Abstract
Castor beans, sometimes used in traditional therapies, contain ricin one of the most toxic substances known. It may cause an acute and potentially fatal gastroenteritis in addition to neurological and ophthalmological lesions. Poisoning may also lead to delayed visceral damages; however, the latter is quite rare. The toxicity is dose related and depends on the amount of castor beans ingested. There is no specific treatment and symptomatic management to reduce the load of the toxin needs to be initiated quickly and early when a case of poisoning is suspected so that serious complications will be avoided. Increasing the awareness of the population to the dangers of ricin would be a way to avoid the utilisation of castor seeds in traditional therapies. Here we are reporting a case of mild poisoning after ingestion of a single castor bean. The patient, who presented at Nizwa Hospital, Oman, fortunately recovered completely as the ingested dose was quite small.
Keywords: Castor beans, Poisoning, Ricin, Case report, Oman
The castor oil plant (Ricinus communis L), is a plant species of the family Euphorbiaceae and the sole member of the genus Ricinus and of the subtribe Ricininae.1 The castor plant is native to the Ethiopian region of east Africa. It grows in tropical and warm temperate regions throughout the world and is becoming an abundant weed in the southwestern United States. Ricinus communis is a perennial, erect, branched, herb, typically less than 2 meters in height.2 The beans are oblong and light brown, mottled with dark brown spots. The seed is only toxic if the outer shell is broken or chewed. Ricin is contained in the bean pulp following the separation of the oil from the beans. No ricin is thought to remain in the oil, and it is inactivated during extraction if done under heated conditions.
Ingested castor beans are generally toxic only if the ricin is released through mastication. Reports on the ricin content of castor beans vary, but it is probably in the range of 1% to 5%.3 Purified ricin is a white powder that is soluble in water and stable over a wide pH range.4
It is a protein toxin (toxalbumin).5 It is a glycoprotein lectin composed of 2 chains, A and B, linked by a disulfide bond.6 The B chain is a lectin and binds to galactose-containing glycoproteins and glycolipids expressed on the surface of cells, facilitating the entry of ricin into the cytosol.7 The A chain inhibits protein synthesis by irreversibly inactivating eukaryotic ribosomes through removal of a single adenine residue from the 28S ribosomal RNA loop contained within the 60S subunit. This process prevents chain elongation of polypeptides and leads to cell death.8
Toxicity results from the inhibition of protein synthesis, but other mechanisms are noted including apoptosis pathways, direct cell membrane damage, alteration of membrane structure and function, and release of cytokine inflammatory mediators.9
The castor bean plant also contains another glycoprotein lectin, the Ricin communis agglutinin, which, unlike ricin, is not directly cytotoxic, but does have affinity for the red blood cell, leading to agglutination and subsequent haemolysis. Ricin communis agglutinin is not significantly absorbed from the gut and causes clinically significant haemolysis only after intravenous administration.10
There are no literature reports of poisoning from ingesting purified ricin. All clinical reports with regard to poisoning refer to castor bean ingestion. Documented mild to lethal clinical symptoms may result from ingesting one half to 30 beans.11 Two castor oil beans were the minimum number found to be associated with death.12 Symptom onset after ingestion is usually within 4 to 6 hours but may be as late as 10 hours.13 Initial symptoms are nonspecific and may include colicky abdominal pain, vomiting, diarrhoea, heartburn, and oropharyngeal pain. Haematemesis and melena are reported less commonly.14 Fluid losses may lead to electrolyte imbalances, dehydration, hypotension, and circulatory collapse.15 Laboratory abnormalities may include leukocytosis, elevated transaminases and creatinine kinase, hyperbilirubinemia, renal insufficiency, and anaemia.16 Information on allergic reactions to ricin is primarily from persons working in or living near castor bean processing plants.17 Allergy patch testing reveals an IgE–mediated inflammatory reaction to ricin although other allergens may be present in the castor bean dust.18
Castor beans have been used traditionally by women in many countries for birth control.19 The use of castor seed oil in India has been documented since 2000 BC for use in lamps and in local medicine as a laxative, purgative, and cathartic in Unani, Ayurvedic and other ethnomedical systems. Castor seed and urine have also been used in China for centuries, mainly prescribed in local medicine for internal use or use in dressings.1
Castor beans have been found in ancient Egyptian tombs dating back to 4000 B.C. According to the Ebers Papyrus, an Egyptian medical text from 1500 BC, Egyptian doctors used castor oil to protect the eyes from irritation. The oil from the bean was used thousands of years ago in facial oils and in wick lamps for lighting.20 In Oman, this is the first patient we have receive in the last 10 years who used castor beans as a traditional treatment for a cough.
CASE REPORT
A 51 year-old Omani man, neither diabetic nor hypertensive and not on any regular medication, was brought to the Accident & Emergency Department of Nizwa Hospital, Oman, by his son. He was in a confused state after ingesting three hours previously one green fruit of castor bean, as stated by the son. He had vomited once at home. There were no other complaints.
On examination the patient was confused, disoriented as to time and place, and was afebrile. His pulse was 108/min regular, blood pressure was 110/68 mmHg and the respiratory rate was 22 per minute.
Examinations of the chest, cardiovascular system and abdomen were unremarkable. He had dryness of mouth and the pupils were bilaterally dilated with sluggish reaction to light. The patient was admitted to the medical ward and treated symptomatically and with activated charcoal.
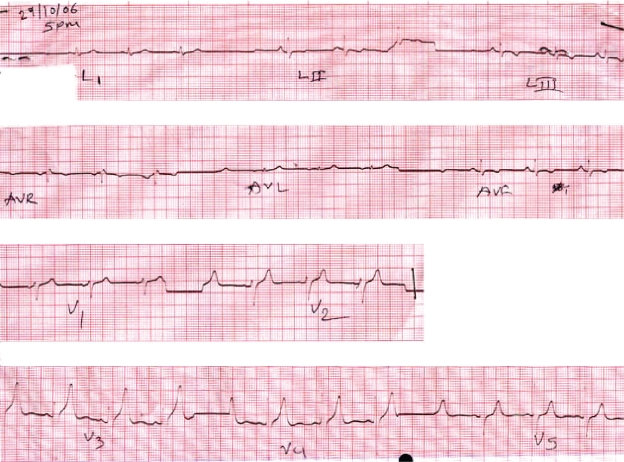
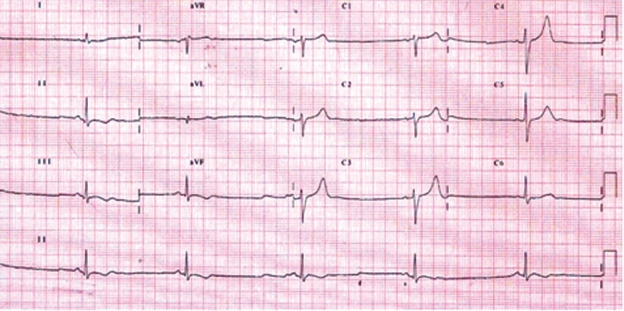
His initial laboratory results showed normal results for renal function tests, electrolytes, liver function tests, coagulation profile, cardiac enzymes, full haemogram and routine urine tests. An initial electrocardiogram showed normal sinus rhythm with T wave inversion in leads II, III and AVF [Fig. 1]. A repeat ECG after 6 hours showed sinus bradycardia with T inversion in inferior leads as well [Fig. 2]. Computed tomography of the brain was normal.
Figure 1:
ECG showing normal sinus rhythm & T wave inversion in LII, LIII, aVF
Figure 2:
ECG of Patient showing sinus bradycardia & T wave inversion LII, LIII, aVF
He stayed in hospital for two days which were uneventful. His pulse rate returned gradually to normal and his conscious level improved and he was discharged in a good condition with the advice to evaluate his cardiac status with an echocardiogram and treadmill test at a later date.
DISCUSSION
In various countries castor beans are the base of many traditional remedies. Our patient believed that they could treat his cough. Ingested castor beans are generally toxic only if ricin is released through mastication or maceration.11 The toxicity results from the inhibition of protein synthesis, but other mechanisms are noted as well.9
The clinical effects of castor bean ingestion can be classified as acute and late effect. In the acute phase, the patient usually develops a gastrointestinal manifestation, while the late phase reflects the cytotoxic effect on liver, kidney, and the adrenal gland, which typically starts 2 to 5 days after exposure. The ricin toxicity affects many of the body systems, and fever may be the major presenting clinical picture.21 The fever may start 30 minutes to two hours after ingesting 1 to 4 beans.22
The ricin may cause miosis14 or mydriasis23; both have been reported. In our patient, the abnormality was a mydriasis. It is also reported that the ricin can produce central nervous system depression, somnolence and loss of consciousness.24 Gastrointestinal haemorrhage due to sloughing of tissue may also be seen in intoxicated persons,21 and vomiting and abdominal pain are frequently reported symptoms.14, 25
Elevated liver enzymes and a transient rise in blood sugar were reported earlier too,26 as well as haematuria, fluid and electrolyte disturbance in association with severe vomiting and diarrhoea.14 A weak pulse is commonly seen, and cardiovascular shock is the commonest cause of death with no direct cardiotoxic effects, but usually secondary to fluid and electrolyte loss.11
Our patient developed somnolence, mydriasis and vomiting, all of them expected symptoms; fortunately, they were mild followed by full recovery. The T inversion in his ECG is most probably not related to the ricin toxicity, but the bradycardia that occurred, may be due to the beta blocking effect of ricin that was reported only once in 1974.23
Gastrointestinal decontamination for potentially toxic ingestions includes the use of activated charcoal. Treatment in general is supportive and symptomatic.27
CONCLUSION
Castor beans contain ricin, one of the most toxic substances known. They may cause an acute and potentially fatal gastroenteritis. Delayed visceral damage is another serious complication; however, the latter is quite rare. The toxicity is dose related and depends on the amount of castor beans ingested. There is no specific treatment and supportive management needs to be started early to reduce the load of the toxin so as to avoid serious complications. Although this is the first case in the last 10 years admitted to Nizwa Hospital with signs and symptoms of castor bean toxicity, there is no database about its prevalence. However, increasing the awareness of the population would be a way to avoid the utilisation of castor seeds in traditional therapies.
REFERENCES
- 1.Castor oil plant From http://en.wikipedia.org/wiki/Castor_bean. Accessed Jan 2008.
- 2.Morton JF. Major Medicinal Plants. In: Morton JF, editor. Springfield, Illinois: CC Thomas; 1977. Botany. [Google Scholar]
- 3.Bradberry SM, Dickers KJ, Rice P, Griffiths GD, Vale JA. Ricin poisoning. Toxicol Rev. 2003;22:65–70. doi: 10.2165/00139709-200322010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Cope AC, Dee J, Cannan RK, Renshaw B, Moore S, et al. Chemical Warfare Agents and Related Chemical Problems - Part I: Summary Technical Report of Division 9. Washington, DC: National Defense Research Committee; 1945. pp. 179–203. [Google Scholar]
- 5.Parker DT, Parker AC, Ramachandran CK. Joint Technical Data Source Book. Vol 6. Part 3. US Dugway Proving Ground. Utah: Joint Contact Point Directorate; 1996. pp. 1–38. DGP No. DPGJCP-961007, [Google Scholar]
- 6.Ishiguro M, Tomi M, Funatsu G, Funatsu M. Isolation and chemical properties of a ricin variant from castor bean. Toxicon. 1976;14:157–165. doi: 10.1016/0041-0101(76)90001-5. [DOI] [PubMed] [Google Scholar]
- 7.Sandvig K, van Deurs B. Entry of ricin and Shiga toxin into cells: molecular mechanisms and medical perspectives. EMBO J. 2000;19:5943–5950. doi: 10.1093/emboj/19.22.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsnes S. The history of ricin, abrin and related toxins. Toxicon. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Day PJ, Pinheiro TJ, Roberts LM, Lord JM. Binding of ricin A-chain to negatively charged phospholipids vesicles leads to protein structural changes and destabilizes the lipid bilayer. Biochemistry. 2002;41:2836–2843. doi: 10.1021/bi012012i. [DOI] [PubMed] [Google Scholar]
- 10.Hegde R, Podder SK. Studies on the variants of the protein toxins ricin and abrin. Eur J Biochem. 1992;204:155–164. doi: 10.1111/j.1432-1033.1992.tb16618.x. [DOI] [PubMed] [Google Scholar]
- 11.Challoner KR, McCarron MM. Castor bean intoxication: review of reported cases. Ann Emerg Med. 1990;19:1177–1183. doi: 10.1016/s0196-0644(05)81525-2. [DOI] [PubMed] [Google Scholar]
- 12.Rauber A, Heard J. Castor bean toxicity reexamined: a new perspective. Vet Hum Toxicol. 1985;27:498–502. [PubMed] [Google Scholar]
- 13.Klaim GJ, Jaeger JJ. Castor Seed Poisoning in Humans: A Review: Technical Report #453. San Francisco, CA: Letterman Army Institute of Research; Jan, 1990. [Google Scholar]
- 14.Malizia E, Sarcinelli L, Andreucci G. Ricinus poisoning: a familiar epidemy. Acta Pharmacol Toxicol (Copenh) 1977;41:351–361. [PubMed] [Google Scholar]
- 15.Koch LA, Caplan J. Castor bean poisoning. Am J Dis Child. 1942;64:485–486. [Google Scholar]
- 16.Wedin GP, Neal JS, Everson GW, Krenzelok EP. Castor bean poisoning. Am J Emerg Med. 1986;4:259–261. doi: 10.1016/0735-6757(86)90080-x. [DOI] [PubMed] [Google Scholar]
- 17.Layton LL, Yamanaka E, Green TW. Multiple allergies to the pollen and seed antigens of Ricinus communis (castor bean) J Allergy. 1962;33:232–235. doi: 10.1016/0021-8707(62)90089-8. [DOI] [PubMed] [Google Scholar]
- 18.Metz G, Bocher D, Metz J. IgE-mediated allergy to castor bean dust in a landscape gardener. Contact Dermatitis. 2001;44:367. doi: 10.1034/j.1600-0536.2001.440609-2.x. [DOI] [PubMed] [Google Scholar]
- 19.Salhab AS, Issa AA, Alhougog I. Pharmaceut Biol. 1997;35:63–65. [Google Scholar]
- 20.Longe JL, editor. Gale Encyclopedia of alternative medicine. 2nd ed. 2005. Castor oil; pp. 369–372. Project ed. [Google Scholar]
- 21.Pillay VV, Bhagyanathan PV, Krishnaprasad R, Rajesh RR, Vishnupriya N. Poisoning due to white seed variety of Abrus precatorius. J Assoc Physicians India. 2005;53:317–319. [PubMed] [Google Scholar]
- 22.Kulkarni ML, Sreekar H, Keshavmurthy KS, Shenoy N. Jatropha curcas - poisoning. Indian J Pediatr. 2005;72:75–76. [PubMed] [Google Scholar]
- 23.Balint GA. Ricin: the toxic protein of castor oil seeds. Toxicology. 1974;2:77–102. doi: 10.1016/0300-483x(74)90044-4. [DOI] [PubMed] [Google Scholar]
- 24.Lampe KF, Fagerstorm R. Plant toxicity and dermatitis. Baltimore, MD: Lippincott Williams & Wilkins; 1968. [Google Scholar]
- 25.Tenenbein M, Cohen S, Sitar DS. Efficacy of ipecac-induced emesis, orogastric lavage, and activated charcoal for acute drug overdose. Ann Emerg Med. 1987;16:838–841. doi: 10.1016/s0196-0644(87)80518-8. [DOI] [PubMed] [Google Scholar]
- 26.Kaszas T. Papp G: Ricinussamen-Vergiftung von Schulkindern (Poisoning of castor oil seeds in schoolchildren) Arch Toxikol. 1960;18:145–150. [PubMed] [Google Scholar]
- 27.Behrman RE, Kliegman RM, Jenson HB. Nelson Textbook of Pediatrics. 17th ed. Philadelphia: WB Saunders; 2003. p. 2375. [Google Scholar]