Abstract
Objectives:
Measurement of outcomes is increasingly employed as an indicator of the quality of clinical care. The most commonly measured outcome in many clinical studies, especially in oncology, still remains the overall survival rate. Sultan Qaboos University Hospital (SQUH), Oman, is striving for excellence through quality management. In seeking continual improvement, quality measurement exercises have been initiated throughout the Hospital. We present the overall survival rate of four of the ten most common cancers diagnosed in Oman.
Methods:
The cancers included non-Hodgkin’s lymphoma (NHL), Hodgkin’s lymphoma (HL), breast cancer, and stomach cancer. The studies were all retrospective and had been conducted previously. For present purposes, only the overall survival was compared with studies both from the region, and with bench-mark studies.
Results:
For NHL, with a median follow-up of 8 months, the 2-year overall survival rate was 64%; 90% for low risk, 55% for intermediate risk, and 15% for high risk groups. For HL, the 5-year overall survival rate was 64%; 76% for low risk and 42% for high risk. For breast cancer, the 5-year survival rate was 67%; percentages were 88%, 75% and 59% for Groups I, II, and III respectively. For gastric cancer, the 5-year survival rate was 16.5 %; 24% for the non-metastatic group.
Conclusion:
The outcome of patients with early stages and fewer adverse prognostic factors is comparable to what has been reported in the international literature; however, the outcome is inferior for patients presenting with advanced stage disease and several adverse prognostic factors.
Keywords: Oncology; Outcome assessment; Quality indicators; Lymphoma; Hodgkin’s; Lymphoma; Non-Hodgkin; Cancer, breast; Cancer, gastric; Oman
Advances in knowledge
This is the first study from Oman and one of the few from the region where the outcomes of cancer care, measured in terms of overall survival, have been used to assess the quality of hospital care provided.
Application to patient care
Clinical audits of this kind are very important to enhance the quality of care at institutions.
The need to measure the results of an intervention in medicine has been recognized for a long time. It has been argued that since hospitals work on the premise that patients should derive benefit from the medical care provided, the end-result of interventions should reflect in outcomes.1 It has also been suggested that if the hospitals provided the care, recorded it in a uniform manner, and then published it, comparisons could be made between the outcomes of different health care institutions.1–3 Hence, measurement of outcomes is increasingly been employed not only in clinical practice, but also as an indicator of quality of clinical care.4
Over the past few decades, scientific methods have been developed to measure the quality of medical care.5–7 More recently, it has been proposed that the quality of care should include dimensions of structure (facilities and organization), process (appropriateness, efficiency, cost-effectiveness) and outcome (mortality, adverse effect, early admissions).8, 9 Since many perceive outcomes to be the ultimate validator of the effectiveness and quality of medical care, outcomes research is rapidly gaining attention. Outcomes can be measured using several indicators, e.g. alleviation of symptoms and quality of life. However, the most commonly measured outcome in many clinical studies, especially in oncology, continues to remain the overall survival of the patient.10
Sultan Qaboos University Hospital (SQUH), Oman, is on the road to achieving excellence in quality, and has already received ISO9001:2000 certification. In an effort to seek continual improvement, quality measurement exercises have been initiated through out the Hospital. The section of Medical Oncology is an integral part of the Department of Medicine, and endeavors to promote teaching, research and clinical service in accordance with the vision and mission of the Hospital. It receives newly diagnosed cancer cases both from within the hospital and referred cases from across the Sultanate of Oman. Breast cancer, gastric cancer, non-Hodgkin’s lymphoma (NHL) and Hodgkin’s lymphoma (HL) are among the ten most common cancers diagnosed in Oman11 and a substantial number of cases are treated at SQUH. Herein, we report the overall survival of these cancers treated at SQUH over the past few years, and compare the results with some bench-mark studies, with a view to using outcome quantified in terms of overall survival as a measure of quality of care.
METHODS
The data on outcomes of NHL, HL, breast cancer, and gastric cancer were collected retrospectively as part of separate studies. The study on NHL aimed to review the clinical features, pathological sub-types (classified according to the recent WHO classification), response to treatment, disease free survival (DFS) and overall survival (OS) of consecutive adult patients (> 14 years of age) diagnosed to have NHL between Jan 2003 and Dec 2004 and seen at the SQUH. Similarly, the study on HL was aimed to review the clinical features, pathological sub-types, response to treatment, disease free survival (DFS) and overall survival (OS) of consecutive adult patients (> 14 years of age) diagnosed to have HL between June 1999 and Dec 2005. The study on breast cancer aimed to study clinico-pathological features and outcomes of treatment using patients treated between January 1996 and June 2002. Details of the methods can be found elsewhere.12 Similarly, the study on gastric cancer aimed to review the clinical features and outcomes of treatment of patients diagnosed between 1993 and 2004. The detailed methods of the study can be found elsewhere.13 A proportion of patients with breast and gastric cancers were diagnosed and treated at the other tertiary care hospital in Oman. The staging for both breast and gastric carcinomas was carried out using the American Joint Committee on Cancer TNM (Tumour, Node, Metastasis) Cancer Staging Manual.14 For all four cancer types, the data from the medical charts were extracted on to pre-designed questionnaires and transferred to SPSS (Statistical Package for the Social Sciences) for analysis. Survival curves were generated using the method of Kaplan and Meier.15 Only the overall survival data are presented in this paper. Details on the rest of the parameters is not within the scope of this paper, and can be found elsewhere.12, 13, 16, 17 All retrospective studies were approved by the Medical Research and Ethics Committee of SQUH.
RESULTS
Table 1 lists the 10 most common cancers diagnosed in the Sultanate of Oman during the year 2004. Brief description of cancers, together with the overall survival results are presented below:
Table 1:
Most common cancers diagnosed in the Sultanate of Oman during 2004. The figures denote the actual number of cases.
| Leukemia | 84 |
| Non-Hodgkin’s lymphoma | 77 |
| Breast Cancer | 66 |
| Stomach Cancer | 49 |
| Thyroid Cancer | 44 |
| Lung Cancer | 36 |
| Cancer of the Uterine Cervix | 33 |
| Brain tumours | 30 |
| Hodgkin’s Lymphoma | 30 |
| Prostate Cancer | 29 |
NON-HODGKIN’S LYMPHOMA
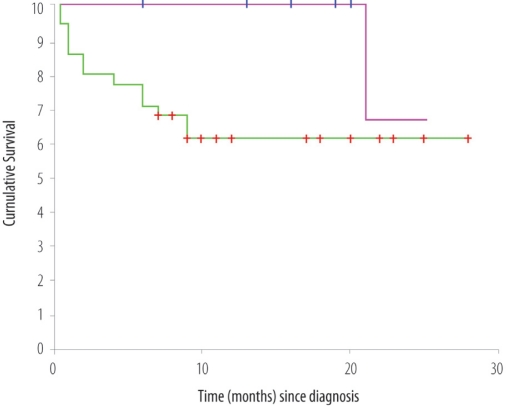
Over the study period of 2 years, a total of 46 new patients were diagnosed to have NHL. The characteristics are as follows: The median age was 53 (14–77) years; there were 27 males and 19 females. The histological sub-types were as follows: diffuse large B cell lymphoma (DLBCL) 31 (67%); anaplastic large cell lymphoma (ALCL) 5 (11%); marginal zone cell lymphoma 3 (one each splenic, cutaneous and intestinal); T-cell acute lymphoblastic lymphoma 2; others 5. Overall, 82% of the patients presented with an aggressive histological sub-type. Nineteen patients presented with primary extra-nodal disease. Thirty-one patients had a high lactate dehydrogenase (LDH). The performance status on the WHO/ECOG (Eastern Cooperative Oncology Group) scale was as follows: 0:1:2:3:4 = 3:17:9:8:9. The WHO/ECOG performance status is a grade on a five point scale (range 0 to 4) at the time of investigation in which ‘0’ denotes normal activity and ‘4’ a patient who is 100% bedridden. Clinical stages were classified according to the Ann-Arbor staging system, the most popular system for classifying NHL, from Stage 1, limited to one lymph node, to Stage 4, extensive in one organ or site. Six patients presented with Stage I disease, 12 with Stage II, 6 with Stage III, and 22 with Stage IV disease. Patients with aggressive histological sub-types were uniformly treated with standard doxorubicin-based chemotherapy (CHOP/R-CHOP). Patients with bulky initial disease, or with residual disease of more than 1 cm after 6–8 cycles of chemotherapy, were also treated with involved field radiation therapy (IFRT) of 36–40 Gy. For aggressive lymphomas, the International Prognostic Index (IPI) score was calculated; 13 patients had 0–1 adverse prognostic factors (low risk = 28%), 7 had 2–3 adverse prognostic factors (intermediate risk = 15%), while 18 patients had 4–5 adverse prognostic factors (high risk = 39%). Three patients were lost to follow up, and 16 patients had died at the time of analysis. With a median follow-up of 8 months, the 2-year survival for the entire cohort is 64% (Figure 1a); 90% for the low risk, 55% for the intermediate risk, and 15% for the high risk groups (Figure 1b).
Figure 1a:
Overall survival (OS) of patients treated for NHL. The top line indicates the OS of patients diagnosed to have indolent lymphomas. The bottom line indicates OS of patients with aggressive lymphomas.
Figure 1b:
Overall survival (OS) of patients with aggressive NHL according to the International Prognostic Index. (Top line: 0–1 adverse prognostic factors; Middle line: 2–3 adverse prognostic factors; Lower line: 4–5 adverse prognostic factors)
HODGKIN’S LYMPHOMA
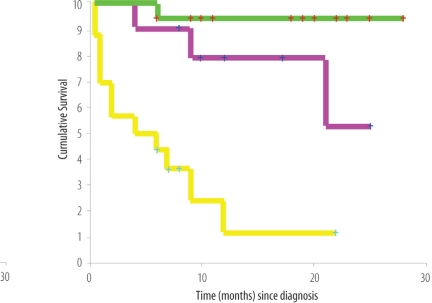
Over the study period of 6.5 years, a total of 40 adult patients were diagnosed to have HL. The median age was 37 years (range 14–76 years). There were 22 men and 18 women. 75% patients presented with B symptoms, and nearly half of the patients presented with symptoms of more than 4 months duration. Histological subtypes according to the REAL (Revised European-American Lymphoma) classification were as follows: Nodular sclerosis 17; Mixed cellularity 14; lymphocyte predominant 4; not otherwise specified 5. Nineteen patients presented with early stage disease (IA–IIB), whereas, 13 and 8 patients (total = 21, 52%) presented with Stage IIIB and IVB disease respectively. Eighteen patients had mediastinal involvement; 3 presented with pulmonary parenchymal involvement, and 4 patients presented with primary infra-diaphragmatic disease. Fifty percent of patients presented with a high LDH. Two patients died before the treatment could be instituted. Patients were treated according to the standard chemotherapy with ABVD (Adriamycin, Bleomycin, Vinblastine and DTIC); COPP/ABV (Cyclophosphamide, Oncovin [Vincristine], Procarbazine, Prednisolone/Adriamycin [Doxorubicin], Bleomycin, VP-16[Etoposide]); BEACOPP (Bleomycin, Etoposide, Adriamycin, Cyclophosphamide, Vincristin [Oncovin], Procarbazine, Prednisone) and VEPEMB (Vinblastine, Cyclophosphamide, Procarbazine, Etoposide, Mitoxantrone and Bleomycin) and with IFRT to the sites of initial bulky or residual disease. There were 38 complete and 2 partial responders. The 2-year overall survival rate was 64% (Figure 2a). International prognostic factor score (IPFS) was applied, and the 2 year survival of patients with 0–2 adverse prognostic factors was 76% compared with 32% for those with 3 or more adverse prognostic factors (Figure 2b).
Figure 2a:
Overall survival (OS) of patients treated for Hodgkin’s lymphoma
Figure 2b:
Overall survival (OS) of patients with advanced Hodgkin’s lymphoma disease according to International Prognostic Factor Score. The top line indicates OS of patients with 0–2 adverse prognostic factors. The bottom line indicates OS of patients with three or more adverse prognostic factors
BREAST CANCER
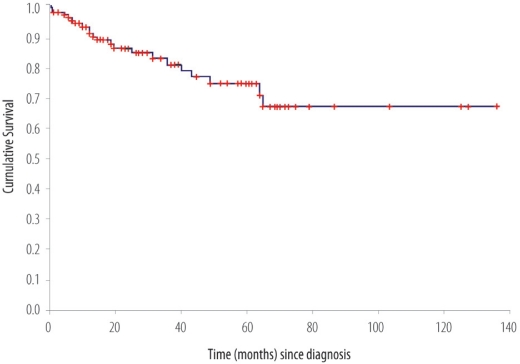
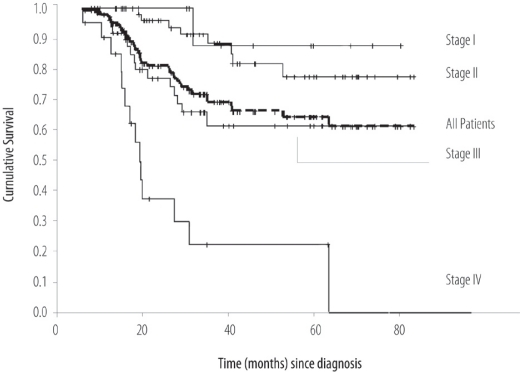
Over the study period of 6 years, a total of 152 patients were treated for invasive breast cancer. The mean age was 48.5 (SD ± 10.8) years. Forty eight percent of the female patients were pre-menopausal and 20.4 % were 40 years of age or younger. The average clinical and pathological sizes of breast tumours were 5.4 cm (SD ± 3.86) and 4.6 cm (SD ± 3.29) respectively. The majority of patients presented with advanced disease: Stage III = 53 (34.9%); Stage IV = 24 (15.8%). The receptor status was available for 107 patients (68%) and of these 62 (58%) and 57 (53.3%) expressed estrogen and progesterone receptors respectively. The majority of patients (65.8%) underwent modified radical mastectomy. Twenty patients (13.2%) received neo-adjuvant chemotherapy, which represents only 37.7% of 53 patients with locally advanced disease. Adjuvant external beam radiotherapy to the breast area was administered to 96 patients (63.1%). During the study period, radiotherapy facilities were not available in Oman; hence, radiotherapy was administered in various centres outside Oman. With a mean follow-up interval of 35.6 months, there were 37 deaths and 6 patients were lost to follow up. The overall 5-year relapse free survival rate was 62%. The cumulative 5-year relapse free survival rates for Stages I, II, and III were 87.5%, 71.6%, and 42.7% respectively (data not shown). The overall 5-year survival rate was 67%. The cumulative 5 year survival rate for Stages I, II, III were 88%, 75%, and 59%, respectively [Figure 3].
Figure 3:
Overall survival (OS) of patients treated for breast cancer
GASTRIC CARCINOMA
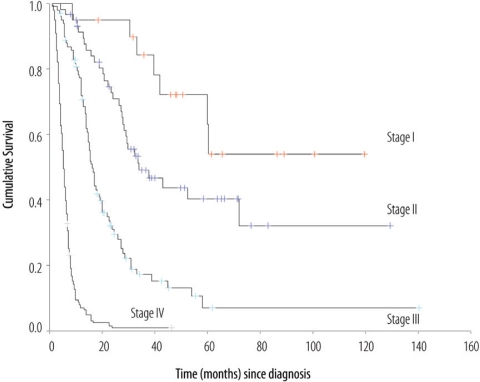
Over the study period, clinico-pathological data were available for 339 patients. There were 221 males and 118 females, with an overall mean age of 59.8 years (range: 14–90 years). The predominant tumour was an ulcerative, intestinal adenocarcinoma. At presentation, most of the tumours had deeply penetrated the stomach wall, with T3 and T4 lesions constituting respectively 55.7% and 14.3% of the cases. Lymph node involvement was found in 75.9% of patients. Advanced Stage III and IV constituted respectively 33.6% and 39.2% of all cases. Two hundred and thirty seven patients (69.9%) were subjected to surgery, of which 158 (46.6%) had complete resections. Sixty-two (26.2%) patients received additional systemic treatment in the form of neo-adjuvant chemotherapy, adjuvant chemotherapy or chemo-radiotherapy. The mean follow-up time for the entire cohort was 29.3 months (range: 3–103 months). There were 256 deaths (75.5%), including 11 early post-operative deaths, while 72 patients (21.2%) remained alive.
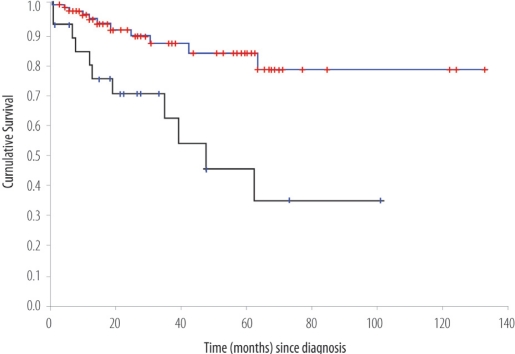
The median survival time for the entire cohort was 12.3 months (95 % CI 9.8–14.8) with a 5-year overall survival rate of 16.5 % [Figure 4]. The median survival time for the non-metastatic group was 23.5 months (95 % CI 18.7–28.3) with a 5-year overall survival rate of 24.1 %.
Figure 4:
Overall survival (OS) of patients with Gastric cancer
DISCUSSION
As outcomes research is a broad concept, there is no consensus on its precise definition. On the one hand, all results are outcomes; thus, all research is outcomes research.18 On the other hand, Donabedian’s concept of quality includes outcome as a part of the “structure, process, and outcome” paradigm.8 The National Library of Medicine makes a distinction between health services research and outcome assessment.19 Whereas health services research is usually concerned with relationships between need, demand, supply, use, and outcome of health services, outcome research is aimed at assessing the quality and effectiveness of health care as measured by the achievement of a planned end result, improved health, and lowered morbidity or mortality.20
Outcome studies have gained attention in oncology literature over the past few years.1, 4, 21, 22 Outcome continues to remain the ultimate validator of the effectiveness and quality of care offered to cancer patients.21–23 It is often hard to define and assess quality of care on the basis of main outcomes; frequently, surrogate end points are used to assess the quality of work in daily oncology practice.24 Although surrogate outcomes of effectiveness are important and often can not be avoided, these may not be related to the main clinical outcomes. An example is tumour response to chemotherapy or radiotherapy, and the ultimate progression free survival or overall survival. For some cancers there is an association between response rates and survival, for others this relationship is either weak or does not exist. Whereas some outcomes are generally unmistakable and easy to measure (death), others are not so clearly defined and can be difficult to measure, e.g. patient attitudes, satisfactions, physical disability etc.18, 22 Survival and quality of life are considered the main patient outcomes in oncology practice.21–24 It has long been suggested that all oncologists should review either their own work or the work in their departments using these two major outcomes.1 We chose to use survival as the measure of outcome. Since our work was based on retrospective data, it was difficult to measure quality of life, especially when it was not recorded, or not recorded uniformly.
The 2-year survival rate was 64% for patients with NHL. Although 2 years is a relatively short period of follow up for aggressive NHL, and relapses continue to occur for further several years, our results at this stage are comparable to what has been reported in the literature.25–29 However, since NHL is a heterogeneous group of lymphomas, with a variable outcome, we sought to study the outcomes according to the risk groups described by IPI (International Prognostic Index).30 IPI describes 5 independent prognostic factors at presentation known to affect the outcome of aggressive NHL. These include old age, advanced stage, poor performance status, a high LDH, and multiple extra-nodal sites of involvement. Analysis of overall survival into the risk categories reveal that, whereas the outcomes for low and intermediate risk is comparable (90% versus 85%, and 55% versus 50% respectively), the outcome for high risk is inferior (15% versus 35%).31 All patients were treated uniformly with anthracyclin based chemotherapy ± rituximab, and IFRT according to the institutional guidelines for patients with initial bulky disease. Slow response was defined as less than partial remission after 4 cycles of chemotherapy; residual disease more than 1.5 cm; positive gallium scan after completion of chemotherapy in not more than 1 location. It is clear that the outcomes of patients with fewer risk factors were comparable to the reported figures. At the same time, the outcomes of patients with several risk factors were found to be inferior to the standards. There are several explanations. Firstly, the sample size is small. Out of a total of 38 patients with aggressive histology, 18 (39%) presented with 4–5 adverse prognostic features. Second, it is well known that there are other important prognostic factors, not included in the IPI, which have recently been shown to have an adverse effect on the outcome, such as: bulky disease, lack of expression of bcl-6 protein, presence of CD10 antigen, bcl-2 antigen etc.32 Of the high risk group of patients, more than 50% in our series presented with bulky disease, which is significantly higher than what has been reported in the literature. The pattern of expression of the pro-apoptotic and anti-apoptotic genes is currently not known in our group of patients, but the work is still in progress. Third, 26% of the patients had co-existing hepatitis B or C virus antibodies. This is significantly higher than the control population in Oman, and also significantly higher than what has been reported in the literature. The outcomes of hepatitis virus associated NHL has been reported to be inferior compared to those in which the viruses are not present.33 Finally, an impaired immune status confers an inferior long-term disease free survival, and it is plausible that patients with higher percentages of advanced disease (61% versus 30–40%), or extra-nodal disease (48% versus 24–29%), may have an impaired immune status. A study to investigate this particular issue is underway at our institution.
For patients with HL, the 5-year survival was 64%. The results are comparable to several reports from around the region.34–38 Like NHL, the outcome of HL is variable and depends on several prognostic factors. IPFS describes 7 independent prognostic factors at presentation which are known to affect the outcome of classical HL.39 Analysis of overall survival into the risk categories reveals that whereas the outcomes for patients with 0–2 adverse prognostic factors was comparable to the European data (76% versus 78%), 39 the outcome for patients with three or more adverse prognostic factors was inferior (42% versus 55%). Like NHL, there are several explanations for this paradox. Firstly, the small numbers mean that the difference is not statistically different. Second, additional prognostic factors, not included in the IPFS may be present. For example, 70% of the patients in our series presented with B symptoms, compared to about 40% reported in the European data which formed the basis for IFPS. Finally, important differences in the biological nature of the disease are known to exist in HL over different parts of the globe. Incorporation of Epstein-Barr virus (EBV) and the presence of Il-10 receptors polymorphisms have been shown to be additional independent prognostic factors, currently unknown in our group of patients.40
For patients with breast cancer, the 5-year survival rate was 67%. A breast cancer study from Oman exemplifies the state of breast cancer presentation, care, and outcome in many developing countries.41–46 Breast cancer in Oman displays established poor prognostic features not only by presenting at a younger age with advanced stage and extensive lymph node involvement, but also with a poor differentiation grade and lack of estrogen and progesterone receptors expression.12 In this series, tumour size of more than 5 cm and tumour differentiation grade were strong predictors of overall survival. In contrast, axillary lymph node status, tumour size of more than 5 cm, and poor differentiation grade were predictors of relapse free survival. Tumour size, lymph node involvement, differentiation grade, and estrogen receptor status were tested in multivariate Cox’s regression analysis for their relationship with overall survival and relapse free survival. Tumour differentiation and 4–9 lymph node involvement retained independent prognostic significance for disease free survival and overall survival respectively12. The survival outcome is worse than for counterparts in the West, but consistent with results from the region. The 5-year survival rate of 67% reported in our series compares favorably with 48% reported by Gajalakshmi et al from India, the 10-year survival rate of 55% reported from the eastern province of Saudi Arabia and a 5-year survival of 68.8% from Bahrain.41, 43, 44 There are several explanations. Outcomes of breast cancers have been shown to be inferior in patients of lower socio-economic groups, and in under-privileged ethnic minorities.47, 48 For example, O’Malley et al have shown the 10-year unadjusted survival rates of 81% for whites, 69% for blacks, 75% for Hispanics, and 79% for Asians. Our results call for immediate steps to increase breast cancer awareness, and introduce breast cancer screening programs in developing countries.49, 50 More importantly, all patients should have easy access to tertiary referring units where multidisciplinary assessment is made on presentation.50 One of the aims should be more breast conserving surgery with greater utilization of neo-adjuvant chemotherapy. Research areas exploring cultural, environmental, and genetic issues should be undertaken in an attempt to explain further the above clinical and pathological features.49, 50
For gastric cancer, the 5-year survival was 16%. This figure compares favorably with 5-year survival rates of 21% from Jordan, and 15–20% worldwide.14, 53 Gastric cancer study exemplifies the issues of presentation, management and prognosis in many developing countries51–55. It exhibits the clinico-pathological features seen in endemic areas where the majority of tumours are likely to be distal, ulcerative, and intestinal adenocarcinomas.56 The most important determinant of outcome is the TNM stage at presentation14. Lymph node involvement and overall TNM stage are independent prognostic factors. Extended surgical approach and adjuvant treatment may modify the survival outcome, but in the current cohort the advanced stages of most presentations appear to have lessened the survival prospects. This finding emphasises the need for detecting gastric cancer early, either by employing screening programmes or having a lower threshold for initiation of upper gastrointestinal endoscopy, especially for the elderly (who comprised the vast majority of our patients), whilst continuing to adopt the current surgical and medical interventions.
Whether outcomes research actually affects clinical practice remains open to discussion.57, 58 For example, randomized clinical trials have been shown to have little influence on patterns of care. Similarly, consensus development conferences were found to have little effect on medical practice.57 On the other hand, there are examples suggesting the application of outcomes research in routine clinical practice.58 For example, algorithms for evaluation of thyroid nodules or abnormal Pap smears, are common in clinical practice. Similarly, efforts to assist patients with pancreatic cancers in clarifying their treatment preferences, especially with the use of current palliative chemotherapy, are increasingly been observed.58 At SQUH, the outcome results have already lead to a study of additional factors which may be responsible for the variable outcomes.
Although increasingly more research is being carried out using outcomes, there are certain limitations to using outcome as the measure of quality.4 Most importantly, the differences in outcomes may reflect variations in the patient population, especially when survival outcomes from one group of patients are compared with another group, as important biological differences are known to occur, as may be the case here. Secondly, measurements of outcome require time delays, and by the time data are available, changes are likely to have occurred either in the treatment of the disease, or in the organisations treating the disease.4 In case of NHL, the significant change in the initial management, has been the addition of Rituximab to the combination chemotherapy, which has already been incorporated into the standard of care.59 Similarly, adjuvant chemo-radiotherapy became the standard care for completely resected gastric cancers in the recent past, and this modality has been uniformly applied to all patients where it was indicated.60 Finally, large sample size may be required to detect small differences in the outcomes, which may occur because of variations in care.
CONCLUSION
Our results suggest that the outcome of patients with early stages and fewer adverse prognostic factors is comparable to what has been reported in the international literature; however, the outcome is inferior for patients presenting with advanced stage disease and several adverse prognostic factors. Studies to investigate the reasons for this dichotomy, including the study of additional adverse prognostic factors, are underway. However, because more patients present with advanced stage, bulky disease and in a state where the patients are moribund, with little hope of treatment let alone cure, this means that the necessity for mass education, awareness raising not only for patients, but also for primary health care providers, and surveillance and early detection should be strongly emphasised.
Acknowledgments
We wish to thank all the physicians, nurses, technical and other staff involved in the care of patients involved in this report. We also acknowledge the grants from Sultan Qaboos University which supported some of the work presented in this report.
REFERENCES
- 1.Lee S, Earle C, Weeks J. Outcomes research in oncology: History, conceptual framework, and trends in the literature. J Natl Cancer Inst. 2000;92:195–204. doi: 10.1093/jnci/92.3.195. [DOI] [PubMed] [Google Scholar]
- 2.Clancy CM, Eisenberg JM. Outcomes research: measure the end results of health care. Science. 1998;282:245–246. doi: 10.1126/science.282.5387.245. [DOI] [PubMed] [Google Scholar]
- 3.Agency for healthcare research and quality Outcomes research fact sheet: what is outcomes research? Available at: http://www.ahrq.gov/clinic/outfact/htm. Accessed Aug 2007.
- 4.Malin JL. ASCO Educational Book 2004. Alexandria, Virginia: American Society of Clinical Oncology; 2004. Moving from guidelines to quality measurement. [Google Scholar]
- 5.Kritchevsky SB, Simmons BP. Continuous quality improvement: concepts and applications for physician care. JAMA. 1991;266:1817–1823. doi: 10.1001/jama.266.13.1817. [DOI] [PubMed] [Google Scholar]
- 6.Brook RH, McGlynn EA, Cleary PD. Quality of Care, Part 2 - Measuring quality of care. N Engl J Med. 1996;335:966–970. doi: 10.1056/NEJM199609263351311. [DOI] [PubMed] [Google Scholar]
- 7.Benbassat J, Taragin M. What is adequate health care and how can quality of care be improved? Int J Health Care Qual Assur. 1998;11:58–64. doi: 10.1108/09526869810206080. [DOI] [PubMed] [Google Scholar]
- 8.Donabedian A. The quality of care: how can it be managed? JAMA. 1988;260:1743–1748. doi: 10.1001/jama.260.12.1743. [DOI] [PubMed] [Google Scholar]
- 9.Clifford PI, Katsavdakis KA, Lyle JL, Fultz J, Allen JG, Graham P. How are You? Further development of a generic quality of life outcome measure. J Mental Health. 2002;11:389–404. [Google Scholar]
- 10.Fossa S, Hjortdahl P. Does the service at a large, oncologic out-patient clinic satisfy the patients’ perceived need? Int J Health Care Qual Assur. 1996;9:24–29. doi: 10.1108/09526869610124209. [DOI] [PubMed] [Google Scholar]
- 11.Ministry of Health Oman . Cancer Incidence in Oman 2004. Muscat: Ministry of Health; 2004. [Google Scholar]
- 12.Al-Moundhri M, Al-Bahrani B, Pervez I, Ganguly SS, Nirmala V, Al-Madhani A, et al. The outcome of treatment of breast cancer in a developing country-Oman. Breast. 2004;13:139–145. doi: 10.1016/j.breast.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Al-Moundhri MS, Al-Bahrani B, Burney IA, Nirmala V, Al-Madhani A, Al Mawaly K, et al. The prognostic determinants of gastric cancer treatment outcome in Omani Arab patients. Oncology. 2006;70:90–96. doi: 10.1159/000092584. [DOI] [PubMed] [Google Scholar]
- 12.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC Cancer Staging Handbook. 6th ed. New York: Springer; 2002. [Google Scholar]
- 13.Kaplan EL, Meier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Burney IA, Ahmed S, Rehman J, Al Kindi S, Nirmala V, Al Moundhri MS. Presentation of non-Hodgkin’s lymphoma in Oman: a prospective study from a single institute. Ann Oncol. 2005;S5:342. [Google Scholar]
- 15.Burney IA, Ganguly SS, Al Kindy S, Al Moundhri MS. Outcomes of Hodgkin’s lymphoma in Oman. Ann Oncol. 2006;S9:212. [Google Scholar]
- 16.Youngs MT, Wingerson L. The 1996 medical outcomes and guidelines source book. New York: Faulkner and Gray Inc; 1995. [Google Scholar]
- 17.National Library of Medicine Medical subject headings. Available from: http://www.nlm.nih.gov/mesh. Accessed Aug 2007.
- 18.Association for Health Services Research Definition of health services research. Available from: http://www.ashr.org/hsproj/define.htm. Accessed Aug 2007.
- 19.American Society of Clinical Oncology, Committee Health series research (HSR) Available from: http://asco.infostreet.com/prof. Accessed Aug 2007.
- 20.Lipscomb J, Donaldson MS. Outcomes research at the National Cancer Institute: measuring, understanding, and improving the outcomes of cancer care. Clin Ther. 2003;25:699–712. doi: 10.1016/s0149-2918(03)80106-6. [DOI] [PubMed] [Google Scholar]
- 21.Ayanian JZ, Chrischilles EA, Fletcher RH, Fouad MN, Harrington DP, Kahn KH, et al. Understanding cancer treatment and outcomes: The Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22:2992–2996. doi: 10.1200/JCO.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 22.National Cancer Institute Applied research: Outcomes research. Available from: http://www-ccps.ims.nci.gov/ARB. Accessed Aug 2007.
- 23.Naresh KN, Advani S, Adde Aziz Z M, Banavali S, Bhatia K, et al. Report of an International Network of Cancer Treatment and Research workshop on non-Hodgkin’s lymphoma in developing countries. Blood Cells Mol Dis. 2004;33:330–337. doi: 10.1016/j.bcmd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Baker H, Al-Jarallah M, Manguno H, Temmim L, Madda JP, Sinowatz F. Clinical characteristics and pathological classification of non-Hodgkin’s lymphoma in Kuwait. Results of a collaborative study with the International Lymphoma Study Group (ILSG) Leuk Lymphoma. 2004;45:1865–1871. doi: 10.1080/10428190410001697386. [DOI] [PubMed] [Google Scholar]
- 25.Shome DK, George SM, Al-Hilli F, Satir AA. Spectrum of malignant lymphomas in Bahrain. Leitmotif of a regional pattern. Saudi Med J. 2004;25:164–167. [PubMed] [Google Scholar]
- 26.Aziz Z, Sana S, Saeed S, Akram M. Applicability of international prognostic index in non Hodgkin’s lymphoma in Pakistan. J Ayub Med Coll Abbottabad. 2004;16:15–20. [PubMed] [Google Scholar]
- 27.Lashari I, Memon S, Siddique U, Gareeb M, Islam M, Burney IA. Outcomes of non-Hodgkin’s lymphoma in Pakistan: Use of international prognostic index. J Pak Med Assoc. 2002;52:23–24. [Google Scholar]
- 28.The International Non-Hodgkin’s Lymphoma Prognostic Factors Project A predictive model for aggressive non-Hodgkin’s lymphoma. N Eng J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 29.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16:2780–2795. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 30.Sehn LH. Optimal use of prognostic factors in non-Hodgkin’s lymphoma. American Society of Hematology Education Program Book. 2006:295–302. doi: 10.1182/asheducation-2006.1.295. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo K, Kusano A, Suguma A, Nakamura S, Tajima K, Mueller NE. Effect of hepatitis C virus infection on the risk of non-Hodgkin’s lymphoma: a meta-analysis of the epidemiological studies. Cancer Sci. 2004;95:745–752. doi: 10.1111/j.1349-7006.2004.tb03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Masri NM. Hodgkin’s lymphoma in North Jordan. Does it have a different pattern? Saudi Med J. 2004;25:1917–1921. [PubMed] [Google Scholar]
- 33.Iraj AK. Hodgkin’s disease: assessment of treatment and survival rates in Iran. Asia Pacific J Cancer Prev. 2004;5:397–382. [PubMed] [Google Scholar]
- 34.Burney IA, Rashid S, Salam A, Siddiqui T. Does prognostic score apply to Pakistani patients with Hodgkin’s disease? Ann Oncol. 2002;13:123. [Google Scholar]
- 35.Al Diab AI, Siddiqui N, Sogiawalla FF, Fawzy EM. The changing trends of adult Hodgkin’s disease in Saudi Arabia. Saudi Med J. 2003;24:617–622. [PubMed] [Google Scholar]
- 36.Glaser SL, Hsu JL. Hodgkin’s disease in Asians: Incidence, patterns and risk factors in population based data. Leukemia Res. 2002;26:261–269. doi: 10.1016/s0145-2126(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 37.Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. N Eng J Med. 1998;339:1506–1514. doi: 10.1056/NEJM199811193392104. [DOI] [PubMed] [Google Scholar]
- 38.Casasnovas R-O, Mounier N, Brice P, Divine M, Morschhauser F, Gabarre J, et al. Plasma Cytokine and Soluble Receptor Signature Predicts Outcome of Patients with classical Hodgkin’s lymphoma. A Study From the Groupe d’Etude des Lymphomes de l’Adulte. J Clin Oncol. 2007;25:1732–1740. doi: 10.1200/JCO.2006.08.1331. [DOI] [PubMed] [Google Scholar]
- 39.Gajalakshmi CK, Shanta V, Swaminathan R, Sankaranarayanan R, Black RJ. A population-based survival study on female breast cancer in Madras, India. Br J Cancer. 1997;75:771–775. doi: 10.1038/bjc.1997.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malik IA. Clinico-pathological features of breast cancer in Pakistan. J Pak Med Assoc. 2002;52:100–104. [PubMed] [Google Scholar]
- 41.Ibrahim EM, Al-Mulhim FA, Al-Amri A, Al-Muhanna FA, Ezzat AA, Stuart RK, et al. Breast cancer in the eastern province of Saudi Arabia. Med Oncol. 1998;15:241–247. doi: 10.1007/BF02787207. [DOI] [PubMed] [Google Scholar]
- 42.Fakhro AE, Fateha BE, Al-Asheeri N, Al-Ekri SA. Breast cancer. Patient characteristics and survival analysis at Salmaniya Medical Complex, Bahrain. East Mediterr Health J. 1999;5:430–439. [PubMed] [Google Scholar]
- 43.Ezzat AA, Ibrahim EM, Raja MA, Al-Sobhi S, Rostom A, Stuart RK. Locally advanced breast cancer in Saudi Arabia: high frequency of Stage III in a young population. Med Oncol. 1999;16:95–103. doi: 10.1007/BF02785842. [DOI] [PubMed] [Google Scholar]
- 44.Ikpatt OF, Kuopio T, Collan Y. Proliferation in African breast cancer. Biology and prognostication in Nigerian breast cancer material. Mod Pathol. 2002;15:783–789. doi: 10.1097/01.MP.0000021764.03552.BD. [DOI] [PubMed] [Google Scholar]
- 45.O’Malley CD, Le GM, Glaser SL, Shema SJ, West DW. Socioeconomic status and breast carcinoma survival in four racial/ethnic groups: a population-based study. Cancer. 2003;97:1303–1311. doi: 10.1002/cncr.11160. [DOI] [PubMed] [Google Scholar]
- 46.Chu KC, Lamar CA, Freeman HP. Racial disparities in breast carcinoma survival rates. Separating factors that affect diagnosis from factors that affect treatment. Cancer. 2003;97:2853–2860. doi: 10.1002/cncr.11411. [DOI] [PubMed] [Google Scholar]
- 47.Sandelin K, Apffelstaedt JP, Abdullah H, Murray EM, Ajuluchuku EU. Breast Surgery International - breast cancer in developing countries. Scand J Surg. 2002;91:222–226. doi: 10.1177/145749690209100302. [DOI] [PubMed] [Google Scholar]
- 48.Anderson BO, Braun S, Carlson RW, Gralow JR, Lagios MD, Lehman C, et al. Overview of Breast Health Care Guidelines for Countries with Limited Resources. Breast J. 2003;9:S42–S50. doi: 10.1046/j.1524-4741.9.s2.3.x. [DOI] [PubMed] [Google Scholar]
- 49.Oluwasola AO, Ogunbiyi JO. Gastric cancer: Aetiological, clinicopathological and management patterns in Nigeria. Niger J Med. 2003;12:177–186. [PubMed] [Google Scholar]
- 50.Hassan HA, Sharma VK, Raufman JP. Changing trends in gastric carcinoma at a university medical center: a twelve-year retrospective analysis. J Clin Gastroenterol. 2001;32:37–40. doi: 10.1097/00004836-200101000-00009. [DOI] [PubMed] [Google Scholar]
- 51.Bani-Hani KE, Yaghan RJ, Heis HA, Shatnawi NJ, Matalka II, Bani-Hani AM, et al. Gastric malignancies in Northern Jordan with special emphasis on descriptive epidemiology. World J Gastroenterol. 2004;10:2174–2178. doi: 10.3748/wjg.v10.i15.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kandasami P, Tan WJ, Norain K. Gastric cancer in Malaysia: the need for early diagnosis. Med J Malaysia. 2003;58:758–762. [PubMed] [Google Scholar]
- 53.Koong HN, Chan HS, Nambiar R, et al. Gastric cancers in Singapore: poor prognosis arising from late presentation. Aust NZ J Surg. 1996;66:813–815. doi: 10.1111/j.1445-2197.1996.tb00755.x. [DOI] [PubMed] [Google Scholar]
- 54.Stemmermann GN, Fenoglio-Preiser C. Gastric carcinoma distal to the cardia: a review of the epidemiological pathology of the precursors to a preventable cancer. Pathology. 2002;34:494–503. doi: 10.1016/s0031-3025(17)30697-9. [DOI] [PubMed] [Google Scholar]
- 55.Kosecoff J, Kanouse DE, Rogers WH, McCloskey L, Winslow CM, Brook RH. Effects of National Institute of Health consensus development program on physicians practice. JAMA. 1998;279:1638–1642. [PubMed] [Google Scholar]
- 56.Weeks JC. Outcomes assessment in the NCCN. Oncology. 1997;11:137–140. [PubMed] [Google Scholar]
- 57.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Eng J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 58.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Eng J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 59.MacDonald JS, Smalley SR, Benedetti, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotheraphy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Eng J Med. 2001;35:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 60.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotheraphy plus ritucimab compared with CHOP alone in elderly patients with diffuse large -B-cell lymphoma. N Eng J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]