The V. parahaemolyticus resuscitation-promoting factor YeaZ has been purified and crystallized by the hanging-drop vapour-diffusion method. A diffraction data set has been collected to 3.1 Å resolution.
Keywords: YeaZ, resuscitation-promoting factors, Vibrio parahaemolyticus
Abstract
Vibrio parahaemolyticus is a human pathogen associated with gastroenteritis caused by the ingestion of contaminated raw seafood. V. parahaemolyticus is able to survive exposure to low temperatures typical of those used for the refrigeration of foods by entering a viable but nonculturable (VBNC) state. The VBNC cells can regain culturability and renewed ability to cause infection upon temperature upshift. The resuscitation-promoting factor protein (Rpf, YeaZ) plays a key role in reactivation of growth. Crystals of V. parahaemolyticus YeaZ have been grown using the hanging-drop vapour-diffusion method with polyethylene glycol as a precipitating agent. The crystals belonged to the primitive monoclinic space group P21, with unit-cell parameters a = 81.7, b = 63.8, c = 82.3 Å, β = 105° and four subunits in the asymmetric unit. A complete X-ray diffraction data set was collected from a single crystal to 3.1 Å resolution.
1. Introduction
Vibrio parahaemolyticus is one of the most significant causes of human foodborne gastroenteritis in countries with long coastlines and high raw-seafood consumption (e.g. China, Japan, Thailand and the USA), where V. parahaemolyticus infections are associated with approximately 50% of total food-poisoning outbreaks (Nair et al., 2007 ▶; Su & Liu, 2007 ▶). Although often self-limiting, V. parahaemolyticus infection may cause septicaemia, which is life-threatening to people with underlying medical conditions such as liver disease or immune disorders (Su & Liu, 2007 ▶). The emergence of the highly virulent strain serotype O3:K6, which was discovered around 1996 in Asia and subsequently spread to the USA, Chile, Russia and Spain, has highlighted the pandemic potential of this species (Okuda et al., 1997 ▶; Daniels et al., 2000 ▶).
V. parahaemolyticus is a versatile organism that naturally inhabits coastal waters worldwide and is frequently isolated from a variety of seafoods (Liston, 1990 ▶). At temperatures below 288 K, corresponding to temperatures used for food storage, V. parahaemolyticus is able to enter a viable but nonculturable (VBNC) state as a survival strategy (Oliver, 2010 ▶). VBNC cells exhibit antibiotic resistance and retain the ability to attach and persist in their environment. Exposing V. parahaemolyticus VBNC cells to a temperature upshift leads to resuscitation; the cells regain culturability and a renewed ability to cause infection. One key biological factor that is thought to be involved in resuscitation is the resuscitation-promoting factor protein (Rpf) that has been identified in both non-spore-forming Gram-positive and Gram-negative bacteria. Whilst Rpfs of Gram-positive bacteria function as peptidoglycan hydrolases (Mukamolova et al., 2006 ▶) that are likely to be involved in cell-wall digestion and thus cell division (Hett et al., 2007 ▶), Rpfs of Gram-negative bacteria belong to a distinctly different class of proteins (named YeaZ) that have an as yet unknown biochemical function (Panutdaporn et al., 2006 ▶; Nichols et al., 2006 ▶). Studies in Escherichia coli and Salmonella typhimurium have shown that the YeaZ homologues expressed by these organisms are essential for bacterial survival (Nichols et al., 2006 ▶; Handford et al., 2009 ▶). The essentiality of YeaZ suggests that it might be a promising target for antimicrobial agents.
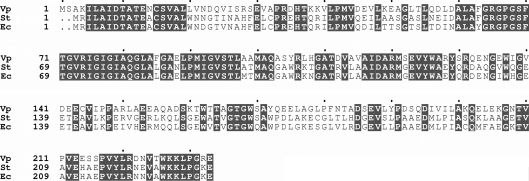
Structural studies of YeaZ homologues from E. coli (PDB code 1okj; Jeudy et al., 2005 ▶), Thermotoga maritima (PDB code 2a6a; Xu et al., 2010 ▶) and S. typhimurium (PDB code 2gel; Nichols et al., 2006 ▶) have revealed that these proteins adopt a two-lobed HSP70/actin-like fold. E. coli YeaZ has been shown to interact with the conserved essential proteins YjeE and YgjD and to act as a protease that specifically degrades YgjD (Handford et al., 2009 ▶). It has been suggested that YeaZ can post-translationally regulate cellular pools of YgjD via proteolytic degradation. A BLAST (Altschul et al., 1990 ▶) sequence-similarity search with known YeaZ sequences against the complete genome of V. parahaemolyticus RIMD 2210633 (Makino et al., 2003 ▶) identified one unambiguous hit (gene name VP0866; UniProt ID Q87RD1). The ORF encodes a polypeptide of 232 residues that shares 57% sequence identity with YeaZ from E. coli K-12 (gene name b1807) and S. typhimurium LT2 (gene name STM1820) (Fig. 1 ▶).
Figure 1.
Alignment of the sequences of YeaZ proteins from V. parahaemolyticus (Vp; UniProt Q87RD1), S. typhimurium LT2 (St; gene name STM1820) and E. coli K-12 (Ec; gene name b1807). Conserved residues are highlighted. Multiple sequence alignments were performed using ClustalW (Thompson et al., 1997 ▶). The figure was prepared using ESPript (Gouet et al., 1999 ▶).
In order to better understand the structure–function relationship in this family of essential proteases, we have expressed, purified and crystallized V. parahaemolyticus YeaZ and collected an X-ray diffraction data set to 3.1 Å resolution. Structural analysis of this protein and elucidation of the three-dimensional structural conservation in YeaZ across Gram-negative species is expected to lead to identification of the putative catalytic site and the enzymatic mechanism of the YeaZ family of proteins, as well as facilitate structure-based drug design.
2. Materials and methods
2.1. Cloning, protein expression and purification
The coding sequence of the yeaZ gene homologue from V. parahaemolyticus was amplified by PCR from genomic DNA of strain NCTC 10884. The oligonucleotide primer sequences that were used (5′-GAGTCATATGAGCGCAAAAATTCTTGCTAT-3′ and 5′-TAATTACTCGAGGCCTGGAAGCTTCTTCCA-3′) were based on the nucleotide sequence of ORF VP0866 located on chromosome 1 of the V. parahaemolyticus RIMD 2210633 genome. The resulting 702 bp DNA fragment was cloned into vector pGEM-T (Promega Corporation, NSW, Australia), excised as an NdeI–XhoI fragment and cloned into similarly digested vector pET21b (Novagen, Merck Biosciences) to yield plasmid pET21bRPF. Plasmid pET21bRPF was used to transform the E. coli expression host BL21 by selecting for resistance to 100 mg l−1 ampicillin. The construct was confirmed by nucleotide-sequence analysis.
Recombinant V. parahaemolyticus YeaZ was expressed with a C-terminal hexahistidine tag (no linker) from plasmid pET21bRPF in E. coli BL21 (DE3). Cells were grown in LB medium containing 100 mg l−1 ampicillin at 310 K until an OD600 of 0.6 was reached, at which point the cell culture was cooled to 303 K. Overexpression of YeaZ was then induced by adding 0.4 mM IPTG and growth was continued at 303 K for a further 3 h. The cells were then harvested by centrifugation at 6000g for 20 min at 277 K.
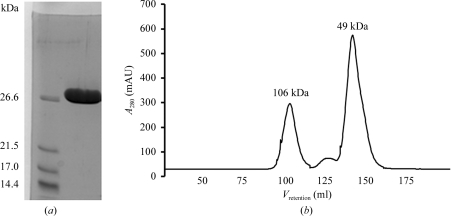
Cells were lysed using the freeze–thaw method followed by sonication in a buffer consisting of 50 mM Tris–HCl pH 7.5, 100 mM NaCl and 1 mM PMSF. Cell debris was removed by centrifugation at 10 000g for 20 min, after which the cell lysate was cleared by further centrifugation at 100 000g for 15 min. NaCl and imidazole were then added to the supernatant to final concentrations of 500 and 10 mM, respectively, and the supernatant was loaded onto a 5 ml Hi-Trap Chelating HP column (GE Healthcare) pre-washed with buffer A (20 mM sodium phosphate pH 7.4, 500 mM NaCl, 10 mM imidazole, 1 mM PMSF). The column was washed with 20 column volumes of buffer B (20 mM sodium phosphate pH 7.4, 500 mM NaCl, 40 mM imidazole) and protein was eluted with buffer B containing 500 mM imidazole. The protein was concentrated to 2 ml in a VivaSpin 10 000 Da cutoff concentrator and loaded onto a Superdex 75 HiLoad 26/60 gel-filtration column (GE Healthcare) equilibrated with 50 mM Tris–HCl pH 7.5, 200 mM NaCl. The fractions containing YeaZ were identified using SDS–PAGE, pooled and dialysed overnight against 30 mM Tris–HCl pH 7.5. The protein purity was estimated to be greater than 98% (Fig. 2 ▶ a).
Figure 2.
Analysis of purified recombinant V. parahaemolyticus YeaZ. (a) Reduced 15% SDS–PAGE Coomassie blue-stained gel. (b) Gel-filtration trace. The molecular weight was determined from a calibration plot of log(M r) versus retention volume available at the EMBL Protein Expression and Purification Core Facility website [http://www.embl.de/pepcore/pepcore_services/protein_purification/chromatography/hiload26-60_superdex75/index.html; V retention (ml) = 631.3 − 104.3 × log (M r)].
2.2. Crystallization and preliminary X-ray analysis
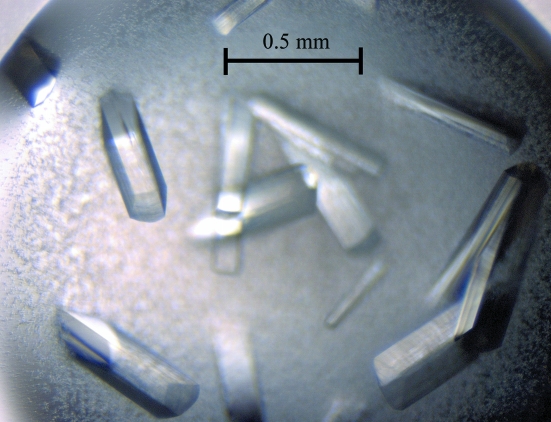
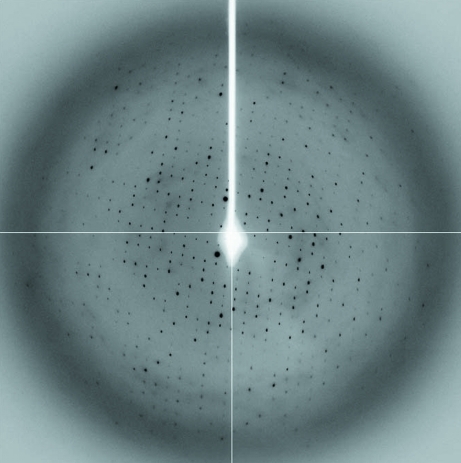
Prior to crystallization, the protein was concentrated to 20 mg ml−1 (based on the Bradford assay; Bradford, 1976 ▶) and centrifuged for 20 min at 13 000g to clarify the solution. Crystallization trials were conducted at 290 K using Crystal Screen, Crystal Screen 2 and PEG/Ion screen (Hampton Research). Typically, a 1 µl aliquot of protein solution was mixed with an equivalent aliquot of reservoir solution and allowed to equilibrate against 0.5 ml reservoir solution. The best crystals, which had a rod-like morphology (Fig. 3 ▶) and dimensions of about 0.1 × 0.1 × 0.5 mm, were obtained using 17–20%(w/v) PEG 3350 and 200 mM lithium acetate dihydrate (PEG/Ion screen condition No. 24). For data collection, crystals were flash-cooled to 100 K in a cold nitrogen stream after soaking them in a cryoprotectant solution equivalent to the reservoir solution supplemented with 25%(v/v) glycerol for 10 s. An X-ray data set was collected from a cryocooled native crystal to 3.1 Å resolution (Fig. 4 ▶) on beamline MX1 at the Australian Synchrotron (Victoria, Australia). A total of 360 images were collected using a 0.5° oscillation width. All data were processed and scaled using the programs MOSFLM (Leslie, 1992 ▶) and SCALA (Winn et al., 2011 ▶). The statistics of data collection are summarized in Table 1 ▶.
Figure 3.
Crystals of V. parahaemolyticus YeaZ.
Figure 4.
A representative 0.5° oscillation image of the data collected from a native V. parahaemolyticus YeaZ crystal using an ADSC Quantum 210r detector at station MX1 at the Australian Synchrotron, Victoria, Australia. The edge of the diffraction image corresponds to a resolution of 3.1 Å.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Space group | P21 |
| Unit-cell parameters (Å, °) | a = 81.7, b = 63.8, c = 82.3, β = 105 |
| Z | 2 |
| VM (Å3 Da−1) | 2.1 |
| Mosaicity (°) | 0.9 |
| No. of crystals | 1 |
| Temperature (K) | 100 |
| Detector | ADSC Quantum 210r |
| No. of images | 360 |
| Rotation per image (°) | 0.5 |
| Wavelength (Å) | 1.0 |
| Resolution range (Å) | 64–3.1 (3.3–3.1) |
| Completeness (%) | 92 (91) |
| Observed reflections | 33648 |
| Unique reflections | 13608 |
| Mean I/σ(I) | 8.9 (2.7) |
| Rmerge† | 0.066 (0.293) |
R
merge = 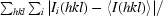
 , where Ii(hkl) is the intensity of the ith observation of reflection hkl.
, where Ii(hkl) is the intensity of the ith observation of reflection hkl.
2.3. Molecular replacement
Molecular replacement was performed with the Phaser program using all data to 3.1 Å resolution (McCoy et al., 2005 ▶). The search model was prepared using CHAINSAW (Stein, 2008 ▶) based on the coordinates of an S. typhimurium YeaZ monomer (PDB code 2gel; Nichols et al., 2006 ▶).
3. Results and discussion
3.1. Overexpression, purification and biochemical characterization
Recombinant V. parahaemolyticus YeaZ carrying a C-terminal hexahistidine tag was expressed from the pET21bRPF plasmid in E. coli BL21 (DE3) upon induction of T7 polymerase. The enzyme was purified to >98% electrophoretic homogeneity based on Coomassie blue staining of SDS–PAGE gels (Fig. 2 ▶ a). The protein migrates on SDS–PAGE with an apparent molecular weight of 27 kDa, which is close to the value calculated from the amino-acid sequence (26.0 kDa).
When subjected to gel filtration most of the protein eluted as a peak with a molecular weight of approximately 49 kDa (Fig. 2 ▶ b), indicating that V. parahaemolyticus YeaZ is mostly dimeric in solution, with only a small fraction forming tetramers. This is in line with previous reports of the molecular packing in the crystals of YeaZ from E. coli (PDB code 1okj; Jeudy et al., 2005 ▶) and S. typhimurium (PDB code 2gel; Nichols et al., 2006 ▶), where molecules were found in dimer or dimer of dimers (tetramer) arrangements, and with the previous gel-filtration studies on E. coli YeaZ in solution (Handford et al., 2009 ▶).
3.2. Crystallization and preliminary crystallographic analysis
Crystals of V. parahaemolyticus YeaZ were obtained using a sparse-matrix crystallization approach in the presence of PEG and lithium salt under conditions similar to those previously described for S. typhimurium YeaZ (Nichols et al., 2006 ▶). Test data collected from a cryocooled crystal of V. parahaemolyticus YeaZ at station MX1 at the Australian Synchrotron showed diffraction to 3.1 Å resolution. Analysis of the diffraction data using the autoindexing routine in MOSFLM (Leslie, 1992 ▶) showed that the crystals belonged to a primitive monoclinic space group, with unit-cell parameters a = 81.7, b = 63.8, c = 82.3 Å, β = 105°. The self-rotation function computed using MOLREP (Vagin & Teplyakov, 2010 ▶) with diffraction data between 10 and 3.1 Å and an integration radius of 32 Å exhibited three orthogonal twofold-symmetry axes [κ = 180° with (θ, ϕ) values of (91, 0°), (0, 173°) and (134, 180°)] with heights of 6.0σ, 6.0σ and 3.2σ, respectively. This suggested that the protein may form a crystallographic tetramer with 222 point-group symmetry. The V M value calculated under the assumption that there is one tetramer of full-length YeaZ in the asymmetric unit was 1.9 Å3 Da−1, corresponding to a solvent content of approximately 36% (Matthews, 1968 ▶). These values are at the low extreme of the range observed by Matthews for protein crystals. A molecular-replacement search was performed in space groups P2 and P21 for the four subunits in the asymmetric unit using Phaser (McCoy et al., 2005 ▶). Four copies of the search model were found in P21, which made up two dimers related by a noncrystallographic twofold axis forming a p222 tetramer. Rigid-body refinement with REFMAC (Murshudov et al., 2011 ▶) using all data to 3.1 Å resolution yielded values of 0.30 and 0.34 for R and R free, respectively, confirming that the molecular-replacement solution is correct. Inspection of the Fourier electron-density maps with the program Coot (Emsley & Cowtan, 2004 ▶) revealed no density for the C-terminal residues beyound Glu213 in all subunits in the asymmetric unit, suggesting that the C-terminus may have been proteolytically removed during crystallization. The V M and solvent-content values calculated under this assumption were 2.1 Å3 Da−1 and 45%, respectively, which fall within the range observed for protein crystals (Matthews, 1968 ▶). Attempts to optimize the crystal quality and collect higher resolution diffraction data and structure refinement are currently in progress.
3.3. Conclusions
We have obtained high-level expression of recombinant V. parahaemolyticus resuscitation-promoting factor YeaZ in E. coli. Similar to YeaZ from S. typhimurium and E. coli, V. parahaemolyticus YeaZ can oligomerize, with the major form being a dimer. Determination of the atomic structure of this protein and elucidation of the three-dimensional structural conservation in YeaZ homologues is expected to provide insights into the mechanism of regulation and catalysis of the YeaZ family of proteins and to facilitate structure-based drug design.
Acknowledgments
We thank the support staff at the Australian Synchrotron for assistance with data collection. This work was supported by an Australian Research Council Research Fellowship to AR.
References
- Altschul, S. F., Gish, W., Miller, W., Myers, E. W. & Lipman, D. J. (1990). J. Mol. Biol. 215, 403–410. [DOI] [PubMed]
- Bradford, M. M. (1976). Anal. Biochem. 72, 248–254. [DOI] [PubMed]
- Daniels, N. A., Ray, B., Easton, A., Marano, N., Kahn, E., McShan, A. L., Del Rosario, L., Baldwin, T., Kingsley, M. A., Puhr, N. D., Wells, J. G. & Angulo, F. J. (2000). JAMA, 284, 1541–1545. [DOI] [PubMed]
- Emsley, P. & Cowtan, K. (2004). Acta Cryst. D60, 2126–2132. [DOI] [PubMed]
- Gouet, P., Courcelle, E., Stuart, D. I. & Métoz, F. (1999). Bioinformatics, 15, 305–308. [DOI] [PubMed]
- Handford, J. I., Ize, B., Buchanan, G., Butland, G. P., Greenblatt, J., Emili, A. & Palmer, T. (2009). J. Bacteriol. 191, 4732–4749. [DOI] [PMC free article] [PubMed]
- Hett, E. C., Chao, M. C., Steyn, A. J., Fortune, S. M., Deng, L. L. & Rubin, E. J. (2007). Mol. Microbiol. 66, 658–668. [DOI] [PubMed]
- Jeudy, S., Stelter, M., Coutard, B., Kahn, R. & Abergel, C. (2005). Acta Cryst. F61, 848–851. [DOI] [PMC free article] [PubMed]
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr. 26
- Liston, J. (1990). Food Technol. 44, 56–62.
- Makino, K. et al. (2003). Lancet, 361, 743–749. [DOI] [PubMed]
- Matthews, B. W. (1968). J. Mol. Biol. 33, 491–497. [DOI] [PubMed]
- McCoy, A. J., Grosse-Kunstleve, R. W., Storoni, L. C. & Read, R. J. (2005). Acta Cryst. D61, 458–464. [DOI] [PubMed]
- Mukamolova, G. V., Murzin, A. G., Salina, E. G., Demina, G. R., Kell, D. B., Kaprelyants, A. S. & Young, M. (2006). Mol. Microbiol. 59, 84–98. [DOI] [PubMed]
- Murshudov, G. N., Skubák, P., Lebedev, A. A., Pannu, N. S., Steiner, R. A., Nicholls, R. A., Winn, M. D., Long, F. & Vagin, A. A. (2011). Acta Cryst. D67, 355–367. [DOI] [PMC free article] [PubMed]
- Nair, G. B., Ramamurthy, T., Bhattacharya, S. K., Dutta, B., Takeda, Y. & Sack, D. A. (2007). Clin. Microbiol. Rev. 20, 39–48. [DOI] [PMC free article] [PubMed]
- Nichols, C. E., Johnson, C., Lockyer, M., Charles, I. G., Lamb, H. K., Hawkins, A. R. & Stammers, D. K. (2006). Proteins, 64, 111–123. [DOI] [PubMed]
- Okuda, J., Ishibashi, M., Hayakawa, E., Nishino, T., Takeda, Y., Mukhopadhyay, A. K., Garg, S., Bhattacharya, S. K., Nair, G. B. & Nishibuchi, M. (1997). J. Clin. Microbiol. 35, 3150–3155. [DOI] [PMC free article] [PubMed]
- Oliver, J. D. (2010). FEMS Microbiol. Rev. 34, 415–425. [DOI] [PubMed]
- Panutdaporn, N., Kawamoto, K., Asakura, H. & Makino, S. I. (2006). Int. J. Food Microbiol. 106, 241–247. [DOI] [PubMed]
- Stein, N. (2008). J. Appl. Cryst. 41, 641–643.
- Su, Y.-C. & Liu, C. (2007). Food Microbiol. 24, 549–558. [DOI] [PubMed]
- Thompson, J. D., Higgins, D. G. & Gibson, T. J. (1997). Nucleic Acids Res. 22, 4673–4680. [DOI] [PMC free article] [PubMed]
- Vagin, A. & Teplyakov, A. (2010). Acta Cryst. D66, 22–25. [DOI] [PubMed]
- Winn, M. D. et al. (2011). Acta Cryst. D67, 235–242.
- Xu, Q. et al. (2010). Acta Cryst. F66, 1230–1236.