Abstract
Facets of the post-natal environment including the type and complexity of environmental stimuli, the quality of parenting behaviors, and the amount and type of stress experienced by a child affects brain and behavioral functioning. Poverty is a type of pervasive experience that is likely to influence biobehavioral processes because children developing in such environments often encounter high levels of stress and reduced environmental stimulation. This study explores the association between socioeconomic status and the hippocampus, a brain region involved in learning and memory that is known to be affected by stress. We employ a voxel-based morphometry analytic framework with region of interest drawing for structural brain images acquired from participants across the socioeconomic spectrum (n = 317). Children from lower income backgrounds had lower hippocampal gray matter density, a measure of volume. This finding is discussed in terms of disparities in education and health that are observed across the socioeconomic spectrum.
Introduction
A growing body of research, conducted mainly in rodents, has found that factors such as the complexity of stimuli present in the post-natal environment, the quality of parenting behaviors, and the amount of stress that occurs during the lifespan can affect neural, emotional and cognitive functioning (for review, see [1], [2]). These findings raise complex questions about how variations in the environment can shape neural development in humans [3]. In particular, an increasing interest is being paid to the effects of socioeconomic status and poverty on brain and behavior, since living in poverty is often characterized by heightened amounts of stress and reductions in environmental stimulation [4].
This study focuses on associations between household income and the hippocampus. The hippocampus is located in the medial temporal lobe of the brain. This region is known to be affected by stress and is tied to cognitive functions such as learning, memory, and behavioral regulation (for review, see [5]). It is difficult to quantify the many facets of an individual's environment; for this reason, we use income as a proxy for a multitude of factors including enriched cultural environment, better schools and neighborhoods, and access to stimulating materials in early childhood.
Non-human animal research has found environmental enrichment is related to greater dendritic branching and wider dendritic fields [6], [7], increased astrocyte number and size [8], and improved synaptic transmission [9] in portions of the hippocampus. Environmental enrichment, in addition, appears to bolster neurobiological resiliency. For example, enriched environments result in increases in neuronal precursor cells in portions of the hippocampus [10] and greater recovery after a lesion in the hippocampus [11]. Stress also exerts long-lasting negative effects on the hippocampus. For example, research has found prolonged maternal separation and brief handling impacts the hippocampus and affects stress regulation and memory ability later in life [12]. Similar effects have been noted in humans. These studies suggest that parental nurturance and environmental stimulation, including both resources such as the number of books in a child's home and parental time spend reading to a child, predict neurocognitive performance on tests related to the hippocampus such as long-term memory [13], [14].
Prior research has linked poverty with a myriad of deleterious outcomes from poor health to lower educational achievement [15], [16], [17], [18]. Yet little is currently understood about the neurobiological mechanisms leading to these socioeconomic disparities. We hypothesized that the morphometric properties of hippocampus would be related to gradients in income. We focus on this brain region both because of its known sensitivity to environmental stress and its role in core adaptive processes such as learning.
Methods
Subjects and MRI acquisition
Behavioral and MRI data were taken from the National Institutes of Health (NIH) MRI study of normal brain development (website: http://nihpd.crbs.ucsd.edu/nihpd/info/index.html and [19]). This public-access database was developed by the NIH to aid in understanding the course of normal brain-behavior development. The database consists of clinical, behavioral and neuroimaging metrics that were acquired at multiple research centers across the US from a large cohort of children and adolescents ages 4 to 18. To participate in the study, subjects had to meet criteria based on demographic, prenatal history, physical, behavioral/psychiatric, family history, and neurological exam cutoffs (Exclusion criteria are listed in Table 1; adapted from [20]). Families whose child met all inclusion and no exclusion criteria were invited to participate in neurological evaluation, neuropsychological testing, and structural MRI imaging, typically performed in one day. Written informed consent from the parents/guardians of all children was obtained in compliance with research standards for human research at Boston Children's Hospital, Cincinnati Children's Hospital, Philadelphia Children's Hospital, Washington University in St. Louis, the University of Texas Health Science Center in Houston, and the University of California in Los Angeles. Children ages 6 to 17, in addition, gave their written assent. These procedures were in accordance with the Helsinki Declaration. The Institutional Review Board at the University of Wisconsin-Madison also approved the analysis of this human subjects data.
Table 1. Exclusionary criteria (originally appeared in [20] © Cambridge Journals, reproduced with permission.).
| Category | Specific criteria |
| Demographic | Children of parents with limited English proficiency. Adopted children excluded due to inadequate family histories. |
| Pregnancy, birth and perinatal history | Intra-uterine exposures to substances known or highly suspected to alter brain structure or function (certain medications, any illicit drug use, smoking >.5 pack per day or >2 alcoholic drinks per week during pregnancy); Hyperbilirubinemia requiring transfusion and0or phototherapy (>2 days); gestational age at birth of <37 weeks or >42 weeks; multiple birth; delivery by high forceps or vacuum extraction; infant resuscitation by chest compression or intubation; maternal metabolic conditions (e.g., phenylketonuria, diabetes); pre-eclampsia; serious obstetric complication; general anesthesia during pregnancy/delivery; C-section for maternal or infant distress |
| Physical/medical or growth | Current height or weight <3rd percentile or head circumference <3rd percentile by National Center for Health Statistics 2000 data (charts at http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/charts.htm); history of significant medical or neurological disorder with CNS implications (e.g., seizure disorder, CNS infection, malignancy, diabetes, systemic rheumatologic illness, muscular dystrophy, migraine or cluster headaches, sickle cell anemia, etc.); history of closed head injury with loss of consciousness >30 min or with known diagnostic imaging study abnormalities; systemic malignancy requiring chemotherapy or CNS radiotherapy; hearing impairment requiring intervention; significant visual impairment requiring more than conventional glasses (strabismus, visual handicap); metal implants (braces, pins) if likely to pose safety or artifact issues for MRI; positive pregnancy test in subject. |
| Behavioral/psychiatric | Current or past treatment for language disorder (simple articulation disorders not exclusionary); lifetime history of Axis I psychiatric disorder (except for simple phobia, social phobia, adjustment disorder, oppositional defiant disorder, enuresis, encopresis, nicotine dependency); any CBCL subscale score ≥70; WASI IQ<70; Woodcock-Johnson Achievement Battery subtest score <70; current or past treatment for an Axis I psychiatric disorder. |
| Family history | History of inherited neurological disorder; history of mental retardation caused by non-traumatic events in any first-degree relative; one or more first degree relatives with lifetime history of Axis I psychiatric disorders; schizophrenia, bipolar affective disorder, psychotic disorder, alcohol or other drug dependence, obsessive compulsive disorder, Tourette's disorder, major depression, attention deficit hyperactivity disorder or pervasive developmental disorder. |
| Neuro examination | Abnormality on neurological examination (e.g., hypertonia, hypotonia, reflex asymmetry, visual field cut, nystagmus, and tics). |
Our analyses focused on the first wave of data collected. MRI scans were acquired using either General Electric or Siemens 1.5 Tesla scanners. Overall, four hundred and thirty-one subjects were recruited for this project. Of this initial sample, one-hundred and fourteen subjects were excluded from our analyses (2 subject had errors in preprocessing, 10 subjects had unusable data due to motion artifacts, 41 subjects did not complete scanning, while 61 subjects were excluded due to lower resolution of their MRI scans which led to a decreased ability to localize the brain structures of interests). The demographic characteristics of the sample are displayed in Tables 2, 3, 4, 5, 6, 7, 8, along with Supplemental Tables S1 & S2. Parents of participants were asked about total household income in the last year, which includes earnings, unemployment compensation, pension or retirement income, interest, dividends, rents, social security, and all other miscellaneous sources. Incomes were then divided into 9 levels: $1–5000, $5001–10000, $10001–15000, $15001–25000, $25001–35000, $35001–50000, $50001–75000, $75001–100000,and $100001+ in the publicly available data. Parents of participants were also asked about their education and responded whether they had completed less than a 6th grade education, less than high school, graduated high school, completed some college, graduated college, obtain some graduate education, or completed graduate school.
Table 2. Demographic Summary for full sample (based on Wave 1 data).
| Age (Average age in months for Wave 1) | 126.13+/−46.59 months |
| Gender (Male) | 207 |
| Total n | 431 |
Table 3. Demographic Summary for full sample (based on Wave 1 data).
| Father Education | Maternal Education | |
| Less than High School | 10 | 4 |
| High School | 86 | 55 |
| Some College | 116 | 131 |
| College | 115 | 144 |
| Some Graduate Level | 19 | 22 |
| Graduate Level | 83 | 73 |
| No Information | 2 | 2 |
| TOTAL | 431 | 431 |
Table 4. Demographic Summary for full sample (based on Wave 1 data).
| Income at Wave 1 | |
| <$5000 | 1 |
| 5001–$10,000 | 2 |
| 10001–15000 | 4 |
| 15001–25000 | 10 |
| 25001–35000 | 21 |
| 35001–50,000 | 82 |
| 50001–75000 | 104 |
| 75001–100,000 | 102 |
| >100001 | 94 |
| No information | 11 |
| TOTAL | 431 |
Table 5. Demographic Variables for Subjects with and without MRI Scans and/or Income.
| Subjects with all variables (n = 317) | Subjects without all variables (n = 114) | ||
| Age (Average age in months for Wave 1) | 133.74+/−45.76 months | 133.74+/−45.76 months | F(1,429) = 44.675, p<.001 |
| Gender (Male) | 146 | 61 | χ2 = .305, p = .642 |
Table 6. Demographic Variables for Subjects with and without MRI Scans and/or Income.
| Father's Education | ||
| Subjects with all variables (n = 317) | Subjects without all variables (n = 114) | |
| Less than High School | 7 | 3 |
| High School | 61 | 25 |
| Some College | 83 | 33 |
| College | 85 | 30 |
| Some Graduate Level | 13 | 6 |
| Graduate Level | 68 | 17 |
| TOTAL | 317 | 114 |
Table 7. Demographic Variables for Subjects with and without MRI Scans and/or Income.
| Maternal Education | ||
| Subjects with all variables (n = 317) | Subjects without all variables (n = 114) | |
| Less than High School | 2 | 3 |
| High School | 45 | 25 |
| Some College | 88 | 33 |
| College | 107 | 30 |
| Some Graduate Level | 16 | 6 |
| Graduate Level | 59 | 17 |
| TOTAL | 317 | 114 |
Table 8. Demographic Variables for Subjects with and without MRI Scans and/or Income.
| Income at Wave 1 | ||
| Subjects with all variables (n = 317) | Subjects without all variables (n = 114) | |
| <$5000 | 1 | 0 |
| 5001–$10,000 | 2 | 0 |
| 10001–15000 | 4 | 0 |
| 15001–25000 | 7 | 3 |
| 25001–35000 | 13 | 8 |
| 35001–50,000 | 53 | 29 |
| 50001–75000 | 76 | 28 |
| 75001–100,000 | 88 | 25 |
| >100001 | 73 | 21 |
| TOTAL | 317 | 114 |
Imaging Analyses
To examine the relationship between income and hippocampal gray matter, we employed a voxel-based morphometry (VBM) analytic framework with region of interest drawing. VBM is a fully automatic imaging analysis strategy which allows for the precise localization of anatomical differences between groups, involves a comparison between two groups of subjects of the local concentration of gray matter (or volume comparison) using Jacobian modulation, and has been applied to the study of various types of pathologies [21], [22], [23], [24]. The steps involved with VBM have recently been improved with the Diffeomorphic Anatomical Registration using Exponentiated Lie algebra (DARTEL) registration method [25]. Previous structural imaging research focused on the hippocampus has often employed manual segmentation protocol. Such procedures require specific anatomical expertise, are operator time consuming and may result in high intra- and inter-rater variability (as noted by [26]). Advancement in registration methods, such as DARTEL, improves the realignment of small brain structures [27], making such an analytic strategy particularly robust for quantifying the hippocampus in such a large dataset.
In this analysis, we used Statistical Parametric Mapping 8 (Wellcome Department of Cognitive Neurology: London, England) with the following steps: first, T1-weighted images were checked for scanner artifacts (e.g., extreme field inhomogeneity). Next, these volumes were segmented using custom a priori brain tissue segmentations generated by the Template-O-Matic toolbox [28]. These custom segmentations were based on the age and gender distributions of the full sample. The first author then checked the accuracy of each subjects' segmentation. If any errors were present, the bounding box or image matrix was adjusted and MRI images were reprocessed. If after this correction segments still contained errors, they were corrected by hand to remove skull, dura, and other non-brain matter.
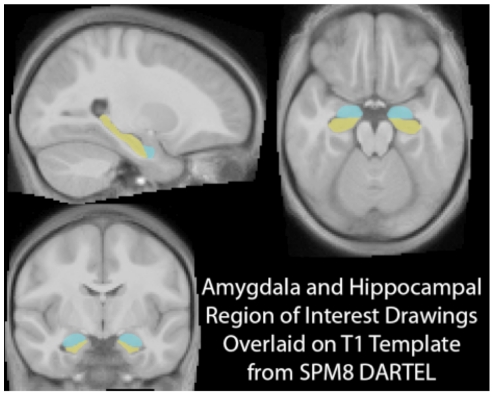
Once segmentation was completed, creation of and registration to study specific templates began. This process first involved rigidly aligning and averaging each tissue class (i.e., grey and white matter segments) for each subject. Using the initial template, an advanced non-linear registration algorithm (DARTEL) was employed to register each participant's segments to the template gray and white matter maps. The results of this registration process were then averaged to create a second template. Averaging and registering of gray and white matter segments was repeated six times. This processing pipeline allows for robust registration, while preserving the topology of the brain via constant velocity flow fields [25]. These processing procedures were recently validated as a robust approach to detecting hippocampal differences [26]. After warping the images to the final template, region of interest drawing was completed on the template through the Anatomical Automatic Labeling Toolbox [29]. The hippocampal and amygdala region of interest drawings used for our analyses are shown in Figure 1. Modulated Segments, adjusted for the non-linear registration were then generated to assess gray matter differences in relation to socioeconomic status (SES) variables.
Figure 1. Hippocampal and amygdala region of interest drawings.
The top left brain slice shows a sagittal brain slice with the hippocampus highlighted in yellow and the amygdala in turquoise, while the top right brain image shows an axial slice (with the hippocampus again highlighted in yellow and the amygdala in turquoise). The bottom left brain picture shows a coronal slice with the amygdala in turquoise and the hippocampus in yellow.
After processing neuroimaging data from each subject with the procedures detailed above, we conducted linear regressions in Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL) controlling for participant age in months, gender (dummy-coded), and whole-brain volumes entered as independent variables. The log-transformed, mid-point for each income category and the approximate number of years of education obtained by parents (<6th grade = 5 years, less than high school = 11 years, high school = 12 years, some college = 14 years, college = 16 years, some grad = 17 years and graduate level = 19 years) were also used as continuous independent variables in these analyses. Gray matter probability for the hippocampus or the amygdala (for total gray matter, as well as for the left and right side separately) was entered as the dependent variable in these regressions. The brain variables in these analyses are the unsmoothed average “modulated” gray matter density in a whole-hippocampal or amygdala region of interest drawing. Recent evaluations of registration algorithms have noted superior performance of DARTEL, with top ratings in overlap and distance measures [30]. Age, gender, whole-brain volume, and parental education were included to isolate the unique effects of income on the medial temporal lobe.
Results
Examining the association between income and the hippocampus
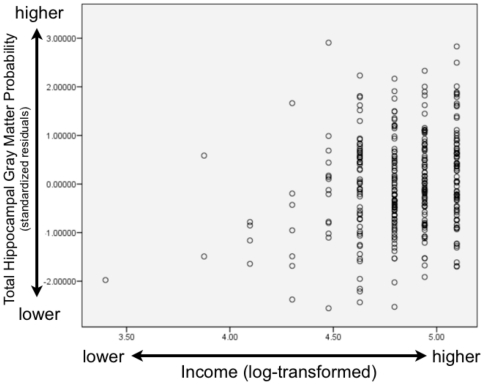
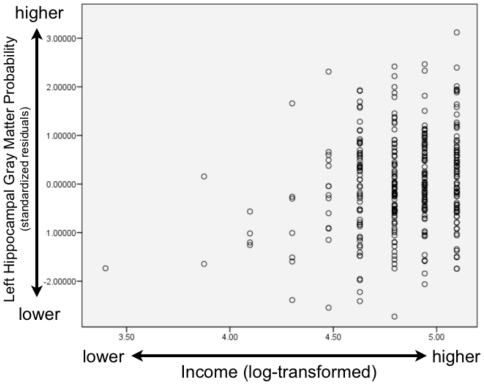
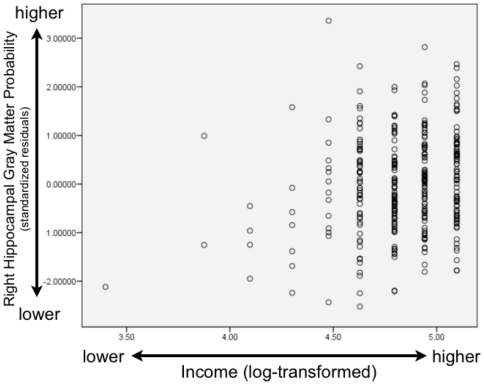
In terms of income and the neurobiological correlates of socioeconomic status, we examined hippocampal and amygdala gray matter across a large income spectrum: participants had annual family incomes of below $5000 to above $100,000 per year. Our lowest income group is composed of families below 150% of the Federal Poverty Line (for 2010 levels, see http://aspe.hhs.gov/poverty/10poverty.shtml). As predicted, there was a relationship between income and the hippocampus, for total hippocampal gray matter (β = .145, t = 2.459, p = .014) as well as left (β = .165, t = 2.773, p = .006) and right (β = .118, t = 1.999, p = .046) hippocampal gray matter separately. Scatterplots of these associations are shown in Figure 2 (total hippocampal gray matter and income), Figure 3 (left hippocampal gray matter and income), and Figure 4 (right hippocampal gray matter and income).These results demonstrate for the first time that the hippocampus is associated with household income, as children from lower SES backgrounds had less gray matter and participants from more affluent backgrounds had greater concentrations of gray matter. All of these models included child gender entered as a dummy coded variable, child age in months, whole brain volume, parental education, and income as continous independent variables, along with the brain area of interest as the dependent variable.
Figure 2. Scatterplot of Total Hippocampal Gray Matter and Income.
This scatterplot shows the association between total hippocampal gray matter probability and income. Total hippocampal gray matter shown on the vertical axis is displayed as a standardized residual controlling for child's age (in months), gender (dummy-coded), and whole brain volume, while log-transformed income is displayed on the horizontal axis. Higher income is associated with greater gray matter probability.
Figure 3. Scatterplot of Left Hippocampal Gray Matter and Income.
This scatterplot shows the association between left hippocampal gray matter probability and income. Left hippocampal gray matter shown on the vertical axis is displayed as a standardized residual controlling for child's age (in months), gender (dummy-coded), and whole brain volume, while log-transformed income is displayed on the horizontal axis. Higher income is associated with greater gray matter probability.
Figure 4. Scatterplot of Right Hippocampal Gray Matter and Income.
This scatterplot shows the association between right hippocampal gray matter probability and income. Right hippocampal gray matter shown on the vertical axis is displayed as a standardized residual controlling for child's age (in months), gender (dummy-coded), and whole brain volume, while log-transformed income is displayed on the horizontal axis. Higher income is associated with greater gray matter probability.
To ensure specificity of these effects, we tested gray matter of the amygdala, a region adjoining the hippocampus. No such association emerged for income and amygdala gray matter (for total amygdala β = .088, t = 1.483, p = .139; for the left amygdala β = .091, t = 1.529, p = .127; for the right amygdala β = .8, t = 1.343, p = .180). The full outputs of our regression models are shown in Tables 9 & 10. Again, all of these models controlled for gender, age, whole-brain volume, and parental education. Also worthy of note, no relationship emerged between income and whole-brain volume (β = −.018, t = −.278, p = .781).
Table 9. Regression Output For Models Examining the Association Between the Hippocampus and Income.
| Region of Interest (Dependent Variable) | Independent Variables | Unstandardized regression coefficients, Standard Error, Standardized regression coefficients, test statistics |
| Total Hippocampus | Maternal Education | B = −0.0001, SE = 0.003, β = −.005, t = 0.08 p = .93 |
| Paternal Education | B = 0.003, SE = 0.002, β = .105, t = 1.785 p = .075 | |
| Income | B = 0.045, SE = 0.018, β = .145, t = 2.459 p = .014 | |
| Left Hippocampus | Maternal Education | B = −0.001, SE = 0.002 β = −.03, t = 0.505 p = .614 |
| Paternal Education | B = 0.003, SE = 0.002, β = .083, t = 1.404 p = .161 | |
| Income | B = 0.052, SE = 0.019, β = .165, t = 2.773 p = .006 | |
| Right Hippocampus | Maternal Education | B = 0.0007, SE = 0.002, β = .02, t = −0.344, p = .73 |
| Paternal Education | B = 0.004, SE = 0.002, β = .122, t = 2.073 p = .039 | |
| Income | B = 0.038, SE = 0.019, β = .118, t = 1.999 p = .046 |
NB: All regression models included child age (in months), gender of the child (dummy-coded), and whole-brain volume as covariates.
Table 10. Regression Output For Models Examining the Association Between the Amygdala and Income.
| Region of Interest (Dependent Variable) | Independent Variables | Unstandardized regression coefficients, Standard Error, Standardized regression coefficients, test statistics |
| Total Amygdala | Maternal Education | B = −0.0003, SE = 0.002, β = −.01, t = −0.17 p = .867 |
| Paternal Education | B = 0.0013, SE = 0.002, β = .040, t = .679 p = .498 | |
| Income | B = 0.031, SE = 0.021, β = .088, t = 1.483 p = .139 | |
| Left Amygdala | Maternal Education | B = −0.001, SE = 0.002, β = −.013, t = −0.22 p = .830 |
| Paternal Education | B = 0.001, SE = 0.002, β = .030, t = 0.509 p = .611 | |
| Income | B = 0.034, SE = 0.022, β = .091, t = 1.529 p = .127 | |
| Right Amygdala | Maternal Education | B = −0.0002, SE = 0.002, β = −.007, t = −0.11 p = .91 |
| Paternal Education | B = 0.002, SE = 0.002, β = .048, t = 0.805 p = .421 | |
| Income | B = 0.029, SE = 0.021, β = .080, t = 1.343 p = .180 |
NB: All regression models included child age (in months), gender of the child (dummy-coded), and whole-brain volume as covariates.
Discussion
This study was designed to examine the possible association between household family income and the hippocampus, a brain region central to many important cognitive and emotional processes. We identified an association with the hippocampus and income, as hypothesized. The hippocampus has previously been found to be associated with quality of environmental input and stress. Taken together, these findings suggest that differences in the hippocampus, perhaps due to stress tied to growing up in poverty, might partially explain differences in long-tern memory, learning, control of neuroendocrine functions, and modulation of emotional behavior. These results are consistent with research on neuropsychological differences across the SES gradient (for review, see [31]). Farah and colleagues [13], [32] along with Rao et al. [14] found environmental stimulation and parental nurturance was related to memory functioning in childhood. Such long-term memory functions are mediated by the hippocampus [33]. Variations in hippocampal size have been associated with memory performance with larger hippocampal volumes being related to better memory performance [34]. In addition, higher levels of chronic life stress appear to be associated with smaller hippocampal volumes in adults [35]. These results add to the modest body of research examining neurobiological associations with socioeconomic status, providing one potential neurobiological mechanism through which the early environment may convey risk for a host of deleterious outcomes.
In contrast to previous research linking amygdala volume and stress [36], we did not observe associations for the amygdala and income. Amygdala quantification is very challenging and even with such a large sample size, automated methods may not be appropriate. Follow-up analyses using a different method of automated segmentation however yielded similar results (see Supplemental Materials S1). In addition, associations between the amygdala and early life stress effects may vary by age of measurement (for discussion, see [37]). For example, increases in amygdala volume may be seen early in development after the experience of stress, while small amygdala volume may occur later in development.
The structural imaging project presented here does not address issues of causation: poverty carries multiple components of environmetal risk and many factors may affect the development of brain structure. Future research should longitudinally assay both brain structure and function, as understanding both factors are likely central to truly understanding associations between neurobiological outcomes and income. Additional work should also include a variety of neuropsychological assessment, as the cognitive tests employed in this study were predominantly “prefrontal-dependent”: tapping rule acquisition and working memory. Subsequent studies must also aim to delineate the effects of household income, environmental stimulation, stress, and other variables such as possible nutritional differences related to poverty with large samples of children living in poverty. Such research designs will further increase understanding the neurobiological correlates of poverty and socioeconomic status.
This study examined a large group of children and adolescents from 5 different research sites around the United States. Although issues of race and ethnicity were not the focus of our study, these factors may be associated with variations in neural development. Preliminary analyses suggested that our effects held for Caucasian and non-Caucasian participants. Future research should focus on exploring ethnic diversity with appropriately sized samples across income categories. Of important note, the NIH data set was also designed with a plan to screenout individuals with mental health issues or very low intelligence. This design skews the sample because psychopathology and learning disorders are disproportionately represented among impoverished children. The present results therefore reflect so-called “normal” children living in poverty. This suggests that the present results likely under-represent the true effects of poverty. Alternatively one could argue that the exclusionary criteria may strengthen the implications of our results as psychopathology or learning disorders as possible explanations of the association can largely be ruled out as factors lying behind the correlation.
Understanding how environmental variations can affect neural, emotional and cognitive functioning in humans has major implications for both basic scientific questions and public policy initiatives. Such knowledge about the neural embedding of socioeconomic status, specifically poverty, may aid in the design and implementation of intervention programs addressing SES-related disparities in a cognitive and health outcomes. We found variations in socioeconomic status were associated with hippocampal volumes (as measured by gray matter probability). This finding suggests a potential neurobiological mechanism through which the early environment may convey risk for a host of deleterious outcomes from poor health to lower educational achievement. In addition to SES-related disparities, such results add to our understanding of human brain development, as we aim to further delineate how post-natal experiences may uniquely shape the brain and change behavior.
Supporting Information
Additional Demographic Summary for full sample (based on Wave 1 data).
(DOC)
Demographic Variables for Subjects with and without MRI Scans and/or Income.
(DOC)
(DOCX)
Acknowledgments
We thank Jay Bhattacharya, Ed Moss, and the Health & SES working book group at the Russell Sage Foundation for helpful discussions.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the US National Institute on Drug Abuse (Grant DA028087 to JLH), the US National Institute of Mental Health (Grants MH61285 and MH68858 to SDP) and the Children's Bureau of the Administration on Children, Youth and Families as part of the Child Neglect Research Consortium. This project was also supported by the Russell Sage Foundation and the University of Wisconsin-Madison Graduate School grants to BLW. The authors also thank the Russell Sage Foundation for their support of Health and SES working group. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Greenough W, Black J. Induction of brain structure by experience: Substrate for cognitive development. In: Gunnar MR, Nelson CA, editors. Minnesota Symposia on Child Psychology 24: Developmental Behavioral Neuroscience. Hillsdale, NJ: Lawrence Erlbaum; 1992. pp. 155–200. [Google Scholar]
- 2.Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–23. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raizada RD, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3. doi: 10.3389/neuro.09.003.2010. Available: http://www.frontiersin.org/human_neuroscience/10.3389/neuro.09/003.2010/abstract Accessed 2011 March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans GW, Kim P. Childhood Poverty and Health Cumulative Risk Exposure and Stress Dysregulation. Psychological Science. 2007;18:953–957. doi: 10.1111/j.1467-9280.2007.02008.x. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–77. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 6.Fiala BA, Joyce JN, Greenough WT. Environmental complexity modulates growth of granule cell dendrites in developing but not adult hippocampus of rats. Exp Neurol. 1978;59:372–83. doi: 10.1016/0014-4886(78)90229-7. [DOI] [PubMed] [Google Scholar]
- 7.Greenough WT, Volkmar FR, Juraska JM. Effects of rearing complexity on dendritic branching in frontolateral and temporal cortex of the rat. Exp Neurol. 1973;41:371–8. doi: 10.1016/0014-4886(73)90278-1. [DOI] [PubMed] [Google Scholar]
- 8.Soffié M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res. 1999;101:37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 9.Green EJ, Greenough WT. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: evidence from hippocampal slices maintained in vitro. J Neurophysiol. 1986;55:739–50. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- 10.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–5. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 11.Will BE, Rosenzweig MR, Bennett EL, Hebert M, Morimoto H. Relatively brief environmental enrichment aids recovery of learning capacity and alters brain measures after postweaning brain lesions in rats. J Comp Physiol Psychol. 1977;91:33–50. doi: 10.1037/h0077306. [DOI] [PubMed] [Google Scholar]
- 12.Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–92. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- 13.Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 14.Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, et al. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2010;49:1144–50. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, et al. Socioeconomic status and health. The challenge of the gradient. Am Psychol. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 16.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65:209–37. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 17.McLoyd VC, Wilson L. Maternal behavior, social support, and economic conditions as predictors of distress in children. New Dir Child Dev Winter. 1990;46:49–69. doi: 10.1002/cd.23219904605. [DOI] [PubMed] [Google Scholar]
- 18.Moffitt TE, Gabrielli WF, Mednick SA, Schulsinger F. Socioeconomic status, IQ, and delinquency. J Abnorm Psychol. 1981;90:152–6. doi: 10.1037//0021-843x.90.2.152. [DOI] [PubMed] [Google Scholar]
- 19.Evans AC Brain Development Cooperative Group. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- 20.Waber DP, De Moor C, Forbes PW, Almli CR, Botteron KN, et al. The NIH MRI study of normal brain development: performance of a population based sample of healthy children aged 6 to 18 years on a neuropsychological battery. J Int Neuropsychol Soc. 2007;13:729–746. doi: 10.1017/S1355617707070841. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 22.Busatto GF, Diniz BS, Zanetti MV. Voxel-based morphometry in Alzheimer's disease. Expert Rev Neurother. 2008;8:1691–702. doi: 10.1586/14737175.8.11.1691. [DOI] [PubMed] [Google Scholar]
- 23.Williams LM. Voxel-based morphometry in schizophrenia: implications for neurodevelopmental connectivity models, cognition and affect. Expert Rev Neurother. 2008;8:1049–65. doi: 10.1586/14737175.8.7.1049. [DOI] [PubMed] [Google Scholar]
- 24.Whitwell JL, Josephs KA. Voxel-based morphometry and its application to movement disorders. Parkinsonism Relat Disord. 2007;13(Suppl 3):S406–16. doi: 10.1016/S1353-8020(08)70039-7. [DOI] [PubMed] [Google Scholar]
- 25.Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Bergouignan L, Chupin M, Czechowska Y, Kinkingnéhun S, Lemogne C, et al. Can voxel based morphometry, manual segmentation and automated segmentation equally detect hippocampal volume differences in acute depression? Neuroimage. 2009;45:29–37. doi: 10.1016/j.neuroimage.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 27.Yassa MA, Stark CE. A quantitative evaluation of cross-participant registration techniques for MRI studies of the medial temporal lobe. Neuroimage. 2009;44:319–27. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 29.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 30.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends Cogn Sci. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, et al. Childhood poverty: specific associations with neurocognitive development. Brain Res. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- 33.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 34.Biegler R, McGregor A, Krebs JR, Healy SD. A larger hippocampus is associated with longer-lasting spatial memory. Proc Natl Acad Sci U S A. 2001;98:6941–4. doi: 10.1073/pnas.121034798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianaros PJ, Jennings JR, Sheu LK, Greer PJ, Kuller LH, et al. Prospective reports of chronic life stress predict decreased grey matter volume in the hippocampus. Neuroimage. 2007;35:795–803. doi: 10.1016/j.neuroimage.2006.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McEwen BS. Stress, sex, and neural adaptation to a changing environment: mechanisms of neuronal remodeling. Ann N Y Acad Sci. 2010;1204(Suppl):E38–59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tottenham N, Sheridan MA. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front Hum Neurosci. 2010;3:68. doi: 10.3389/neuro.09.068.2009. Available: http://www.frontiersin.org/human_neuroscience/10.3389/neuro.09/068.2009/abstract Accessed 2011 March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Demographic Summary for full sample (based on Wave 1 data).
(DOC)
Demographic Variables for Subjects with and without MRI Scans and/or Income.
(DOC)
(DOCX)