overexpressed either the full-length βarr1 (to increase adrenal βarr1 levels/activity) or the βarr1ct (to inhibit adrenal βarr1 activity in vivo) specifically in the adrenals of the post-MI rats. Experimental animals were randomized to 3 different groups: 1 group receiving adrenal gene transfer of AdGFP (control group), 1 receiving full-length wild-type βarr1 (Adβarr1), and 1 receiving the βarr1ct (Adβarr1ct). One day before adrenal gene transfer, all groups were analyzed by echocardiography to confirm presence of similar levels of left ventricular dysfunction and HF before gene delivery. All groups were then studied over the course of the following 7 days (i.e., up to 3 weeks after MI).
In vivo expression of the respective transgenes in the adrenal glands of the animals at 7 days after gene delivery was confirmed by Western blotting (Online Fig. 2). Of note, the adrenal-targeted gene transfer methodology employed results in no ectopic transgene expression (17) (data not shown). As expected, plasma circulating aldosterone levels at 7 days after gene delivery were found markedly elevated in control AdGFP-treated post-MI rats (470 ± 20 pg/ml, approximately 2-fold of the aldosterone levels of normal AdGFP-treated rats) (15), compared with normal (i.e., sham-operated) AdGFP-treated rats, indicating marked MI-induced aldosterone elevation. Importantly, adrenal βarr1 overexpression resulted in an even more pronounced aldosterone elevation after MI, on top of that normally present due to the occurrence of MI (845 ± 150 pg/ml in Adβarr1-treated versus 470 ± 20 pg/ml in control AdGFP-treated post-MI rats, n = 6, p <0.05) (Fig. 1). In contrast, levels in Adβarr1ct-treated rats (350 ± 30 pg/ml, n = 6, p < 0.05 vs. AdGFP) were significantly lower than in control AdGFP-treated post-MI rats (Fig. 1). Aldosterone levels in post-MI AdGFP rats were similar to saline-treated post-MI rats (data not shown), indicating no nonspecific effects of the adenoviruses used on plasma aldosterone values.
Figure 1. Regulation of Plasma Aldosterone Levels by Adrenal βarr1.
Plasma aldosterone levels in AdGFP-, Adβarr1-, or Adβarr1ct-treated 2-week post-myocardial infarction rats at 7 days after in vivo gene delivery. *p < 0.05 versus AdGFP, **p < 0.05 versus Adβarr1, n = 6 rats/group. AdGFP = adenovirus green fluorescent protein; Adβarr1 = adenoviruses that encode full-length wild-type beta-arrestin 1; Adβarr1ct = adenoviruses that encode rat beta-arrestin 1 C-terminal fragment.
Consistent with the aforementioned findings, βarr1 over-expression led to significant up-regulation of adrenal StAR protein, the most critical enzyme in adrenocortical biosynthesis of aldosterone (as well as of the other adrenal steroids) (15), compared with control AdGFP-treated post-MI rats, indicating enhanced aldosterone synthesis in vivo, whereas overexpression of βarr1ct reduced adrenal StAR levels below the levels of the control rats (Online Fig. 2). Taken together, these results indicate that adrenal βarr1 promotes post-MI-associated hyperaldosteronism, and inhibition of its activity reduces aldosterone production and plasma circulating aldosterone levels after MI in vivo.
In vivo cardiac function and dimensions at 7 days after gene delivery
Next, we examined the impact of this adrenal βarr1-mediated hyperaldosteronism on the post-MI myocardium. Indeed, we found that ejection fraction (EF) was markedly reduced in Adβarr1-treated post-MI rats at 7 days after gene delivery, compared with control AdGFP-treated post-MI rats (41.4 ± 1.2% vs. 48.7 ± 1.1%, respectively, n = 7, p < 0.05) (Fig. 2A). The EF in both groups was similar before gene delivery, and EF of AdGFP-treated rats at 7 days after gene delivery was slightly but significantly reduced compared with pre-gene delivery, as expected, given that cardiac function deteriorates over time after MI, although at 3 weeks after MI (when post-gene delivery measurements were taken) there is limited dysfunction with this model (Fig. 2A). Indeed, previous studies by us have shown that this model in the rat does not lead to significant cardiac dysfunction before approximately 10 weeks after MI (21). Furthermore, left ventricular end diastolic diameter (LVEDD), a marker of cardiac dimensions, was significantly increased in Adβarr1-treated rats at 3 weeks after MI compared with control AdGFP post-MI rats, in which heart enlargement was less pronounced at 3 weeks after MI (Fig. 2B). This indicates that adrenal βarr1 overexpression significantly accelerates the progression of cardiac hypertrophy by promoting aldosterone elevation after MI. Of note, EF and LVEDD of saline-treated, 3-week, post-MI rats were similar to those of control AdGFP-treated post-MI rats at 7 days after gene delivery, indicating no nonspecific effects of the adenoviral gene delivery on cardiac function (data not shown).
Figure 2. Effect of Adrenal βarr1-Mediated Hyperaldosteronism on Cardiac Function, Dimensions, and Contractility.
(A) Ejection fraction (EF%) of Adβarr1- and control AdGFP-treated post-myocardial infarction (MI) rats before and after gene delivery (see also Table 1). *p < 0.05 versus AdGFP after gene delivery or Adβarr1 before gene delivery; **p < 0.05 versus AdGFP-pre-gene delivery, n = 7 rats/group. (B) Left ventricular end diastolic diameter (LVEDD) of these rats. *p < 0.05 versus AdGFP after gene delivery or Adβarr1 before gene delivery, n = 7 rats/group. (C) The EF% and (D) LVEDD of Adβarr1-treated post-MI rats administered either with saline (vehicle) or with eplerenone (Adβarr1-Eplerenone) for 7 days, at 1 week after gene delivery (3 weeks after MI). AdGFP post-MI rats (treated with vehicle) are also shown at 1 week after gene delivery (3 weeks after MI) for comparisons. *p < 0.05 versus either AdGFP or Adβarr1-Eplerenone; no significant difference between AdGFP and Adβarr1-Eplerenone was observed at p = 0.05, n = 5 rats/group. (E, F) Basal and maximal dose of isoproterenol (Max. Iso)-stimulated +dP/dtmax (E) and −dP/dtmin (F) responses of Adβarr1- and control AdGFP-treated post-MI rats at 7 days after adrenal gene delivery (see also Table 1). *p < 0.05 versus AdGFP, n = 7 rats/group. Abbreviations as in Figure 1.
Importantly, these adrenal βarr1-induced cardiac alterations are aldosterone-mediated (i.e., due to the elevated aldosterone levels caused by adrenal βarr1 overexpression in vivo), because EF reduction and LVEDD increase are prevented (i.e., are similar to control AdGFP-treated rats) by treatment of post-MI Adβarr1 rats with the aldosterone antagonist eplerenone (Figs. 2C and 2D), although this drug—as expected—has no effect on the plasma aldosterone increase caused by the Adβarr1 treatment of the adrenals of these post-MI animals (Online Fig. 3). Of note, eplerenone prevented the effects of adrenal βarr1 overexpression at 2 completely different doses (a high dose, 100 mg/kg/day [Figs. 2C and 2D], and a low dose, 5 mg/kg/day [data not shown]). Thus, the cardiac effects observed upon adrenal βarr1 overexpression are indeed mediated by circulating aldosterone.
Finally, hemodynamic analysis revealed that Adβarr1-treated post-MI rats exhibited significantly reduced basal and maximal dose of isoproterenol-induced cardiac contraction and relaxation indexes, compared with control AdGFP-treated rats (Figs. 2E and 2F). At this early post-MI time point, when cardiac dysfunction has not yet manifested as HF, echocardiographic and hemodynamic parameters of Adβarr1ct-treated post-MI rats did not display statistically significant differences from those of control AdGFP-treated post-MI rats, as one might expect, although there was some trend toward functional improvement in the Adβarr1ct group (see Table 1 for complete in vivo cardiac functional parameters in all 3 post-MI groups at 1 week after gene delivery). These results show that the adrenal βarr1-mediated hyperaldosteronism indeed results in significantly accelerated deterioration of function of the post-MI rat heart.
Table 1.
In Vivo Cardiac Functional Parameters After Adrenal Gene Delivery
| Sham-Operated (n = 6) | AdGFP (n = 7) | Adβarr1 (n = 7) | Adβarr1ct (n = 7) | |
|---|---|---|---|---|
| Echocardiographic parameters after gene delivery | ||||
| LVIDs (mm) | 3.76 ± 0.08 | 6.6 ± 0.04* | 7.2 ± 0.04*† | 6.0 ± 0.04*‡ |
| LVEDD (mm) | 6.36 ± 0.15 | 8.7 ± 0.25* | 9.5 ± 0.15*† | 7.9 ± 0.5*‡ |
| FS (%) | 37.09 ± 1.39 | 25.6 ± 0.7* | 21.3 ± 0.7*† | 28.6 ± 2.7*‡ |
| EF (%) | 65.01 ± 1.77 | 48.7 ± 1.1* | 41.4 ± 1.2*† | 52.3 ± 2.9*‡ |
| PWTd (mm) | 1.25 ± 0.08 | 1.6 ± 0.04* | 2.0 ± 0.2*† | 1.4 ± 0.24*‡ |
|
| ||||
| Basal LV hemodynamic measurements | ||||
| HR (beats/min) | 354.2 ± 13.9 | 352.8 ± 16.2 | 363.6 ± 7.8 | 356.8 ± 14.2 |
| LV +dP/dtmax (mm Hg/s) | 9,997 ± 446 | 7,549 ± 512* | 5,820 ± 88.5*† | 8,149 ± 512*‡ |
| LV −dP/dtmin (mm Hg/s) | −8,598 ± 248 | −6,157 ± 392* | −5190 ± 110*† | −6,657 ± 392*‡ |
| LVESP | 120.6 ± 1.1 | 115.0 ± 4.7 | 104.2 ± 4.4*† | 118.0 ± 3.7‡ |
| LVEDP | 1.7 ± 0.6 | 2.8 ± 0.4 | 8.2 ± 1.0*† | 2.3 ± 0.4‡ |
|
| ||||
| Hemodynamic measurements after maximal isoproterenol (333 ng/kg BW) | ||||
| HR (beats/min) | 434.2 ± 13.2 | 437 ± 15.0 | 452 ± 13.0 | 447 ± 16.0 |
| LV +dP/dtmax | 15,128 ± 391 | 11,200 ± 668* | 9,742 ± 291*† | 11,900 ± 668*‡ |
| LV −dP/dtmin | −9,981 ± 115 | −7,509 ± 496* | −6,646 ± 171*† | −8,209 ± 496*‡ |
| LVESP | 127.9 ± 1.8 | 122.0 ± 4.9 | 111.2 ± 2.3*† | 124.0 ± 4.6‡ |
| LVEDP | 1.9 ± 0.9 | 3.1 ± 0.4 | 8.8 ± 0.9*† | 2.8 ± 0.4‡ |
|
| ||||
| Phenotypic data | ||||
| HW/BW ratio (mg/g) | 2.03 ± 0.16 | 2.7 ± 0.16* | 3.3 ± 0.21*† | 2.5 ± 0.11*‡ |
Adrenal gene delivery of adenovirus green fluorescent protein (AdGFP), adenoviruses that encode full-length wild-type beta-arrestin 1 (Adβarr1), or adenoviruses that encode rat beta-arrestin 1 C-terminal fragment (Adβarr1ct) was performed at 2 weeks after myocardial infarction, and the parameters in the table were measured 7 days after gene delivery (direct adrenal gland injection). Values of age-matched, sham-operated animals are shown for comparisons. Analysis of variance with Bonferroni test was performed among all groups. Data are presented as mean ± SEM.
p < 0.05 versus sham-operated;
p < 0.05 versus AdGFP;
p < 0.05 versus Adβarr1.
BW = body weight; EF = ejection fraction; FS = fractional shortening; HR = heart rate; LV +dP/dtmax = maximal first derivative of left ventricular pressure rise; LV −dP/dtmin = minimal first derivative of left ventricular pressure fall; LVEDD = left ventricular end-diastolic diameter; LVEDP = left ventricular end diastolic pressure; LVESP = left ventricular end systolic pressure; LVIDs = left ventricular inner diameter during systole; PWTd = posterior wall thickness in diastole.
Cardiac remodeling and functional biomarkers at 7 days after gene delivery
We also performed molecular and structural evaluation of the post-MI rat hearts at 7 days after gene delivery. Real time PCR in total messenger RNA isolated from these hearts showed—consistent with the in vivo functional data—a marked up-regulation of collagen types 1α1 and 3α1, markers of cardiac fibrosis, and of ANP and B-type natriuretic peptide, markers of cardiac hypertrophy, in the post-MI hearts of Adβarr1-treated rats, compared with control AdGFP-treated animals (Figs. 3A to 3D). Conversely, up-regulation of all these markers was prevented in Adβarr1ct-treated rats (Figs. 3A to 3D), even though this group did not show significant improvement in cardiac function, which is not surprising given the early post-MI time point at which these measurements were taken. Thus, lowering of circulating aldosterone levels by adrenal βarr1 inhibition in vivo causes a marked reduction in the expression of adverse remodeling-related genes, which might help halt the post-MI cardiac decline at later time points. Additionally, heart-weight/body-weight ratio measurements also confirmed the accelerated cardiac hypertrophy (i.e., enhanced at 1 week after adrenal gene delivery, compared with control AdGFP-treated) displayed by Adβarr1-treated post-MI rats (Table 1, see also preceding text, Fig. 2B).
Figure 3. Effect of Aldosterone Levels on Cardiac Remodeling Markers.
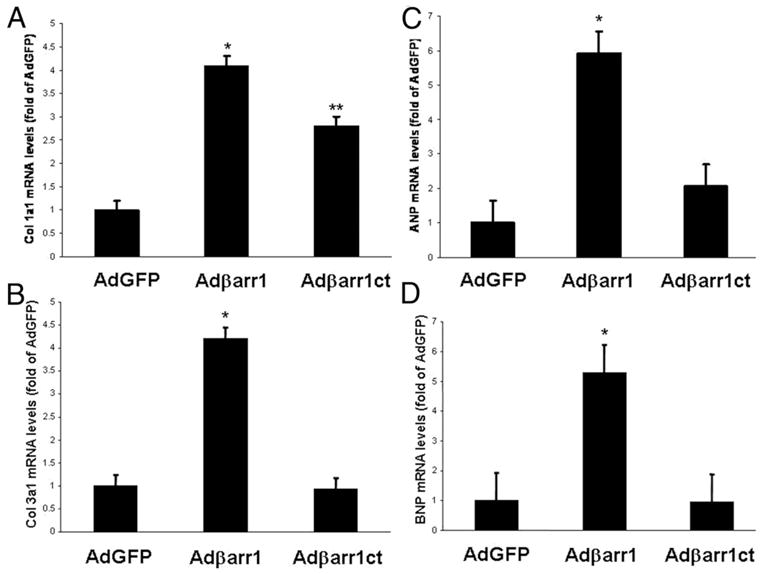
Heart messenger ribonucleic acid (mRNA) levels of (A) collagen I (Col1a1); (B) collagen III (Col3a1); (C) atrial natriuretic peptide (ANP); and (D) brain natriuretic peptide (BNP) in all experimental groups at 7 days after gene delivery (3 weeks after myocardial infarction). All values were standardized to amplified 18S ribosomal RNA. Data are presented as mean ± SEM and plotted as fold of AdGFP values. *p < 0.05 versus AdGFP or Adβarr1ct; **p < 0.05 versus AdGFP, n = 5 rat hearts/group. Abbreviations as in Figures 1 and 2.
Cardiac fibrosis at 7 days after gene delivery
Masson-trichrome staining for cardiac fibrosis at 3 weeks after MI (7 days after gene delivery) showed markedly increased fibrosis in Adβarr1-adrenal-treated rat hearts compared with control AdGFP-treated rat hearts, whereas fibrosis was almost completely absent in Adβarr1ct-adrenal-treated rat hearts (Figs. 4A and 4B). As expected, no fibrosis was detectable in sham-operated rat hearts (Fig. 4A). In addition, eplerenone treatment markedly reduced fibrosis in Adβarr1-adrenal-treated rat hearts (Online Fig. 4), thus providing another indication that the cardiac effects of βarr1 are aldosterone-dependent.
Figure 4. Impact of Aldosterone Levels on Cardiac Fibrosis and Adverse Remodeling Mediators.
(A) Trichrome-Masson’s staining in myocardial cross sections from AdGFP-, Adβarr1-, or Adβarr1ct-treated post-MI rats at 7 days after adrenal gene delivery. Blue denotes collagen fibers, red denotes muscle fibers, and black represents cell nuclei. Representative images are shown from 5 to 6 rat hearts stained/group, along with staining in sham rat hearts, in which no blue staining was detectable. (B) Quantification of the % fibrotic area visualized upon Trichrome-Masson’s staining. *p < 0.05 versus AdGFP; **p < 0.05 versus Adβarr1, n = 5 to 6 rat hearts/group. (C) Western blotting for cardiac plasminogen activator inhibitor (PAI)-1 and transforming growth factor-β (TGF-β1) in AdGFP-, Adβarr1-, or Adβarr1ct-treated post-MI rats at 7 days after gene delivery, including glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as loading control. (D) Densitometric analysis of 5 heart samples tested/group. *p < 0.05 versus AdGFP; **p < 0.05 versus Adβarr1, n = 5 rat hearts/group. Abbreviations as in Figures 1, 2, and 3.
Cardiac mediators of aldosterone at 7 days after gene delivery
Immunoblotting in cardiac protein extracts revealed a marked up-regulation of cardiac PAI-1 and TGF-β—2 of the most important molecular mediators of the cardiac fibrotic and adverse remodeling actions of aldosterone (5)—in the post-MI hearts of Adβarr1-treated rats compared with control AdGFP-treated rats (Figs. 4C and 4D). In contrast, in the hearts of Adβarr1ct-treated rats, not only was up-regulation of PAI-1 and TGF-β prevented but the levels of these proteins were actually lowered below the levels of control AdGFP-treated rats (Figs. 4C and 4D). Taken together, these results indicate that adrenal βarr1-mediated hyperaldosteronism accelerates cardiac adverse remodeling and progression to HF after MI and that these effects can be reciprocally mitigated by adrenal βarr1 inhibition, which significantly reduces circulating aldosterone levels.
Angiotensin antagonism and βarr1-mediated aldosterone levels after MI
Finally, we examined whether adrenal βarr1 can affect the efficacy of AT1R antagonism at curbing AngII-induced aldosterone production. For this purpose, we treated post-MI rats with the prototypic AT1R antagonist losartan (23,24) for the entire 7-day post-gene delivery period at a dose of 50 mg/kg/day. As expected, in control AdGFP-treated post-MI rats, losartan produced a small but significant plasma aldosterone reduction (from 470 ± 20 pg/ml in saline-treated to 402 ± 10 pg/ml in losartan-treated rats, p < 0.05, n = 6) (Fig. 5). In Adβarr1-treated post-MI rats however, losartan is virtually unable to lower aldosterone levels (845 ± 150 pg/ml in saline-treated vs. 880 ± 88 pg/ml in losartan-treated rats, not significant at p < 0.05, n = 6) (Fig. 5). In the Adβarr1ct-treated group, no significant aldosterone reduction by losartan was observed, probably because plasma aldosterone levels were already reduced below the levels of AdGFP-treated rats by Adβarr1ct alone. Consistent with this, losartan seems also incapable of reducing the cardiac fibrosis induced by adrenal βarr1-mediated hyperaldosteronism (Online Fig. 4). However, levels in both the saline- and losartan-treated Adβarr1ct rats were significantly lower than in vehicle-administered control AdGFP post-MI rats (Fig. 5). These results strongly suggest that the post-MI aldosterone-lowering effects of losartan are antagonized by adrenal βarr1; therefore adrenal βarr1 inhibition can potentiate the hypoaldosteronic actions of this drug in post-MI HF. Effects of losartan in AdGFP-treated and saline-treated post-MI rats were similar (data not shown).
Figure 5. Adrenal βarr1-Dependent Aldosterone Levels and Losartan.

Plasma aldosterone levels 7 days after adrenal gene delivery of post-myocardial infarction rats after concomitant vehicle (−Los) or losartan (+Los) treatment. *p < 0.05 versus AdGFP/−Los or Adβarr1/+Los, n = 5 rats/group/ treatment. Abbreviations as in Figures 1, 2, and 3.
Discussion
We recently reported that adrenal βarr1 promotes AngII-dependent aldosterone production in vitro in human AZG cells, independently of G-proteins (15). Additionally, adrenal-specific βarr1 overexpression in vivo resulted in marked elevation of circulating aldosterone levels in otherwise normal animals (15). In the present study, we sought to investigate whether adrenal βarr1 plays any role in regulation of circulating aldosterone levels in post-MI HF progression. We found that adrenal βarr1 is indeed a crucial regulator of circulating aldosterone levels in vivo during post-MI HF progression, in that increased adrenal βarr1 levels/activity promote aldosterone elevation after MI, resulting in accelerated cardiac adverse remodeling and deterioration of function, whereas blockade of its activity in vivo lowers post-MI aldosterone levels, attenuating or even preventing these detrimental effects of aldosterone on the failing heart.
These findings strongly suggest that blockade of adrenal βarr1 action on AT1R might serve as a novel therapeutic strategy for lowering aldosterone levels after MI and in HF. This is particularly important, because aldosterone has been shown to exert some of its actions (its so-called “non-genomic” actions) independently of the mineralocorticoid receptor (MR), its molecular target that normally mediates its cellular actions (4,5). These MR-independent actions are unaffected by the currently available MR antagonists, such as eplerenone and spironolactone, used in the treatment of HF (9,10). Therefore, curbing aldosterone production at its major source (i.e., the adrenal cortex) by inhibiting βarr1 actions could presumably be more effective therapeutically than inhibiting the actions of aldosterone at its receptor level.
In addition, because adrenal βarr1 seems necessary for up-regulation of StAR, the enzyme that regulates synthesis of all adrenal steroids, its inhibition presumably leads to suppression of the production of the other adrenocortical steroids as well (i.e., of glucocorticoids and corticosterone) (15). Of note, glucocorticoids have been reported to actually occupy the cardiac MRs under normal conditions instead of aldosterone (25). Therefore, adrenal βarr1 inhibition, by suppressing production of glucocorticoids and mineralocorticoids alike, has the unique potential of keeping cardiac MRs completely at bay. For this very same reason, adrenal βarr1 emerges as a much superior target for post-MI cardiac remodeling and HF treatment than MR inhibition (e.g., with eplerenone) or aldosterone synthase inhibition, given that the latter strategies cannot counter all the adverse effects of all adrenal steroids after MI, as suppression of all adrenal steroid production via adrenal βarr1 inhibition is projected to do.
Another important ramification of the present study is that pathological situations that cause elevation of adrenal βarr1 activity toward receptors can lead to abnormally high AngII-induced aldosterone production and hyperaldosteronism. Indeed, we recently reported that in chronic HF, adrenal GRK2—a protein kinase that induces receptor-βarr coupling—is dramatically up-regulated, resulting in chronically enhanced catecholamine production by the adrenal medulla (16). Thus, it is entirely plausible that, driven by the enhanced GRK2 activity, adrenal βarr1 activity toward receptors—including the AT1Rs—is also increased in chronic HF or during progression from MI to HF, which could mediate (at least in part) the chronically elevated circulating levels of aldosterone that precipitate this disease. Importantly, we have previously shown that GRK2 can desensitize AngII receptors in the heart in vivo (26) and that overexpression of GRK2 in rat adrenal glands also causes elevation of plasma aldosterone (15). Both of these findings argue in favor of the aforementioned scenario.
Furthermore, it is now well-established that, in addition to the circulatory renin-angiotensin-aldosterone system (RAAS), there are also several other local RAASs in peripheral tissues, including the heart (intracardiac RAAS) and the kidneys (intrarenal RAAS), and these systems also hyperfunction in HF contributing to the HF-associated hyperaldosteronism (27,28). Therefore, it would be worth investigating whether βarr1 is involved in aldosterone production by these local RAASs and whether it contributes to their increased aldosterone output during HF as well. In fact, specifically for the intracardiac RAAS, this possibility is very likely, given the elevated cardiac GRK2 levels in HF (29).
One of the major physiological effects of aldosterone is an increase in blood pressure via salt and water retention (4,5). Thus, alterations in mean arterial pressure by the elevated aldosterone levels caused by adrenal βarr1 overactivity might very well have contributed to the observed cardiac phenotype of adrenal βarr1-overexpressing post-MI rats. It should be noted here, however, that βarr1 knockout mice do not show any changes in blood pressure compared with wild-type age-matched control mice (30). Additionally, the direct effects of aldosterone on cardiac tissue are bound to have played the most important role in the observed cardiac phenotype of the post-MI animals, given the relatively small time period (only 7 days) between genetic manipulation of adrenal βarr1 levels that raises aldosterone levels (i.e., gene delivery) and the day of cardiac measurements/examination, which is rather insufficient for blood pressure to dramatically affect cardiac function and remodeling. Besides, whether changes in blood pressure play any role in the cardiac effects of aldosterone is still an open question in its own right, because there are several reports in the published literature showing aldosterone to affect cardiac function and fibrosis in post-MI rats independently of changes in mean blood pressure (31,32). Indeed, no differences in systemic mean arterial pressure among the 3 post-MI treatment groups of the present study (i.e., AdGFP, Adβarr1, Adβarr1ct) were observed at 1 week after gene delivery (data not shown), further supporting the notion that blood pressure did not play any major role in the observed cardiac effects of βarr1-dependent aldosterone at this early post-MI time point (3 weeks).
The last finding of the present study is that the aldosterone-lowering actions of losartan, the prototypic drug of the class of AT1AR antagonists (sartans) (23,24), are countered by adrenal βarr1. Although at normal βarr1 levels (control AdGFP-treated post-MI rats) it is capable of producing a small but significant plasma aldosterone lowering as expected, when adrenal βarr1 is overactive (Adβarr1-treated post-MI rats), losartan does not decrease plasma aldosterone at all. This finding implies that inhibition of adrenal βarr1 in vivo can facilitate the inhibitory effects of losartan (and possibly also of the other sartans) on AngII-induced aldosterone production. Of note, limited efficacy of losartan and other sartans at lowering aldosterone levels in HF patients and in experimental animals, the so-called “aldosterone escape,” has been reported (20,33,34). Therefore, the finding that the effects of losartan on aldosterone production can be antagonized by adrenal βarr1-AT1R coupling might explain (at least in part) this reported limited efficacy of losartan and related drugs at curbing aldosterone levels. By contrast, increased activity of the βarr1 co-factor GRK2 on cardiac AT1Rs also attenuates the pro-contractile signaling of these receptors (26). Therefore, the development of novel, functionally selective (or “biased”) AT1R ligands (35,36)—which would inhibit AT1R-induced GRK2/βarr1 activation, at least as effectively as AT1R-induced G-protein activation—might prove extremely beneficial in the treatment of HF-related hyperaldosteronism and decreased cardiac function.
Clinical implications
We have found that circulating aldosterone levels are reciprocally regulated by adrenal βarr1 activity in vivo, in that they are directly proportional to βarr1 activity toward AngII receptors in the adrenal glands. Therefore, inhibiting adrenal βarr1 action markedly decreases circulating aldosterone and attenuates its detrimental effects on the post-MI heart—such as fibrosis, hypertrophy, and dilation—thereby preventing or even reversing adverse remodeling after MI and maintaining cardiac function in the face of post-MI-driven cardiac decline. Additionally, losartan, a classical AngII receptor antagonist drug used in the treatment of hypertension, seems unable to counter this adrenal βarr1-promoted hyperaldosteronism after MI. Taken together, the present findings suggest adrenal βarr1 as a major driving force behind post-MI aldosterone elevation, whose inhibition in vivo—either via gene therapy or pharmacologically—could potentially be of enormous therapeutic value in the management of post-MI HF patients. Finally, from the pharmacotherapeutic standpoint, an evaluation of the whole class of AT1R antagonists (sartans) in terms of their efficacy at antagonizing βarr1-driven hyperaldosteronism is highly warranted, because it could help explain some well-known existing differences in therapeutic efficacy and also identify the most efficacious agents at lowering post-MI aldosterone, within this very important cardiovascular drug class.
Conclusions
The present study reports that adrenal βarr1 promotes the well-documented post-MI-associated elevation of circulating aldosterone, and thus direct inhibition of its activity via adrenal-targeted gene therapy or via development of novel AT1R “biased” or “functionally selective” ligands that can prevent/reduce GRK2/βarr1 activation by the AT1R might be of therapeutic value in post-MI ensuing HF as well as in already established chronic HF—both of which are precipitated by the cardiotoxic actions of elevated aldosterone.
Supplementary Material
Acknowledgments
This work was supported in part by a Scientist Development Grant from the American Heart Association (AHA #09SDG2010138, National Center) to Dr. Lymperopoulos; National Institutes of Health Grants HL56205, HL61690, HL085503, and HL075443 (Project 2) and P01-HL091799 to Dr. Koch; and post-doctoral fellowships to Drs. Lymperopoulos and Rengo from the American Heart Association (Great Rivers Affiliate). The authors have reported that they have no relationships to disclose.
Abbreviations and Acronyms
- Adβarr1
adenoviruses that encode full-length wild-type beta-arrestin 1
- Adβarr1ct
adenoviruses that encode rat beta-arrestin 1 C-terminal fragment
- AdGFP
adenovirus green fluorescent protein
- AngII
angiotensin II
- AT1R
angiotensin II receptor type I
- AZG
adrenocortical zona glomerulosa
- βarr1
beta-arrestin 1
- βarr1ct
beta-arrestin 1 C-terminal fragment
- EF
ejection fraction
- GPCR
G-protein coupled receptor
- GRK2
G-protein coupled receptor kinase-2
- HF
heart failure
- LVEDD
left ventricular end diastolic diameter
- MI
myocardial infarction
- MR
mineralocorticoid receptor
- PAI
plasminogen activator inhibitor
- PCR
polymerase chain reaction
- RAAS
renin-angiotensin-aldosterone system
- RNA
ribonucleic acid
- RT
reverse transcription
- StAR
steroidogenic acute regulatory (protein)
- TGF
transforming growth factor
References
- 1.Thomas S, Rich MW. Epidemiology, pathophysiology, and prognosis of heart failure in the elderly. Heart Fail Clin. 2007;3:381–7. doi: 10.1016/j.hfc.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaye DM, Krum H. Drug discovery for heart failure: a new era or the end of the pipeline? Nat Rev Drug Disc. 2007;6:127–9. doi: 10.1038/nrd2219. [DOI] [PubMed] [Google Scholar]
- 3.Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689–97. doi: 10.1056/NEJMra000050. [DOI] [PubMed] [Google Scholar]
- 4.Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1–20. doi: 10.1677/joe.1.06017. [DOI] [PubMed] [Google Scholar]
- 5.Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond) 2007;113:267–78. doi: 10.1042/CS20070123. [DOI] [PubMed] [Google Scholar]
- 6.Zhao W, Ahokas RA, Weber KT, Sun Y. ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol. 2006;291:H336–43. doi: 10.1152/ajpheart.01307.2005. [DOI] [PubMed] [Google Scholar]
- 7.Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L for the CONSENSUS Trial Study Group. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. Circulation. 1990;82:1730–6. doi: 10.1161/01.cir.82.5.1730. [DOI] [PubMed] [Google Scholar]
- 8.Rouleau JL, Packer M, Moyé L, et al. Prognostic value of neurohumoral activation in patients with an acute myocardial infarction: effect of captopril. J Am Coll Cardiol. 1994;24:583–91. doi: 10.1016/0735-1097(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 9.Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–21. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, Zannad F, Remme WJ, et al. for the Randomized Aldactone Evaluation Study Investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–17. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 11.Ganguly A, Davis JS. Role of calcium and other mediators in aldosterone secretion from the adrenal glomerulosa cells. Pharmacol Rev. 1994;46:417–47. [PubMed] [Google Scholar]
- 12.De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–72. [PubMed] [Google Scholar]
- 13.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512–7. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 14.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 15.Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. An adrenal β-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:5825–30. doi: 10.1073/pnas.0811706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315–23. doi: 10.1038/nm1553. [DOI] [PubMed] [Google Scholar]
- 17.Lymperopoulos A, Rengo G, Zincarelli C, Soltys S, Koch WJ. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol Ther. 2008;16:302–7. doi: 10.1038/sj.mt.6300371. [DOI] [PubMed] [Google Scholar]
- 18.Wan W, Powers AS, Li J, Ji L, Erikson JM, Zhang JQ. Effect of post-myocardial infarction exercise training on the renin-angiotensin-aldosterone system and cardiac function. Am J Med Sci. 2007;334:265–73. doi: 10.1097/MAJ.0b013e318068b5ed. [DOI] [PubMed] [Google Scholar]
- 19.Ishiyama Y, Gallagher PE, Averill DB, Tallant EA, Brosnihan KB, Ferrario CM. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–6. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 20.Mihailidou AS, Mardini M, Funder JW, Raison M. Mineralocorticoid and angiotensin receptor antagonism during hyperaldosteronemia. Hypertension. 2002;40:124–9. doi: 10.1161/01.hyp.0000025904.23047.27. [DOI] [PubMed] [Google Scholar]
- 21.Rengo G, Lymperopoulos A, Zincarelli C, et al. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–9. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMurray JJ. Angiotensin inhibition in heart failure. J Renin Angiotensin Aldosterone Syst. 2004;5 (Suppl 1):S17–22. doi: 10.3317/jraas.2004.019. [DOI] [PubMed] [Google Scholar]
- 24.Díez J. Review of the molecular pharmacology of Losartan and its possible relevance to stroke prevention in patients with hypertension. Clin Ther. 2006;28:832–48. doi: 10.1016/j.clinthera.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Qin W, Rudolph AE, Bond BR, et al. Transgenic model of aldosterone-driven cardiac hypertrophy and heart failure. Circ Res. 2003;93:69–76. doi: 10.1161/01.RES.0000080521.15238.E5. [DOI] [PubMed] [Google Scholar]
- 26.Rockman HA, Choi DJ, Rahman NU, Akhter SA, Lefkowitz RJ, Koch WJ. Receptor-specific in vivo desensitization by the G protein-coupled receptor kinase-5 in transgenic mice. Proc Natl Acad Sci U S A. 1996;93:9954–9. doi: 10.1073/pnas.93.18.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–87. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 28.Silvestre JS, Heymes C, Oubénaïssa A, et al. Activation of cardiac aldosterone production in rat myocardial infarction: effect of angiotensin II receptor blockade and role in cardiac fibrosis. Circulation. 1999;99:2694–701. doi: 10.1161/01.cir.99.20.2694. [DOI] [PubMed] [Google Scholar]
- 29.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–12. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 30.Conner DA, Mathier MA, Mortensen RM, et al. beta-Arrestin1 knockout mice appear normal but demonstrate altered cardiac responses to beta-adrenergic stimulation. Circ Res. 1997;81:1021–6. doi: 10.1161/01.res.81.6.1021. [DOI] [PubMed] [Google Scholar]
- 31.Nehme JA, Lacolley P, Labat C, et al. Spironolactone improves carotid artery fibrosis and distensibility in rat post-ischaemic heart failure. J Mol Cell Cardiol. 2005;39:511–9. doi: 10.1016/j.yjmcc.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Benetos A, Lacolley P, Safar ME. Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1997;17:1152–6. doi: 10.1161/01.atv.17.6.1152. [DOI] [PubMed] [Google Scholar]
- 33.Borghi C, Boschi S, Ambrosioni E, Melandri G, Branzi A, Magnani B. Evidence of a partial escape of renin-angiotensin-aldosterone blockade in patients with acute myocardial infarction treated with ACE inhibitors. J Clin Pharmacol. 1993;33:40–5. doi: 10.1002/j.1552-4604.1993.tb03901.x. [DOI] [PubMed] [Google Scholar]
- 34.Struthers AD. Aldosterone escape during ACE inhibitor therapy in chronic heart failure. Eur Heart J. 1995;16(Suppl N):103–6. doi: 10.1093/eurheartj/16.suppl_n.103. [DOI] [PubMed] [Google Scholar]
- 35.Violin JD, Lefkowitz RJ. Beta-arrestin-biased ligands at seven-transmembrane receptors. Trends Pharmacol Sci. 2007;28:416–22. doi: 10.1016/j.tips.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Neubig RR. Missing links: mechanisms of protean agonism. Mol Pharmacol. 2007;71:1200–2. doi: 10.1124/mol.107.034926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.





