Abstract
The mammalian target of rapamycin (mTOR) pathway is important for regulating protein translation. The present study characterized the role of mTOR-dependent translation in the dorsal hippocampus (DH) during the consolidation and reconsolidation of contextual fear memory. We first showed that fear conditioning resulted in increased phosphorylation of p70s6 kinase (p70s6K) in the DH and that infusion of the mTOR inhibitor rapamycin (RAP) into the DH immediately after training disrupted formation of long-term contextual fear memory. Additionally we showed that p70s6K was activated after retrieval of a previously stored fear memory, and inhibition of mTOR by DH infusion of RAP blocked the reconsolidation of contextual fear memory. Together these results demonstrate that within the DH translational control through the mTOR pathway is important for consolidation as well as the stability of fear memory after retrieval.
Keywords: Fear conditioning, mTOR pathway, hippocampus, reconsolidation, consolidation, rapamycin
1
Pavlovian fear conditioning, a procedure in which a novel stimulus paired with an aversive event comes to elicit a fear response, has been extensively used to study memory consolidation. The formation of a fear memory in this paradigm requires de novo gene transcription and protein synthesis in several brain areas (for review see, Helmstetter et al., 2008). Reconsolidation refers to the finding that a consolidated memory again becomes sensitive to disruption after a brief reminder treatment (Misanin et al., 1968, Nader et al., 2000). This phenomenon has been demonstrated in multiple species in a variety of behavioral paradigms and is reliant upon protein synthesis (for review see, Tronson and Taylor, 2007). Evidence for the importance of protein synthesis in memory reconsolidation comes from studies showing disruption of fear memory with delivery of protein synthesis inhibitors around the time of memory retrieval (Nader et al., 2000; Debeic et al., 2002; Suzuki et al., 2004; Parsons et al., 2006a). Recent work has suggested that specific signaling pathways controlling translation may play a pivotal role in the initial consolidation process (Kelleher et al., 2004; Costa-Mattioli et al., 2005; Parsons et al., 2006b; Bekinschtein et al., 2007; Blundell et al., 2008) as well as reconsolidation following memory retrieval (Parsons et al., 2006b; Blundell et al., 2008; Glover et al., 2010).
The mTOR pathway is one such signaling pathway and it regulates mRNA translation through its downstream targets p70S6 kinase (p70s6K) and the elongation factor binding protein 4EBP1 (for review see Hoeffer and Klann, 2009). Measurement of phosphorylated p70s6K and/or phosphorylated 4EBP1 is often used as an indicator of mTOR activity. Several studies have shown that mTOR signaling is critical for activity-dependent synaptic plasticity in a variety of systems (Casadio et al., 1999; Tang et al., 2002; Gong et al., 2006). A number of recent reports indicate that components of the mTOR pathway are engaged following learning (Parsons et al., 2006b; Bekinschtein et al., 2007; Slipczuk et al., 2009; Belelovsky et al., 2009; Glover et al., 2010; Qi et al., 2010). Many of these same studies and others (Blundell et al., 2008) have shown that rapamycin (RAP), an inhibitor of the mTOR pathway, disrupts contextual fear memory consolidation when the drug is given around the time of learning.
Translational regulation by mTOR might also be involved in the reconsolidation of memory following retrieval. Prior work from our lab showed that delivery of RAP into the amygdala after fear memory retrieval disrupted performance on subsequent tests (Parsons et al., 2006b). Other work has shown that systemically administered RAP given just after retrieval disrupts reconsolidation (Blundell et al., 2008; Glover et al., 2010) and that this disruption is long lasting (Blundell et al., 2008). Finally, mTOR signaling in the dorsal hippocampus (DH) might also be involved in reconsolidation of contextual fear memory, as targeted infusions of RAP disrupt the reconsolidation of hippocampal-dependent object recognition memory (Myskiw et al., 2008).
Here we addressed whether the mTOR pathway is activated in response to hippocampally dependent contextual fear learning and retrieval. We showed that rats trained using contextual fear conditioning had enhanced phosphorylation of p70s6K in the DH 60 minutes later, and that blockade of mTOR activity in the DH after fear conditioning disrupted the formation of contextual, but not cued, fear memory. Animals that were trained and subsequently retrieved a contextual fear memory showed increased phosphorylated p70s6K in the DH. Finally, we show that RAP infused into the DH prior to retrieval disrupted memory when tested the following day. These findings suggest that mTOR signaling is normally required within the DH during consolidation and reconsolidation of contextual fear memory.
2. Experimental Procedures
2.1 Subjects
Naive male Long Evans rats (N=114) obtained from Harlan (Madison, WI) weighing approximately 300-350 grams served as subjects. All animals were individually housed in stainless steel hanging cages in a room maintained on a 14:10 hour light/dark cycle. Experiments took place during the light portion of the cycle. Food and water were available ad libitum. Animals were handled prior to all experiments over 3 days. All procedures were carried out with approval of the Institutional Animal Care and Use Committee.
2.2 Surgery
Animals were handled for several days prior to surgery. Rats that underwent surgery (N=51) were implanted with bilateral cannulae aimed at the dorsal hippocampus (AP -3.5, L +/ - 2.6, V -3.0). Coordinates were chosen based on a rat brain atlas (Paxinos and Watson, 1998). Before surgery animals were anesthetized with systemic injections of ketamine HCL (100mg/kg body weight) and sodium pentobarbital (2.5 mg/kg/rat). The cannulae were anchored to the skull using stainless steel screws and acrylic cement. To prevent blockade of the cannulae 33-gauge obdurators remained in the guide cannulae when not in use. Animals were habituated to the handling and transport procedure for 2 minutes each day for 4 days.
2.3 Apparatus
Fear conditioning was conducted in Context A which was made of Plexiglas and stainless steel observation chambers illuminated with white light and housed in sound attenuating chambers. The floor was comprised of 18 stainless steel bars 5 mm in diameter spaced 12 mm apart and connected to a shock generator. Ventilation fans produced 62-64 decibel (dB) of background noise. Each chamber was equipped with a speaker centered in the middle of one end of the chamber. Before training or testing of each animal, Context A was cleaned with a 5% ammonium hydroxide solution.
The novel chamber (Context B) had a different shape, olfactory cue (2% acetic acid solution) and flooring (Plexiglas) than Context A. Fans provided background noise (∼58 dB) and the chambers were enclosed in a sound attenuating box illuminated with white light.
2.4 Drug Preparation & Infusion Procedure
Prior to behavioral testing animals with cannulae in the DH were habituated to transport and the microinjection procedure for 4 days. Each rat was restrained in a towel for several minutes, their obdurators were removed and their scalp was cleaned. During this time the infusion pump to be used during the experiment was activated in order to habituate the animals to the sound it produces. After this was complete, the obdurators were replaced and the animal was returned to its home cage.
Rats received a bilateral infusion into the DH (1μl/side) given over 60 seconds. The injection cannulae remained in place for an additional 90 seconds to ensure diffusion away from the injector tip. The injection cannulae were cut to extend approximately 0.5 mm beyond the guide cannulae to ensure they infused into fresh tissue. Animals either received rapamycin (Calbiochem, San Diego, CA) dissolved in 100% Dimethyl Sulfoxide (Sigma) to 1, 2 or 5 μg/μl or the same volume of 100% DMSO. Rats were returned to their home cage after infusions.
2.5 Behavioral Procedures
Animals shocked immediately after placement into a context do not learn contextual fear conditioning (Fanselow, 1990). This finding is referred to as the immediate shock deficit. Using the immediate shock deficit group as a control that received the same shock exposure and time in the context as the experimental group, we compared phosphorylated p70s6K expression in animals shocked (1.3 mA, 2 seconds) immediately (IMM; N = 6) to animals shocked after a 2 minutes delay (DLY; N = 7) after placement in the context. Both groups of animals remained in Context A for a total of 3 minutes. A homecage (HC) group (N=6) remained in the vivarium. IMM and DLY animals received a lethal dose of sodium pentobarbital (100 mg/kg) 60 minutes after training. HC animals were euthanized throughout the day along with IMM and DLY animals. Brains were removed and stored at -80C until DH tissue could be collected and prepared for western blot analysis.
To verify that rats did not learn with the immediate shock parameters we used in Experiment 1, we tested whether immediate shock resulted in memory formation in a separate experiment (N = 19). In Experiment 2 we took advantage of the finding that the immediate shock deficit can be reversed in animals pre-exposed to the training context the day before training (Rudy et al., 2002). We pre-exposed animals to either Context A (A/IMM; N=10) or Context B (B/IMM; N=9) for 8 minutes. Twenty four hours later all animals received immediate shock (2.0 mA, 1second) in Context A and remained in Context A for a total of 3 minutes. The following day all animals were tested for 5 minutes in Context A.
For Experiment 3 all rats (N = 42) were placed in the training chambers and after 6 minutes they were given 4 pairings of white noise (72 dB, 10 seconds) and shock (1.3 mA, 1 second) 90 seconds apart. Immediately after training animals were infused with DMSO (N=16), 1 (N= 12), 2 (N= 6) or 5 (N=8) μg/μl of RAP into the DH. Approximately 24 hrs after training rats were tested for retention of the white noise cue and training context in a counterbalanced order. The testing sessions were separated by approximately 4 hours for all animals. For context testing, rats were placed in the training chambers (Context A) for 15 minutes. For auditory cue testing, rats were placed into the novel Context B and exposed to the white noise for 5 minutes after a 6 minutes baseline.
Experiment 4 was done to examine the expression of phosphorylated p70s6K after the retrieval of a contextual fear memory (N=23). In this experiment one group of animals was trained with shocks (+) in Context A and re-exposed to Context A 24 hours later (A+/A, N = 5). Three control groups were included in this experiment to verify that any difference in protein expression was due to retrieval of the contextual fear memory rather than to some other aspect of the procedure. These control groups included a group exposed to Context A without shock and re-exposed to Context A on day 2 (A/A, N = 7); a group that was trained with shocks but exposed to the novel Context B on day 2 (A+/B, N = 5); and a home cage group (HC, N = 6).
After a 4 minute baseline animals in groups A+/A and A+/B received 3 shocks (1.3mA, 1 second) separated by 20 seconds and were returned to their home cage after a 3 minute post-shock period. Rats in group A/A were placed into Context A for an equivalent period of time as A+/A and A+/B but did not receive shock. Twenty four hours later animals were exposed to Context A (A+/A, A/A) or Context B (A+/B) for 90 seconds. Sixty minutes later brains were removed and stored at -80C until DH tissue could be collected and prepared for western blot analysis.
Experiment 5 was done to determine whether contextual fear memory reconsolidation was sensitive to disruption of the mTOR pathway using RAP (N = 9). Animals were trained as in Experiment 4. Twenty four hours later animals were returned to Context A for a 90 second retrieval session. Thirty minutes prior to the retrieval session rats were brought into an adjacent room and given 5 μg/μl of RAP (N=4) or DMSO (N=5) into the DH (1μl/side). Drug was infused 30 minutes prior to retrieval based on previous work showing that drug infusion at this time point disrupted reconsolidation (Suzuki et al., 2004; Doyere et al., 2007). Twenty four hours after retrieval animals were returned to Context A for an 8 minute test.
2.6 Western Blot Procedure
DH tissue was dissected out and homogenized in buffer (all in 100 mL DDH20; Tris-HCL .605g; Sodium Deoxycholate .25g; NaCl .876g; EDTA .038g; .0042g NaF; PMSF 1μg/ml; leupeptin 1μg/ml; aprotinin 1μg/ml; 10ml 10% SDS, 1mM sodium orthovanadate) and immediately placed on dry ice. Samples were stored at −80 C until further processing. Samples were thawed and then centrifuged at 4k rpm for 20 minutes; the supernatant was removed and measured using a Bradford protein assay kit (Bio-Rad laboratories, Hercules, CA). Protein samples were normalized and 50 μg/μl of protein from each sample was loaded onto a 6% SDS/PAGE gel. Proteins were transferred from the gel to a membrane using a semidry transfer apparatus (Bio-Rad Laboratories). Membranes were incubated in blocking buffer for 2 hours and then incubated overnight at 4°C in primary antibody for phosphorylated p70s6K (Thr 412) (1:1000 Millipore). Non-phosphorylated-p70s6K (1:1000, Millipore) was used to verify equal amounts of protein were added to each lane. Total p70s6K has been shown to be unaffected by memory formation at the time point used here (Qi et al., 2010; Slipzcuk et al., 2009). Following primary antibody exposure, all membranes were incubated in secondary antibody (Millipore, 1:5000) for 90 minutes. Membranes were washed thoroughly, placed in a chemiluminescence solution for 3 minutes (Santa Cruz Biotechnology) and exposed to autoradiographic film (Hyperfilm MP). An image of the film was taken and the appropriately sized band was measured in gray scale and subtracted from a background measurement from the same film resulting in the relative optical density (ROD) score. The ROD was derived by dividing each rats ROD by the average of the HC group ROD and multiplying it by 100. This percentage of control score was then statistically analyzed using One-way ANOVA and Fisher's LSD post hoc tests.
2.7 Histology
Animals were euthanized with an injection of sodium pentobarbital (100mg/kg). Animals were transcardially perfused with saline followed by 10% buffered formalin solution. Heads, with cannulae intact, were placed in 10% formalin solution for at least 24 hours. The brains were then extracted from the skull and placed in a 30% sucrose formalin solution until they were ready to section. Frozen sections (40-μm) were collected throughout the hippocampus, mounted on slides, and stained with cresyl violet. Injection sites were then determined with the aid of a rat brain atlas (Paxinos and Watson, 1998).
3. Results
3.1 Experiment 1: Contextual fear conditioning increases the phosphorylation of p70s6K in the hippocampus after training
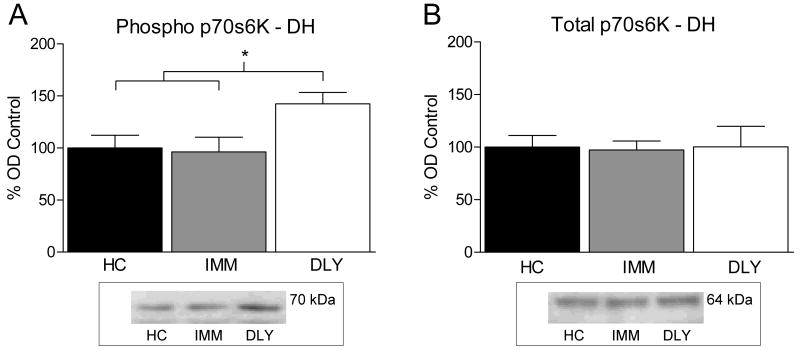
Experiment 1 examined whether the mTOR pathway is activated after contextual fear conditioning. We measured phosphorylated p70s6K as an indicator of mTOR pathway activation. Rats were trained with a single shock delivered immediately (IMM) or 2 minutes after (DLY) placement into the training chamber. One way ANOVA performed on the level of phosphorylated p70s6K in DH tissue after training revealed a significant group difference (F (2, 15) = 4.020, P < 0.05). Fisher's LSD post hoc tests indicated that phosphorylated p70s6K immunoreactivity was significantly increased for the DLY group compared to HC and IMM groups (P's < 0.05, Figure 1A). The same samples were also analyzed for total p70s6K (Figure 1B) as a loading control to measure any differences in total protein loaded. One way ANOVA showed no significant difference in total p70s6K (F (2, 15) = .014, P > 0.05).
Figure 1.
(A) Bars represent mean optical density values (±SEM) expressed as a percentage of home cage control. Rats sacrificed 60 minutes after delay shock (DLY, white bar) showed significantly increased phosphorylated p70s6K in the DH compared to immediate shock (IMM, gray bar) or animals sacrificed from their home cage (HC, black bar). (B) No difference was seen in total p70s6K expression. A representative western blot image is pictured below each graph of western blot data.
3.2 Experiment 2: Immediate shock does not result in formation of contextual fear memory
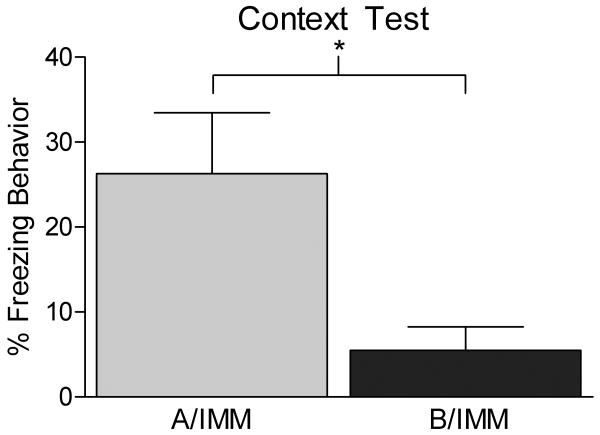
To verify that our immediate shock paradigm did not lead to conditioning in the immediate shock group, a separate cohort of animals was pre-exposed to either the context in which they were to be shocked (Context A, A/IMM) or a novel context (Context B, B/IMM). The following day all animals were trained with immediate shock in Context A and tested twenty four hours later. A two sample t test showed that animals pre-exposed to Context B (B/IMM) froze significantly less than animals pre-exposed to the Context A (A/IMM) when given an immediate shock (t (10) = 2.587, p < 0.05, Figure 2). This finding supports the assertion that our immediate shock protocol does not lead to formation of contextual fear memory, unless the rats were pre-exposed to the training context.
Figure 2.
Bars represent mean percentage freezing behavior (±SEM). Using similar parameters to our immediate shock control in Experiment 1 we showed minimal freezing when a group of animals was pre-exposed to a different context (Context B) from the training context (B/IMM, dark gray bar) and given an immediate shock in Context A the following day. In contrast, animals that received pre-exposure to Context A and an immediate shock in that context the next day showed significant fear memory (A/IMM, light gray bar). This experiment demonstrates that with parameters similar to those used in Experiment 1 we attain the immediate shock freezing deficit.
3.3 Experiment 3: Rapamycin infused into the dorsal hippocampus selectively blocks contextual memory consolidation
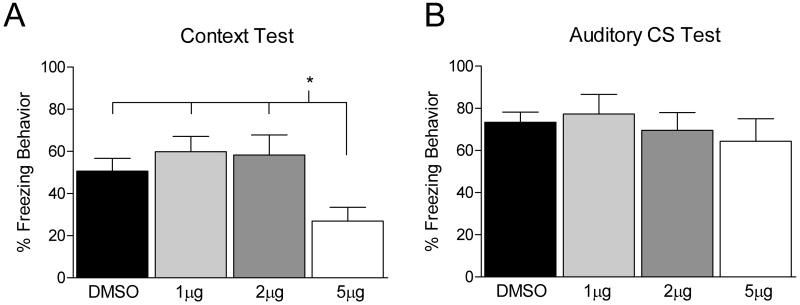
Since phosphorylated p70s6K was increased in the DH in Experiment 1 we next tested if inhibition of the mTOR pathway into the DH would result in disruption of contextual fear memory. Animals were trained and immediately after received an infusion of DMSO vehicle or one of the following doses of RAP (1, 2 or 5 μg/μl) into the DH. The following day animals were tested for fear retention to the white noise cue and training context. While there were no significant differences during training (data not shown, F (3, 38) = 2.410, p > 0.05), there was a significant difference between groups during the test for memory to the context the following day (F (3, 38) = 3.090, p < 0.05). Fisher LSD post hoc analysis showed the group given 5 μg/μl of RAP showed a significant disruption in freezing compared to all other groups (Figure 3A). These same animals showed normal responding to the auditory cue (Figure 3B) indicating they are able to perform the freezing response similarly to controls and that the disruption is selective to the hippocampally dependent contextual memory. Visual inspection of the tissue surrounding the infusion site indicated no damage to the DH from any dose of RAP or DMSO.
Figure 3.
Bars represent mean percentage freezing behavior (±SEM). Rats were trained with 4 white noise – shock pairings (data not shown). Immediately after training animals received an infusion of DMSO vehicle or 1, 2 or 5 μg/μl RAP into the DH. Animals that received DMSO (black bar) or RAP (1μg/μl, light gray bar; 2μg/μl, dark gray bar; 5μg/μl, white bar) were tested 24 hours later to the training context (A) as well as to the white noise in a novel context (B). The group that received 5μg/μl of RAP showed significantly disrupted freezing to the context 24 hours later. No differences in freezing behavior were found during exposure to the white noise cue.
3.4 Experiment 4: Retrieval of contextual fear conditioning increases activation of p70s6K
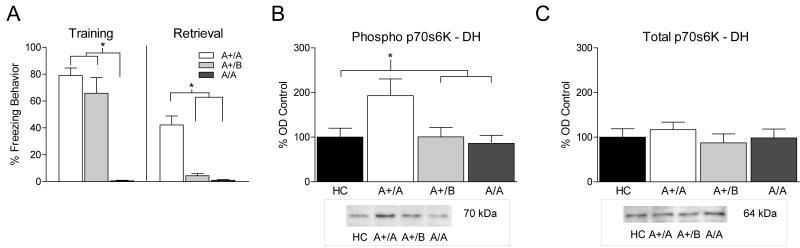
Since phosphorylation of p70s6K in the DH was selectively increased after acquisition of a contextual fear memory we next asked whether the same would be true after memory retrieval. In this experiment one group of animals was trained with shocks (+) in Context A and re-exposed to Context A 24 hours later (A+/A); a second group exposed to Context A without shock and re-exposed to Context A on day 2 (A/A); a third group was trained with shocks but exposed to a novel Context B on day 2 (A+/B); and a fourth group remained in the home cage (HC).
A significant difference was seen in freezing behavior during training (F (2, 14) = 32.421, p < 0.05). As shown in Figure 4A (left), Fisher LSD post hoc analysis showed that animals that were shocked (A+/A, A+B) froze significantly more than those that were not (A/A) (p < 0.01). Twenty four hours later groups A+/A and A/A were returned to Context A while group A+/B was placed in Context B. A One way ANOVA on behavioral data from the retrieval test (Figure 4A, right) showed a significant effect of group (F (2, 14) = 24.297, p < 0.05). Follow up LSD post hoc analysis showed that the A+/A group showed significantly more freezing behavior (p's < 0.01) than groups A+/B or A-/A during the 90 second retrieval test.
Figure 4.
(A, Left) Bars represent mean percentage freezing behavior (±SEM) after presentation of shock during the training session. Animals that were trained with shocks (A+/A, white bar; A+/B, light gray bar) showed significantly more freezing during training than those not shocked (A/A, dark gray bar). (A, Right) Freezing behavior during retrieval was significantly higher for animals that were previously shocked and tested in Context A the following day (A+/A) compared to animals trained in Context A and tested in a novel context (A+/B) or animals that were never shocked (A/A). (B, C) Bars represent mean optical density values (±SEM) expressed as a percentage of home cage control. (B) Western blots conducted on rats sacrificed 60 minutes after retrieval of a contextual fear memory showed increased phosphorylated p70s6K compared to HC (black bar), A+/B and A/A conditions. (C) As a loading control membranes were exposed to antibody against total p70s6K and no differences were observed. A representative western blot image is pictured below graphs of western blot data.
One way ANOVA on data from western blot analysis showed a significant group difference in phosphorylated p70s6K expression after retrieval (F (3,19) = 3.452, p < 0.05, Figure 4B). Fisher LSD post hoc tests showed that group A+/A had increased phosphorylated p70s6K in the DH compared to all other conditions (p's < 0.05). As shown in Figure 4C no differences were found in expression of total p70s6K in the same samples indicating there was no change in the levels of total kinase following retrieval (F (3,19) = 1.084, p > 0.05).
3.5 Experiment 5: Blocking mTOR prior to retrieval disrupts reconsolidation
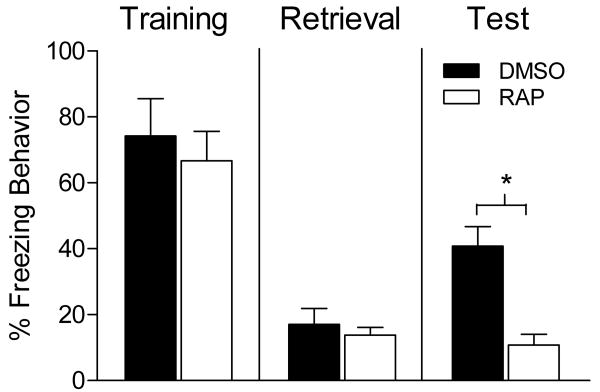
The increase in phosphorylated p70s6K after retrieval suggests that mTOR-dependent translation in DH neurons is activated in response to retrieval of a contextual fear memory. In our final experiment we tested whether this increase was necessary for maintaining the stability of memory after retrieval. As shown in Figure 5 no significant differences in freezing behavior were seen between groups during training (t test, p > 0.05) or retrieval (t test, p > 0.05). However, animals given an infusion of RAP into the DH 30 minutes prior to contextual fear memory retrieval showed attenuated freezing at test 24 hours later (t = 4.196, p < 0.05) compared to vehicle controls. These findings provide support for the idea that the increase in mTOR pathway activation in the DH is necessary for maintaining memory stability. The finding further supports a potential role for mTOR signaling in reconsolidation of a contextual fear memory.
Figure 5.
Bars represent mean percentage freezing behavior (±SEM). Animals were trained (Left) and returned to the training context 24 hours later for a 90 second retrieval session (middle). Thirty minutes prior to the retrieval session animals received an intra hippocampal infusion of vehicle (black bar) or RAP (white bar). While no differences in freezing behavior were seen during retrieval, the following day animals that received RAP showed significantly less freezing to the reactivated context compared to controls (right).
4. Discussion
Our results add to a growing body of work indicating mTOR signaling is critical for synaptic plasticity and memory formation. We showed that phosphorylated p70s6K was increased 60 minutes after contextual fear conditioning in the DH of animals that received delayed presentation of shock. The immediate shock control condition shows that the increase in phosphorylated p70s6k was not due to some non-associative effect of training such as placement into the context, amount of time in the context, or receipt of the shock. We next showed that RAP selectively disrupts consolidation of a contextual fear memory when infused into the DH immediately after fear conditioning without affecting the ability of the animal to show fear behavior to an auditory cue. Intact auditory fear conditioning argues against a general deficit in freezing behavior in RAP treated rats, and underscores the importance of the DH in contextual, but not cued, fear conditioning.
Next we assessed whether phosphorylated p70s6K is increased after retrieval of a contextual fear memory. Animals trained and then tested the following day in the same context showed an increase in phosphorylated p70s6K 60 minutes after retrieval compared to controls. The control conditions included in this experiment speaks to two important issues. First, simply training animals does not lead to activation of the mTOR pathway 24 hours later, as indicated by group A+/B. This argues against the possibility that in our final experiment we disrupted ongoing memory consolidation 24 hours after training with our infusion of RAP prior to retrieval. Also, although several markers of plasticity are increased in the DH between 12 and 24 hours after training (e.g. Bekinschtein et al., 2010), mTOR does not appear to be one of these, as DH infusions of rapamycin do not block the protracted cellular consolidation events that are thought to underlie persistence of memory (Bekinschtein et al., 2008).
Second, the fact that we found no increase in phosphorylated p70s6K after re-exposing rats to a context they had explored the previous day suggests that exploration of an environment and replacement into that same environment the following day does not engage the same sort of cellular processes as exposure to a fearful context. This is consistent with work by Biedenkapp and Rudy (2004) showing that anisomycin infused into the DH after retrieval did not disrupt reconsolidation of a basic contextual memory. It also agrees with the finding that expression of zif268 mRNA in the DH correlates best to retrieval of contextual fear memories and not simply re-exposure to a context (Hall et al., 2001). However, this data should not be taken as evidence that the DH is involved in context-shock associations, as there is considerable evidence indicating that the DH is involved in the formation of contextual memory, but is not necessary for the association between context and shock (e.g. Matus-Amat et al., 2004). Thus, the increase in phosphorylated p70s6K expression after retrieval in the A+/A group might reflect the fact that there is new learning because the context is presented without the shock during retrieval. Further, the finding of no change in p70s6K activity in the A/A group may be due to animals learning nothing new during retrieval. These two possibilities cannot be ruled out with the present data.
Recent studies have begun to tackle how the mTOR pathway becomes engaged and what the downstream targets are through which it affects synaptic plasticity and memory. BDNF is a crucial mediator of synaptic plasticity and memory (Rattiner et al., 2005; Alonso et al., 2002; Tyler et al., 2002) and is known to activate the mTOR pathway (Takei et al., 2004; Schratt et al., 2004). One recent study showed that BDNF likely acts through mTOR to facilitate the synaptic expression of GluR1 containing AMPA receptors during memory consolidation (Slipczuk et al., 2009). The effect RAP has on memory might also involve the regulation of other synaptic proteins including PSD-95, as a recent study showed that levels of this protein are reduced following RAP infusion (Belelovsky et al., 2009). Other studies have provided evidence that extracellular signal-regulated kinase (ERK) might play a role in mTOR activation (Kelleher et al. 2004; Jaworski and Sheng 2006). ERK is involved in LTP and memory and can be regulated by dopaminergic and glutamatergic signals (Brami-Cherrier et al. 2002; Papadeas et al. 2004; Lenz and Avruch 2005; Banko et al. 2005; 2006; Nagai et al. 2007). Within the hippocampus in vitro studies show that there is an interaction of ERK and mTOR in which they increase the translational capacity after LTP-inducing stimulation and activate neuromodulatory transmitter receptors (Gelinas et al. 2007; Tsokas et al. 2007). These studies highlight some potential mechanisms through which mTOR may be affecting synaptic plasticity and memory.
5. Conclusions
To date only a limited number of studies have demonstrated the involvement of the mTOR pathway in memory. Our current findings demonstrate that mTOR signaling in the DH is engaged after consolidation, retrieval and reconsolidation of contextual fear memory. Along with previous published works, our results highlight the burgeoning understanding of mRNA translation and its role in memory at the synaptic and behavioral level.
Acknowledgments
This work was supported by grants from the National Institutes of Health (MH069558 to F.J.H.)
Abbreviations
- mTOR
mammalian target of rapamycin
- p70S6K
p70s6 kinase
- RAP
rapamycin
- DH
dorsal hippocampus
- DMSO
dimethyl sulfoxide
- ROD
relative optical density
- db
decibel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alonso M, Vianna MR, Izquierdo I, Medina JH. Signaling mechanisms mediating BDNF modulation of memory formation in vivo in the hippocampus. Cell Mol Neurobiol. 2002;22:663–674. doi: 10.1023/A:1021848706159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Poulin F, Hou L, DeMaria CT, Sonenberg N, Klann E. The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus. J Neurosci. 2005;25:9581–9590. doi: 10.1523/JNEUROSCI.2423-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk LN, Igaz LM, Cammarota M, Izquierdo I, Medina JH. mTOR signaling in the hippocampus is necessary for memory formation. Neurobiol Learn Mem. 2007;87:303–307. doi: 10.1016/j.nlm.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, Izquierdo I, Medina JH. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci U S A. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekinschtein P, Katche C, Slipczuk L, Gonzalez C, Dorman G, Cammarota M, Izquierdo I, Medina JH. Persistence of long-term memory storage: new insights into its molecular signatures in the hippocampus and related structures. Neurotox Res. 2010;18:377–385. doi: 10.1007/s12640-010-9155-5. [DOI] [PubMed] [Google Scholar]
- Belelovsky K, Kaphzan H, Elkobi A, Rosenblum K. Biphasic activation of the mTOR pathway in the gustatory cortex is correlated with and necessary for taste learning. J Neurosci. 2009;29:7424–7431. doi: 10.1523/JNEUROSCI.3809-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedenkapp JC, Rudy JW. Context memories and reactivation: constraints on the reconsolidation hypothesis. Behav Neurosci. 2004;118:956–964. doi: 10.1037/0735-7044.118.5.956. [DOI] [PubMed] [Google Scholar]
- Blundell J, Kouser M, Powell CM. Systemic inhibition of mammalian target of rapamycin inhibits fear memory reconsolidation. Neurobiol Learn Mem. 2008;90:28–35. doi: 10.1016/j.nlm.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brami-Cherrier K, Valjent E, Garcia M, Pagès C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci. 2002;22:8911–8921. doi: 10.1523/JNEUROSCI.22-20-08911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille JC, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debiec J, LeDoux JE, Nader K. Cellular and systems reconsolidation in the hippocampus. Neuron. 2002;36:527–538. doi: 10.1016/s0896-6273(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Doyère V, Debiec J, Monfils MH, Schafe GE, LeDoux JE. Synapse-specific reconsolidation of distinct fear memories in the lateral amygdala. Nat Neurosci. 2007;10:414–416. doi: 10.1038/nn1871. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Factors governing one-trial contextual conditioning. Anim Learn Behav. 1990;18:264–270. [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Glover EM, Ressler KJ, Davis M. Differing effects of systemically administered rapamycin on consolidation and reconsolidation of context vs. cued fear memories. Learn Mem. 2010;17:577–581. doi: 10.1101/lm.1908310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Park CS, Abbassi NR, Tang SJ. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J Biol Chem. 2006;281:18802–18815. doi: 10.1074/jbc.M512524200. [DOI] [PubMed] [Google Scholar]
- Hall J, Thomas KL, Everitt BJ. Cellular imaging of zif268 expression in the hippocampus and amygdala during contextual and cued fear memory retrieval: selective activation of hippocampal CA1 neurons during the recall of contextual memories. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Parsons RG, Gafford GM. Macromolecular synthesis, distributed synaptic plasticity, and fear conditioning. Neurobiol Learn Mem. 2008;89:324–337. doi: 10.1016/j.nlm.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, 3rd, Govindarajan A, Jung HY, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Lenz G, Avruch J. Glutamatergic regulation of the p70S6 kinase in primary mouse neurons. J Biol Chem. 2005;280:38121–38124. doi: 10.1074/jbc.C500363200. [DOI] [PubMed] [Google Scholar]
- Matus-Amat P, Higgins EA, Barrientos RM, Rudy JW. The role of the dorsal hippocampus in the acquisition and retrieval of context memory representations. J Neurosci. 2004;24:2431–2439. doi: 10.1523/JNEUROSCI.1598-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misanin JR, Miller RR, Lewis DJ. Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science. 1968;160:554–555. doi: 10.1126/science.160.3827.554. [DOI] [PubMed] [Google Scholar]
- Myskiw JC, Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, Nakanishi Y, Murai M, Mizoguchi H, Nabeshima T, Yamada K. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14:117–25. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadeas ST, Blake BL, Knapp DJ, Breese GR. Sustained extracellular signal-regulated kinase 1/2 phosphorylation in neonate 6-hydroxydopamine-lesioned rats after repeated D1-dopamine receptor agonist administration: implications for NMDA receptor involvement. J Neurosci. 2004;24:5863–5876. doi: 10.1523/JNEUROSCI.0528-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Baruch DE, Riedner BA, Helmstetter FJ. Long-term stability of fear memory depends on the synthesis of protein but not mRNA in the amygdala. Eur J Neurosci. 2006a;23:1853–1859. doi: 10.1111/j.1460-9568.2006.04723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RP, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin (mTOR) pathway is critical for the formation and stability of long term fear memory in amygdala neurons. J Neurosci. 2006b;26:12977–12983. doi: 10.1523/JNEUROSCI.4209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. San Diego: Academic; 1998. [DOI] [PubMed] [Google Scholar]
- Qi S, Mizuno M, Yonezawa K, Nawa H, Takei N. Activation of mammalian target of rapamycin signaling in spatial learning. Neurosci Res. 2010;68:88–93. doi: 10.1016/j.neures.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Rattiner LM, Davis M, Ressler KJ. Brain-derived neurotrophic factor in amygdala-dependent learning. Neuroscientist. 2005;11:323–33. doi: 10.1177/1073858404272255. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Barrientos RM, O'Reilly RC. Hippocampal formation supports conditioning to memory of a context. Behav Neurosci. 2002;116:530–538. doi: 10.1037//0735-7044.116.4.530. [DOI] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME. BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci. 2004;24:7366–7377. doi: 10.1523/JNEUROSCI.1739-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S. Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci. 2004;24:4787–4795. doi: 10.1523/JNEUROSCI.5491-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci USA. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Taylor JR. Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci. 2007;8:262–275. doi: 10.1038/nrn2090. [DOI] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]