Abstract
The use of medications to treat substance use disorders (SUDs) has emerged as a potentially central part of the treatment armamentarium. In this paper we present data from several recent US national surveys showing that despite the clinical promise of these medications, there has been limited adoption of pharmacotherapies in the treatment of SUDs. The data reveal variable patterns of use of disulfiram, buprenorphine, tablet naltrexone, acamprosate and injectable naltrexone. After examining the environmental and institutional context for the adoption of pharmacotherapies, the specific organizational facilitators and barriers of medication adoption are considered. The paper concludes with a discussion of the minimal clinical and administrative guidance available to enhance adoption, the lack of client and consumer knowledge of medications that puts a brake on their adoption and availability, and the difficulties that must be surmounted in bringing new medications to market.
1. INTRODUCTION
The use of medications to treat substance use disorders (SUDs) has emerged as a potentially central part of the treatment armamentarium. While disulfiram and methadone have relatively long histories, over the past decade medications with what might be called greater sophistication of action have become prominent. Given the intensity of concern in the U.S. with “the drug problem” and its management, it would be expected that new treatments (e.g., buprenorphine, acamprosate, injectable naltrexone) would be greeted with enthusiasm accompanied by rapid diffusion and adoption. In the case of these new medications, that has yet to happen. It is the objective of this paper to investigate potential explanations for this limited adoption in specialty treatment programs.
In this paper we explore “policy and procedure gaps” in the diffusion, adoption, and implementation of medications, the category of evidence-based practices (EBPs) that is indeed the most revolutionary and most challenging to traditional practice. We present data from several nationally representative surveys as well as from treatment programs attached to a clinical trials network specifically designed to promote innovation adoption. These data demonstrate the limited adoption of pharmacotherapies in the treatment of SUDs. After considering these data, we use the explanation of these patterns to highlight several macroscopic processes that could be applicable in understanding the use of EBPs SUD treatment organizations generally.
1.1 The policy emphasis on evidence-based practices
Few issues in substance abuse treatment have more prominence than the perceived urgency of adopting and implementing EBPs. Within a broader mission based on a declared universal need for enhancing the quality of treatment for SUDs, EBPs now represent the panacea for the ambiguous and ambivalent attitudes toward substance abuse treatment within the broader medical care community. Compared to other policy issues raised in the recent past, such as service integration to accommodate the dually diagnosed or the enhancement of treatment staff credentials, there is remarkable consensus among field leadership about the marked importance of SUD treatment programs’ openness to adopting particular innovations.
There has, however, been a tendency to frame this issue along a single dimension: achievement of the goal of enhancing treatment quality through innovation is defined by greater degrees of adoption behavior by treatment organizations. Thus, some policies have used simple reporting of adoption behavior as the criterion for conformity to new standards of innovativeness. Experience is demonstrating, however, that it is a major mistake to place all EBPs in one generic category, and/or to regard multiple adoptions as some kind of measure of treatment quality. Further, attention has not yet been given to long-term implementation and the extent to which implementation actually follows from adoption.
While there is yet to be an accepted typology categorizing EBPs relevant to SUDs treatment, there can be no doubt that they are not identical in their contingencies for implementation within treatment organizations. Specifically, the requirements for organizational change embedded in the design of EBPs vary widely, with some implementations flowing quickly and easily and others potentially causing major disruption of an organization’s treatment delivery. Outside the category of medications, an apt example may be the relative investments required by motivational interviewing versus contingency management. Further, important innovations such as electronic health records may require extensive reorganization. Some innovations affect patients directly, while others are implemented with absolutely no direct effect on patient care.
The common dichotomy between psychosocial and pharmacological innovations actually captures little, as the “psychosocial” category needs to be broken into subcategories of innovations involving treatment delivery, treatment management, and organizational management. Further, perhaps as a means to facilitate acceptance, there is widespread consensus that the successful implementation of pharmacological treatments requires not only concomitant psychosocial interventions, but also requires multiple psychosocial considerations in assuring long-term treatment success associated with such treatments. Curiously, recent empirical evidence regarding the effectiveness of buprenorphine protocols appears to challenge this assumption.
1.2 Pharmacotherapy and the mainstreaming of the treatment of SUDs
Given the high level of institutionalization of 12-step practices in alcoholism treatment and traditional “drug-free” SUDs treatment, the use of pharmacotherapy introduces what might be seen as a totally new paradigm, providing biochemical aid in the reduction of craving for substances in the case of several of the medications. The consequences of introducing pharmacotherapy in SUD treatment on a widespread basis may have sweeping implications that are not yet well understood. Doubtless the introduction of such treatment and its possible movement to primacy is a dramatic paradigm shift, moving SUD treatment much closer to “mainstream” medical practice. A basic implication centers on the staffing requirements for effective implementation, and the manner of balance between medication-based treatments and physicians and nurses, the treatment organization, and the more-or-less organized systems of SUD treatment that presently exist at the level of the state or territory, linked to the single state “authority” in each of these geographic settings.
Together with the emphasis on EBPs, SUDs field leadership continues to heavily stress the critical importance of integration of the identification and some level of treatment of SUDs into primary care. Thus, the use of medications may be seen as a “second point of entry” into both primary care medicine as well as specialties of medicine such as pediatrics and obstetrics that parallel emerging addiction medicine. The first point of entry, currently being pursued vigorously, is Screening, Brief Intervention and Referral to Treatment (SBIRT) in which physicians are urged to take on roles in screening for SUDs, followed by different levels of possible intervention (O’Connor, Nyquist & McLellan, 2011).
To the extent that these efforts to engage primary care are successful, the use of pharmacotherapies to treat patients with SUDs who present in primary care offers a modality of treatment that is consistent with emphases of training in the larger arena of medical practice. Such an introduction has been “forced” by Federal regulations through the significant limitation of the use of buprenorphine by specially trained physicians in individual practices. While the enrollment in credentialing for buprenorphine administration has been substantial and appears very promising, the success of this effort remains to be rigorously evaluated. Of considerable significance is that the use of pharmacotherapy in SUDs treatment is supportive of the chronic disease/brain disease model of SUDs based in neurochemistry and possible altering of structural configurations in the brain. Acceptance of such a model within medical education and practice is probably critical to the effective mainstreaming of SUDs treatment into medical care.
The introduction of broad pharmacotherapeutic opportunities may offer several significant advantages to therapeutic regimens. First, there can be little doubt that the overall quality of treatment will be enhanced with the addition of treatment alternatives. Persons who have been unresponsive to psychosocial regimens may be assisted by medications, leading to positive treatment outcomes not otherwise possible. In a related fashion, candidates for treatment who are resistant to the limited alternatives of psychosocial regimens (and who may refuse to enter or re-enter treatment) may be attracted to treatments centered heavily on medications. Further, the potential availability of medication-based treatments through either primary or specialty care physicians may attract a part of the potential patient population currently unwilling to access SUD treatment.
As stated earlier, it would be expected that these promising features of pharmacotherapies would facilitate enthusiastic adoption and implementation. Such an outcome is not supported by survey data.
2. METHODS
Data for this study are taken from the National Treatment Center Study (NTCS), a family of national studies of substance abuse treatment programs in the United States. This study includes data from three separate NTCS samples. The first study includes two waves of onsite data collected between 2002–2004 and 2007–2008 via face-to-face interviews with administrators and/or clinical directors of a nationally representative sample of privately funded treatment programs. Private sector treatment programs were defined as programs that receive at least 50% of their annual operating revenues from commercial insurance, patient fees, and income sources other than block grant funding such as government grants or contracts. Medicaid and Medicare were not regarded as “block” funding because these reimbursements are received by programs on an individual patient basis. See Abraham and Roman (2010) for study details.
The second study includes two waves of data collected from a nationally representative sample of publicly funded treatment programs. Programs were defined as publicly funded if they received at least 50% of their annual operating revenues from government grants and contracts, including block grant funds and criminal justice contracts. The first wave of data was collected via face-to-face interviews with administrators and/or clinical directors of public treatment programs between 2002–2004. The second wave of data (2009–2010) was collected via mailed surveys and telephone interviews with the administrator/clinical directors of the programs as part of separately funded study. See Knudsen, Abraham, and Oser (under review) for more details.
For both studies, treatment programs were selected via a two-stage random sampling design (Knudsen, Ducharme & Roman, 2007). To be eligible for both the private and public studies, programs were required to offer alcohol and drug treatment at a level of intensity at least equivalent to American Society of Addiction Medicine (ASAM) Level 1 outpatient services (Mee-Lee et al., 2006). Counselors in private practice, halfway houses, and transitional living facilities were ineligible for the study. Eligibility requirements also excluded programs offering exclusively methadone maintenance, court-ordered driver education classes, detoxification-only services, and programs located in correctional and Veteran’s Health Administration facilities.
The third study includes two waves of onsite data collected from community treatment programs (CTPs) participating in the National Institute on Drug Abuse Clinical Trials Network (CTN) collected between 2002–2004 and 2008–2009. For the purpose of the CTN study, eligible community treatment programs (CTPs) were defined as an organizational unit with an autonomous administrator with discretionary control over the unit’s budget. To be eligible for the study, CTPs were required to provide a minimum level of care at least equivalent to American Society of Addiction Medicine (ASAM)-defined outpatient services (Mee-Lee et al., 2006) or to be a methadone maintenance treatment program. Programs received a $150 cash donation for participation. See Roman et al. (2010) for a complete description of the research methods. Study procedures for all three studies were approved by the University of Georgia’s Institutional Review Board. Data analyses were limited to programs that participated in both waves of data collection resulting in N = 223 privately funded programs, N = 192 publicly funded programs, and N = 97 CTN programs.
2.1 Analytic Strategy
To assess adoption of SUD medications over time, we calculated the percentage of programs that reported using each medication at each wave of data collection for the three NTCS samples. Adoption of acamprosate and injectable naltrexone are reported only at the later timepoint since acamprosate was FDA approved in late 2004 and injectable naltrexone was FDA approved in 2006. We also provide an overview of the facilitators and barriers of the adoption and implementation of AUD medications and buprenorphine based on our cumulative body of NTCS research.
3. RESULTS
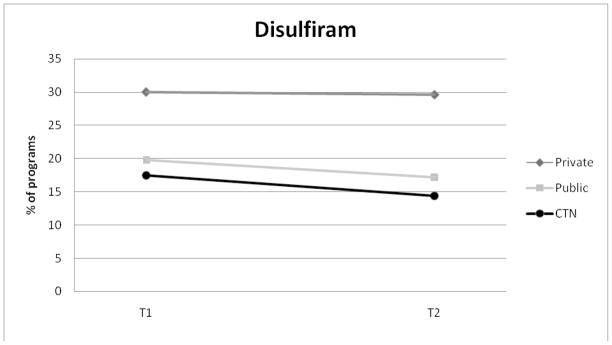
Figures 1 – 5 display the percentage of programs in each NTCS sample adopting SUD medications (Private N=223; Public N = 197; CTN N= 97). The use of disulfiram remained stable in private programs with 30% of programs reporting use at Wave 1 and 29.6% of programs reporting use at Wave 2. Disulfiram use in publicly funded programs decreased from 19.8% at Wave 1 to 17.2% at Wave 2. For CTN programs, disulfiram use declined by approximately 3%.
Figure 1.
Adoption of Disulfiram for Private, Public, and CTN Programs.
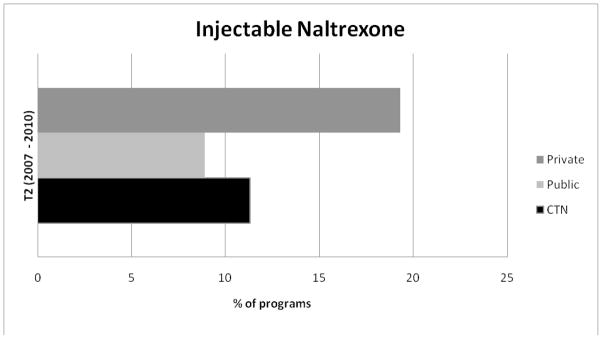
Figure 5.
Adoption of Injectable naltrexone at W3 for Private, Public, and CTN Programs.
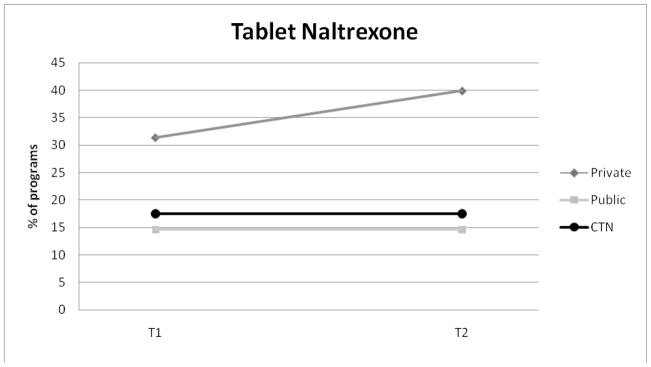
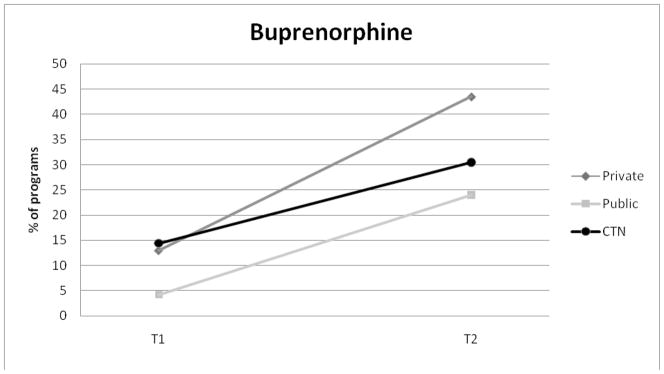
Tablet naltrexone adoption was highest for private programs and increased over the study period from 31.4% to 39.9%. Tablet naltrexone use was the same for public programs (14.6%) and CTN programs (17.5%) at Waves 1 and 2. Buprenorphine adoption steadily increased in all three samples between the two waves of study.
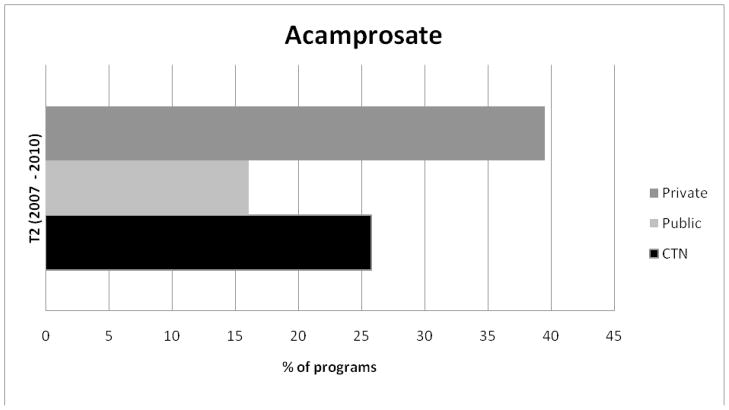
Figures 4 and 5 display the percentage of programs in each sample using acamprosate and injectable naltrexone at Wave 2. Privately funded treatment programs reported the highest levels of acamprosate and injectable naltrexone use and publicly funded programs reported the lowest levels of adoption of each medication.
Figure 4.
Adoption of Acamprosate for Public and CTN Programs.
3.1 Facilitators of organizational SUD medication adoption
Our cumulated body of research identifies a number of organizational characteristics that facilitate the adoption of SUD medications in substance abuse treatment settings. Data indicate that for-profit programs are more likely to adopt disulfiram, tablet naltrexone, and buprenorphine (Knudsen et al., 2007; Oser and Roman, 2007, Knudsen et al., 2006). Research also shows that programs located in hospital settings are more likely to adopt AUD medications (i.e., disulfiram, tablet naltrexone, and acamprosate (Knudsen et al, 2005b; Knudsen et al., 2007, Ducharme et al., 2006). Larger programs (measured by the number of full-time equivalents) are more likely to adopt tablet and injectable naltrexone, as well as buprenorphine (Roman and Johnson, 2002; Abraham et al., 2010; Knudsen et al., 2009). The odds of adopting tablet naltrexone, acamprosate and buprenorphine are greater for programs accredited by the Joint Commission (JC) and/or the Commission on Accreditation of Rehabilitation Facilities (CARF) (Oser and Roman, 2007; Ducharme et al., 2006; Knudsen et al., 2006).
Programs that offer detoxification services are more likely to report using disulfiram, tablet naltrexone, and buprenorphine (Knudsen et al., 2006; Ducharme et al., 2007; Knudsen et al., 2009; Knudsen et al., 2007). Use of other medications such as SSRIs also facilitates adoption of SUD medications including tablet naltrexone, acamprosate, injectable naltrexone and buprenorphine (Oser and Roman, 2007; Abraham et al., 2010; Knudsen et al., 2006; Ducharme et al., 2006). Participation in research including the CTN has been shown to facilitate adoption of tablet naltrexone, acamprosate, and buprenorphine (Abraham et al., 2010; Ducharme et al., 2007; Knudsen et al., 2009). Finally, programs with access to a prescribing physician were more likely to adopt each of the five SUD medications (Knudsen et al., 2007; Ducharme et al., 2006; Abraham et al., 2010; Ducharme et al., 2007).
Our data indicate that a number of organizational characteristics are associated specifically with the adoption of AUD medications. Government owned programs are more likely to report adopting disulfiram and tablet naltrexone (Knudsen et al., 2007; Abraham et al., 2010) and programs with a greater percentage of Master’s level or higher counselors are more likely to adopt disulfiram, tablet naltrexone, and acamprosate. Our data also indicate that treatment ideology significantly influences adoption AUD medications (Knudsen, et al. 2005b; Knudsen et al., 2007; Oser and Roman 2007, 2008). Specifically, programs that place a greater emphasis on the 12-step model are less likely to adopt disulfiram and tablet naltrexone.
Revenues from private insurance and/or the percentage of patients paying for treatment with private insurance is a positive predictor of AUD medication adoption as well (Ducharme et al., 2006; Roman and Johnson, 2002; Abraham and Roman, 2010). Finally, percentage of referrals from the criminal justice system is a negative predictor of tablet naltrexone and acamprosate adoption (Ducharme et al., 2006). In other words, programs with a higher percentage of referrals from the criminal justice system were less likely to adopt these AUD medications.
3.2 Barriers to the adoption of SUD medications
Our research also identifies common barriers to the adoption of SUD medications. Treatment ideology (i.e., emphasis on a 12-step model) is a salient barrier to the adoption of SUD medications (Ducharme et al., 2006; Abraham and Roman, 2010). Both organizational-level analyses and counselor-level analyses support this finding (Knudsen et al., 2005a; Knudsen et al., 2007; Oser and Roman, 2007, 2008; Knudsen et al., 2005b; Abraham et al., 2009). Programs that place a greater emphasis on the 12-step model were less likely to adopt SUD medications. Counselors in recovery and those that holding a strong 12-step ideology are less likely to rate SUD medications as effective and acceptable for use in treatment (Abraham et al., 2009; Knudsen et al., 2005a).
Lack of access to a prescribing physician is also a major barrier to adoption of SUD medications (Abraham and Roman, 2010; Knudsen et al., 2010; Ducharme et al., 2006; Ducharme et al., 2007; Knudsen et al., 2009). To further examine this barrier, we determined the percentage of programs within each sample that did not have access to a prescribing physician. The percentage of programs that lacked access to a prescribing physician was greatest in the public sector (38%), followed by CTN programs (27%) and private programs (23%).
We also determined the percentage of programs with access to a physician that did not prescribe any of the four FDA approved AUD medications or buprenorphine. Among private sector programs, 41% of programs with access to a physician did not prescribe any AUD medications and 49% did not prescribe buprenorphine. Among public programs, 82% with access to a physician did not prescribe AUD medications and 67% did not prescribe buprenorphine and among CTN programs, 54% did not prescribe AUD medications and 36% with access to a physician did not prescribe buprenorphine (excluding OTPs). These findings suggest that while access to a physician is certainly necessary for adoption, it is not sufficient since a sizeable proportion of programs with physicians do not offer medications as an option among their treatment services.
4. DISCUSSION
Adoption of pharmacotherapies in the US SUD treatment system remains low, with less than 45 percent of treatment programs found to prescribe any single SUD medication. If it is reasonable to assume that the endorsement of new treatments will occur first through use in the specialty SUD treatment sector before diffusing elsewhere, then these data suggest a lack of fulfillment of the various “promises” of pharmacotherapeutic treatment for mainstreaming SUD treatment into the larger medical care system. On the other hand, we have no comparison for the expected rate of use at this point of time, given the recency of approval for several of the medications, and what we might call the embeddedness of the 12-step and drug-free models within the treatment of SUDs Thus, it is impossible to offer an absolute judgment of the observed rate of adoption; the “adoption glass” may be one-third full or two-thirds empty.
4.1 Lack of clinical and administrative guidance
In addition to the combinations of facilitators and resistances to adoption that were generated in the data analyses, there are several other contextual considerations that must be elaborated. At the level of the treatment program, decision-makers are faced with the publication of conflicting findings about medications to treat AUDs, leaving the impression that disulfiram, acamprosate and tablet naltrexone are generally ineffective.
Assuming that they move beyond this information, local decision-makers have little guidance for treatment selection of patients given that the more recently approved medications have expanded the number of treatment options. For the medications that are designed to reduce craving, it is emphasized that patients may need to have a period of sustained abstinence prior to beginning the regimen, and that client motivation for recovery may be critical for the regimen to succeed. A suggested length of administered dosage does not appear to have a strong research foundation.
Further, while it is emphasized that psychosocial support and intervention are essential concomitants for medication regimens in SUD treatment, their content is not well specified from the base of empirical data. At the organizational level, there is little data-based guidance for the effective steps necessary for implementation of supportive counseling to occur in concert with medication administration. Relatedly, there is no empirically-based guidance for the overall implementation of medication-assisted treatment into the overall operation of a SUD treatment organization.
Furthermore, despite the availability of the NIDA Clinical Trials Network, there are few data on organizational outcomes and none available through carefully controlled studies ranging across heterogeneous treatment populations. Thus, from a management perspective, there is no guidance as to whether medication implementation is going to impact the bottom line. The promise of “societal” benefits through diversification of treatment offerings appears to be a public good, but SUDs treatment organizations’ daily challenges are centered on survival in a turbulent, unpredictable and often non-supportive environment. Perhaps more evidence of distinctive managerial and survival benefits will be necessary before widespread implementation is observed. Significantly, this outcome may not wait upon research, but upon the observations of success among the SUDs organizations that engage in significant long-term implementation of pharmacotherapy.
4.2 Lack of consumer knowledge
Organizations provide products and services in response to what markets expect and demand. Rarely are SUD treatment organizations considered in this context. Since SUD treatment is part of the health care system, it is important to qualify that bald statement by the fact that professional organizations do not simply provide what the public demands but base service delivery on professional judgment. However, services provided by any organization that are unknown to its potential clients cannot possibly be demanded by clients.
We argue that such is the case with medications for the treatment of SUDs. While we do not have survey data, we assert that the public knows very little about naltrexone, acamprosate, or buprenorphine. Doubtless there is public knowledge of disulfiram, given its availability for over 60 years, but this medication is not central in the current armamentarium, nor does its action parallel that of the medications directed at craving reduction. Over the past decade, mass media advertising of prescription medications has escalated sharply, in particular through magazines and television, yet such advertising of medications for SUDs is rare.
If information were appropriately diffused to the public regarding the newly approved medications, we hypothesize that demand for its use would increase rapidly, and that in turn, its availability at the treatment center level would increase. It is indeed puzzling to observe the absence of government-supported design and support for public information campaigns to specifically diffuse knowledge about the FDA-approved status of these medications and the nature of their action. There is also a likelihood that such knowledge could contribute to a narrowing of the “treatment gap,” i.e. the apparent resistance of many in need of SUD treatment to seek it. Enhancing the image of SUD treatment as medical care and broadening the image of SUD treatment to include more than just one modality of treatment could increase access to treatment. In any event, the absence of consumer or client demand for these interventions may go a long way to explaining their relatively modest availability.
4.3 Future development of medications
The medications available to treat SUDs are, on the one hand, the foundation for a potential paradigm shift in SUD treatment, but on the other hand represent a very narrow set of choices for the clinician compared with those available in psychiatric treatment. From the perspective of what has been invested in scientific work geared toward pharmacotherapy for SUDs, this range may be disappointing. The literature of the last decade is marked by many early discoveries of pharmacological impact (e.g., topirimate, ondansetron), but the SUD medications currently available are the only ones that have passed through the numerous hurdles necessary before reaching the marketplace.
This describes a dysfunctional and perhaps tragic disconnect between science and practice that cannot be closed by the protracted rhetoric about the importance of the adoption of EBPs. While Federal agencies and private foundations dedicated to reducing the public health impact of SUDs can invest in pharmacological research, they cannot bring promising medications through all the stages necessary to make them available to the patient. Such movement is almost totally governed by decisions among investors in the marketplace, the primary issue being ultimate profitability. Given the level of adoption in the data presented here, to say nothing about full-scale implementation, the commercial attractiveness of new SUDs medications is definitely open to question.
Figure 2.
Adoption of Tablet Naltrexone for Private, Public, and CTN Programs.
Figure 3.
Adoption of Buprenorphine for Private, Public, and CTN Programs.
Acknowledgments
The authors gratefully acknowledge the continued support of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Role of Funding Source
Funding for this study was provided by NIDA Grant R01DA14482, NIDA Grant R01DA13110, NIAAA Grant R01AA015974, and the Robert Wood Johnson Foundation’s Substance Abuse Policy Research Program Grant 65111. NIDA, NIAAA, and RWFJ had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the paper; or in the decision to submit the paper for publication.
Footnotes
Contributors
All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham AJ, Ducharme LJ, Roman PM. Counselor attitudes toward pharmacotherapies for alcohol dependence. Journal of Studies on Alcohol and Drugs. 2009;70:628–635. doi: 10.15288/jsad.2009.70.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AJ, Knudsen HK, Rothrauff TC, Roman PM. The adoption of alcohol pharmacotherapies in the Clinical Trials Network: The influence of research network participation. Journal of Substance Abuse Treatment. 2010;38:275–283. doi: 10.1016/j.jsat.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AJ, Roman PM. Early adoption of injectable naltrexone for alcohol use disorders: Findings from the private treatment sector. Journal of Studies on Alcohol and Drugs. 2010;71:460–466. doi: 10.15288/jsad.2010.71.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM. Trends in the adoption of medications for alcohol dependence. Journal of Clinical Psychopharmacology. 2006;26:S13–S19. doi: 10.1097/01.jcp.0000246209.18777.14. [DOI] [PubMed] [Google Scholar]
- Ducharme LJ, Knudsen HK, Roman PM, Johnson AJ. Innovation adoption in substance abuse treatment: Exposure, trialability, and the Clinical Trials Network. Journal of Substance Abuse Treatment. 2007;32:321–329. doi: 10.1016/j.jsat.2006.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Johnson JA, Roman PM. Buprenorphine adoption in the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2009;37:307–312. doi: 10.1016/j.jsat.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Abraham AJ, Oser CB. Barriers to the Implementation of Medication-assisted Treatment for Substance Use Disorder: The important of funding policies and medical infrastructure. Evaluation and Program Planning. doi: 10.1016/j.evalprogplan.2011.02.004. (Under review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. Early adoption of buprenorphine in substance abuse treatment centers: Data from the private and public sectors. Journal of Substance Abuse Treatment. 2006;30:363–373. doi: 10.1016/j.jsat.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM. The adoption of medications in substance abuse treatment: associations with organizational characteristics and technology clusters. Drug and Alcohol Dependence. 2007;87:164–174. doi: 10.1016/j.drugalcdep.2006.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen HK, Ducharme LJ, Roman PM, Link T. Buprenorphine diffusion: The attitudes of substance abuse treatment counselors. Journal of Substance Abuse Treatment. 2005a;29:95–106. doi: 10.1016/j.jsat.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Knudsen HK, Roman PM, Ducharme LJ, Johnson JA. Organizational predictors of pharmacological innovation adoption: The case of disulfiram. Journal of Drug Issues. 2005b;35:559–574. [Google Scholar]
- Knudsen HK, Roman PM, Oser CB. Facilitating factors and barriers to the use of medications in publicly funded addiction treatment organizations. Journal of Addiction Medicine. 2010;4:99–107. doi: 10.1097/ADM.0b013e3181b41a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mee-Lee DL, Gartner L, Miller MM, Shulman GD, Wilford BB. Patient placement criteria for the treatment of substance-related disorders. 2. Chevy Chase, MD: American Society of Addiction Medicine; 2006. [Google Scholar]
- Oser CB, Roman PM. Organizational-level predictors of adoption across time: naltrexone in private substance-use disorders treatment centers. Journal of Studies on Alcohol and Drugs. 2007;68:852–86. doi: 10.15288/jsad.2007.68.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oser CB, Roman PM. A categorical typology of naltrexone-adopting private substance abuse treatment centers. Journal of Substance Abuse Treatment. 2008;34:433–442. doi: 10.1016/j.jsat.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Nyquist JG, McLellan AT. Integrating addiction medicine into graduate medical education in primary care: the time has come. Annals of Internal Medicine. 2011;154:56–59. doi: 10.7326/0003-4819-154-1-201101040-00008. [DOI] [PubMed] [Google Scholar]
- Roman PM, Abraham AJ, Rothrauff TR, Knudsen HK. A longitudinal study of organizational formation, innovation adoption, and dissemination activities within the National Drug Abuse Treatment Clinical Trials Network. Journal of Substance Abuse Treatment. 2010;38:S44–S52. doi: 10.1016/j.jsat.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman PM, Johnson JA. Adoption and implementation of new technologies in substance abuse treatment. Journal of Substance Abuse Treatment. 2002;22:211–218. doi: 10.1016/s0740-5472(02)00241-6. [DOI] [PubMed] [Google Scholar]