Abstract
Summary
Background and objectives
dense deposit disease (DDD) is the prototypical membranoproliferative glomerulonephritis (MPGN), in which fluid-phase dysregulation of the alternative pathway (AP) of complement results in the accumulation of complement debris in the glomeruli, often producing an MPGN pattern of injury in the absence of immune complexes. A recently described entity referred to as GN with C3 deposition (GN-C3) bears many similarities to DDD. The purpose of this study was to evaluate AP function in cases of GN-C3.
Design, setting, participants, & measurements
Five recent cases of MPGN with extensive C3 deposition were studied. Renal biopsy in one case exhibited the classic findings of DDD. Three cases showed GN-C3 in the absence of significant Ig deposition; however, the classic hallmark of DDD—dense deposits along the glomerular basement membranes and mesangium—was absent. The remaining case exhibited features of both DDD and GN-C3.
Results
Evidence of AP activation was demonstrable in all cases and included increased levels of soluble membrane attack complex (all cases), positive AP functional assays (four cases), and a positive hemolytic assay (one case). Autoantibodies were found to C3 convertase (two cases) and to factor H (one case). Factor H mutation screening identified the H402 allele (all cases) and a c.C2867T p.T956M missence mutation (one case). Laser microdissection and mass spectrometry of glomeruli of GN-C3 (two cases) showed a proteomic profile very similar to DDD.
Conclusions
These studies implicate AP dysregulation in a spectrum of rare renal diseases that includes GN-C3 and DDD.
Introduction
Membranoproliferative glomerulonephritis (MPGN) is a pattern of glomerular injury that most frequently results from the mesangial and subendothelial deposition of immune complexes. These complexes trigger activation of complement and a phase of acute injury in the glomerular mesangium and capillaries. The acute injury phase is followed sequentially by an inflammatory phase, with influx of inflammatory cells (proliferative phase) and then a reparative phase. The reparative phase is characterized by the formation of new basement membranes in the mesangium and between the immune deposits and old basement membranes (membrane phase), resulting in mesangial expansion and double contours, respectively (1,2).
Dysregulation of the alternative pathway (AP) of complement with deposition of complement factors in the mesangium and along the capillary walls also results in MPGN in the absence of immune complexes. The prototypical example of this form of MPGN is dense deposit disease (DDD; also referred to as MPGN type II). DDD is characterized by an MPGN pattern of injury, C3 deposits on immunofluorescence microscopy, and the characteristic sausage-shaped wavy osmiophilic electron dense deposits along the glomerular basement membranes (GBMs) and mesangium for which the disease is named (3–5).
We have noted, however, some cases of MPGN in which C3 deposition along the mesangium and capillary walls is extensive and immune deposits are absent, but electron microscopy (EM) fails to show the typical sausage-shaped wavy intramembranous and mesangial deposits of DDD. Instead, we have observed deposits very similar to those seen with immune-complex-mediated MPGN. The terms “glomerulonephritis with isolated C3 deposits (GN-C3)” and “C3 glomerulopathy” have been used for this recently described entity (6–8).
In this manuscript, we describe one recent case of DDD, three cases of GN-C3, and one case that exhibits features of both DDD and GN-C3. Complement assays show that, in all cases, there is AP dysfunction and loss of regulatory control of the complement cascade. Thus, AP dysfunction results in not only DDD, but in a type of MPGN that lacks the classic electron microscopic hallmarks of DDD. Based on these findings, we propose a simple classification for these diseases that places both GN-C3 and DDD under the umbrella of C3 glomerulopathies.
Materials and Methods
Renal biopsies from three Mayo Clinic patients and two renal biopsies sent to the Renal Biopsy Service at the Mayo Clinic were evaluated. In all cases, light microscopy, immunofluorescence microscopy, and EM were performed. Clinical information was obtained from the charts. Institutional Review Board approval was obtained to evaluate the AP of complement, which was performed as follows.
C3 Nephritic Factors
The presence of C3 nephritic factors (C3Nefs), autoantibodies to the alternative pathway C3 convertase (C3bBb), was analyzed by ELISA as described previously (9).
AP Functional Assay (APFA)
AP complement activity was evaluated using the Wieslab complement AP assay kit as described previously (9). In brief, the method combines principles of the hemolytic assay for complement activation with the use of labeled antibodies specific for neoantigen produced as a result of complement activation. The amount of neoantigen generated is proportional to the functional activity of the AP. Results were expressed as a percentage of normal (normal reference range, 65 to 130% based on 50 normal sera samples).
Hemolytic Assay
The sheep erythrocyte lysis assay was completed as described previously (9). This assay measures complement-mediated lysis of sheep erythrocytes secondary to AP activation. Sheep erythrocytes are nonactivators of complement-mediated lysis in human serum. Any observed lysis was graded as follows: normal (<3%); 1+ (3 to 20%); 2+ (20 to 40%); 3+ (40 to 60%); 4+ (60 to 80%); 5+ (80 to 100%, complete hemolysis).
Factor H and Factor B Autoantibodies
Factor H and factor B autoantibodies (FHAA, FBAA) were analyzed by ELISA as described previously (9). An OD of +2 SD above normal based on 50 healthy controls was considered positive (normal reference range: <300 units).
Mutational Analysis
Coding regions and intron–exon boundaries of CFH (MIM#134370; NM_000186), CFHR5 (MIM#608593; NM_030787.3), CFI (MIM#217030; NM_000204.3), CD46 (MIM#120920; NM_002389.3), CFB (MIM#138470; NM_001710.5), C3 (MIM#120700; NM_000064.2), and THBD (MIM#188040; NM_000361.2) were amplified and screened for mutations and polymorphisms using bi-directional sequencing. PCR amplification of genomic DNA was completed using 20 ng genomic DNA, 2× NH4 buffer, 3 mM MgCl2, 400 μM each dNTP, 50 U/ml Taq polymerase, and 10% DMSO. Reaction conditions were as follows: an initial denaturation at 95°C for 5 minutes; 35 cycles of denaturation at 95°C for 30 seconds; annealing at 60°C for 30 seconds; extension at 72°C for 30 seconds; and a final extension at 72°C for 10 minutes.
Specimen Preparation, Laser Microdissection, and Mass Spectrometry Proteomic Analysis
Results
Clinical and Laboratory Findings
Five cases of MPGN with extensive C3 deposits in the absence of significant immune deposits were diagnosed in the renal biopsy service at the Mayo Clinic (Tables 1 and 2). Based on biopsy findings, three cases were diagnosed as GN-C3, one case was diagnosed as DDD, and one case was considered intermediary between GN-C3 and DDD (see next section). Although the age range was wide (9 to 73 years), the case of DDD was in the youngest patient (age 9 years), whereas the patients with GN-C3 were older (≥38 years); the patient with GN-C3/DDD was 19 years old. At presentation, all patients had varying degrees of proteinuria and hematuria, four patients had hypertension, and three patients had lower extremity edema. Three of the five cases had an ophthalmological examination that showed no evidence of Drusen. A brief clinical history for each patient follows.
Table 1.
Clinical findings
| Gender | Age (years) | Hypertension | Lower Extremity Edema | Other | |
|---|---|---|---|---|---|
| Case 1 | Male | 73 | Present | Present | CABG, dyspnea |
| Case 2 | Male | 38 | Present | Present | Obstructive sleep apnea |
| Case 3 | Male | 62 | Present | Negative | MGUS, renal calculi |
| Case 4 | Male | 19 | None | None | Hematuria as a child |
| Case 5 | Male | 9 | Present | Present | Positive ANA and ASO titers at presentation |
CABG, coronary artery bypass graft; MGUS, monoclonal gammopathy of undetermined significance; ANA, anti-nuclear antibody; ASO, anti-streptolysin titer.
Table 2.
Laboratory findings
| Serum Creatinine |
Urinalysis | Urine Protein (g/day) at Presentation | C3 and C4 (mg/dl) | Hepatitis and Lupus Serology | Other | ||
|---|---|---|---|---|---|---|---|
| At Presentation | Follow-Up | ||||||
| Case 1 | 1.9 | 1.3 | 3 + protein | 1.7 | 39 | Negative | None |
| (6 months) | 2 + blood | 33 | |||||
| Case 2 | 1.1 | 0.9 | 3 + protein | 9.7 | 93 | Negative | Essential thrombocythemia |
| (2 years) | 1 + blood | 14 | |||||
| Case 3 | 1.1 to 1.6 | 1.3 | 2 + protein | 1.2 | 48 | Negative | MGUS |
| (3 years) | 1 + blood | 23 | |||||
| Case 4 | 1.8 | 1.5 | 1 + protein | 0.23 | Low C3 | Negative | None |
| (2 years) | 1 + blood | Normal C4 | |||||
| Case 5 | 2.5 | 1.0 | 1 + protein | 10 | 44 | Negative | None |
| (6 months) | 3 + blood | 28 | |||||
Urinalysis: 1+ = 3 to 10 RBC/HPF, 2+ = 10 to 25/HPF, 3+ = > 25 RBC/HPF. C3 and C4 normal range: 75 to 175 and 14 to 40 mg/dl, respectively. S Cr, serum creatinine (mg/dl); MGUS, monoclonal gammopathy of undetermined significance.
Case 1 is a 73-year-old man who presented with dyspnea, lower extremity edema, and renal failure of 3-week duration. His serum creatinine had increased from a baseline of 1.2 mg/dl to 2.3 mg/dl with an estimated GFR (eGFR) of 35 ml/min. Urinalysis showed red blood cells (RBCs) and RBC casts, and urinary protein was 1.7 g/24 h. His medical history was significant for coronary artery disease.
Case 2 is a 38-year-old man with a history of hypertension who was found to have significant proteinuria 2 years earlier, at which time his serum creatinine was 1.1 mg/dl with an eGFR of 58 ml/min and urinary protein of 9.7 g/24 h. Renal biopsy showed proliferative glomerulonephritis with features of postinfectious glomerulonephritis. Although his serum creatinine has remained stable, significant proteinuria (11.4 g/24 h) has persisted and therefore a second renal biopsy was performed. His medical history was significant for essential thrombocythemia with JAK2 mutations and obstructive sleep apnea.
Case 3 is a 62-year-old man who was in excellent health until 3 years ago when he developed hematuria. Renal biopsy at the time suggested postinfectious glomerulonephritis. Serum creatinine was 1.1 mg/dl but gradually increased to 1.7 mg/dl before improving somewhat and stabilizing at 1.3 mg/dl. Because of persistent hematuria and proteinuria, a second renal biopsy was performed. His medical history was significant for monoclonal gammopathy of undetermined significance.
Case 4 is a 19-year-old man who presented with hematuria. Serum creatinine was 1.8 mg/dl and urinary protein was 229 mg/24 h. His medical history was significant for gross hematuria as a child.
Case 5 is a 9-year-old boy who presented with renal failure, hypertension, and anasarca with a serum creatinine of 2.5 mg/dl and proteinuria of 10 g/d. The initial working diagnosis was poststreptococcal glomerulonephritis; however, his renal function continued to decline, and a renal biopsy was performed.
Renal Biopsy Findings
Light microscopy.
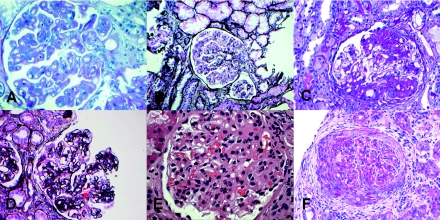
All cases showed a predominantly MPGN pattern of injury with mesangial expansion and increased mesangial cellularity, endocapillary proliferation, thickened glomerular basement membranes, double contour formation, and lobular accentuation of the glomerular tufts (Table 3). One case (5) also showed crescent formation (Figure 1F). The tubulointerstitial scarring varied from minimal (5%) to moderate (25%). None of the biopsies showed features of a thrombotic microangiopathy. Representative glomerular morphology is shown in Figure 1.
Table 3.
Renal biopsy findings
| Light Microscopy | Glomerulia | Crescents/Fibrinoid Necrosis | Tubulo-Interstitial Scarring | IF | EM | |
|---|---|---|---|---|---|---|
| Case 1 | MPGN | 3/0/0 | None | 25% | C3 (3+) Mes, CW | DC, SE, Mesb |
| Case 2 | MPGN, FSGS | 23/1/3 | None | 20% | C3 (3+) Mes, CW | DC, SE, Mes |
| Case 3 | MPGN | 18/0/0 | None | 10% | C3 (3+) Mes, CW | DC, SE, SU Mes |
| Case 4 | MPGN | 16/0/0 | None | 5% | C3 (3+)Mes, CW | DC, SE, SU Mes Sausage-shaped wavy intramembranous deposits |
| Case 5 | MPGN | 30/2/0 | Present | 20% | C3 (3+) Mes, CW | DC, Sausage-shaped wavy intramembranous and mesangial deposits |
Total/global/segmental sclerosis.
DC, double contour; SE, subendothelial; SU, subepithelial; Mes, mesangial electron dense deposits.
Figure 1.
Light microscopy showing an MPGN pattern of injury in cases 1 (A), 2 (B), 3 (D), 4 (E), and case 5 (F). Segmental sclerosis is also seen in case 2 (C), and in case 5, crescents are noted (F).
Immunofluorescence microscopy.
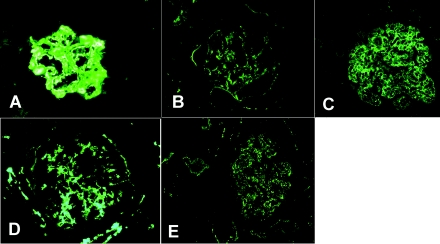
All cases showed marked C3 (3+) deposition along the glomerular capillary walls, in the mesangium, and along some tubular basement membranes. No staining was observed for immunoglobulins (Igs). Kappa and lambda light chain staining was uniformly negative. Representative glomerular C3 staining is shown in Figure 2.
Figure 2.
Immunofluorescence microscopy showing glomerular capillary wall C3 staining in cases 1 (A), 2 (B), 3 (C), 4 (D), and 5 (E).
Electron microscopy.
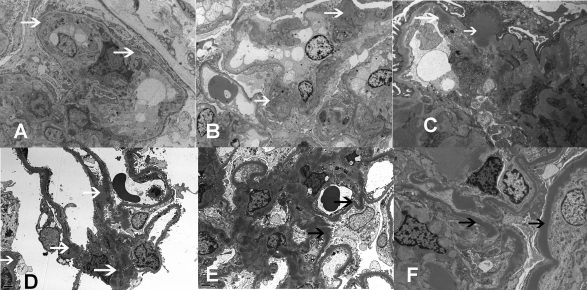
The striking difference between cases 1 to 3 and case 5 was in the EM findings. Cases 1 to 3 showed immune-type electron-dense deposits in the mesangium and along capillary walls in a subendothelial location reminiscent of MPGN type I, although a few subepithelial and intramembranous deposits were noted. Case 5 showed the classic sausage-shaped wavy deposits of DDD along the GBM and in the mesangium. Case 4 was intermediate between the two and showed mesangial, subepithelial, and subendothelial electron-dense deposits along the GBM that lacked the typical sausage-shaped wavy morphology of DDD (Figure 3D), whereas other loops showed the classic wavy sausage-shaped intramembranous deposits of DDD (Figure 3E). Deposits were also noted along tubular basement membranes in cases 4 and 5. Representative EM findings are shown in Figure 3.
Figure 3.
Electron microscopy showing (A) double contours with subendothelial deposits and new basement membranes in case 1; (B) mesangial deposits in case 2; (C) subepithelial, subendothelial, and mesangial deposits in case 3; (D) mesangial, subepithelial, and subendothelial deposits in case 4; and (E and F) osmiophilic wavy sausage-shaped intramembranous deposits and mesangial deposits in cases 4 and 5, respectively (white arrows, electron dense deposits; black arrows, dense deposits along basement membrane).
Kidney biopsy diagnosis.
Based on renal biopsy findings cases 1 to 3 were diagnosed as GN-C3, case 5 was diagnosed as DDD, and case 4 was diagnosed as GN-C3/DDD.
Alternative Pathway Evaluation and Genetic Screening
In all cases, activation of the complement cascade was shown by increased levels of soluble membrane attack complex (sMAC) (mean levels, 0.61 mg/dl; normal <0.30 mg/dl; Table 4). Other abnormal tests of AP function included the APFA, which was positive in four cases, and a hemolytic assay, which was positive in one case. Identified autoantibodies included FHAA (case 1) and C3Nefs (cases 2 and 5). In case 4, genetic testing was positive for a CFH mutation (c.2867 C>T) that results in a p.Thr956Met missense mutation in short consensus repeat 16 of the FH. All cases carried at least one copy of the DDD-associated CFH H402 allele. No mutations were found in C3, CFB, CFI, THBD, MCP, or CFHR5.
Table 4.
AP and genetic evaluation
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | |
|---|---|---|---|---|---|
| CFH | 1 copy H402 | 1 copy H402 | 2 copies of H402 | 2 copies of H402; c0.2867 C>T p. Thr956Met | 1 copy of H402 |
| CFI | No mutations | No mutations | No mutations | No mutations | No mutations |
| MCP | No mutations | No mutations | No mutations | No mutations | No mutations |
| C3 | No mutations | No mutations | No mutations | No mutations | No mutations |
| CFB | ND | No mutations | No mutations | ND | No mutations |
| CFHR5 | ND | No mutations | No mutations | ND | No mutations |
| THBD | No mutations | No mutations | No mutations | ND | No mutations |
| APFA | 6.6% very low | 109.0% normal | 1.0% very low | 5.5% very low | 1.0% very low |
| Hemolytic Assay | Normal | 1+ | Normal | Normal | Normal |
| FHAA | 1:200 positive | Negative | Negative | Negative | Negative |
| FBAA | Negative | Negative | Negative | Negative | Negative |
| C3Nefs | Negative | 1:200 positive | Negative | Negative | 1:800 positive |
| sMAC | 0.46 mg/L | 0.41 mg/L | 1.23 mg/L | 0.93 mg/L | 0.31 mg/L |
ND, not done.
Laser Dissection and Mass Spectrometry of GN-C3
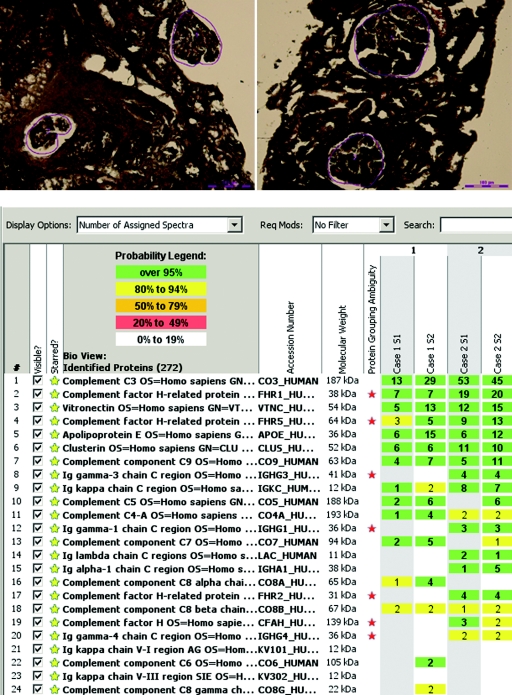
We performed laser microdissection (LMD) and mass spectrometry (MS) on two cases of GN-C3 (cases 1 and 2). Glomeruli were selected for LMD as shown by the red line, leaving a vacant space on the slide after dissection (Figure 4A). Each LMD contained an area of 50,000 to 60,000 μm2, and two to four microdissections were analyzed for each case. Figure 4B shows the MS results in duplicate (S1 and S2) for cases 1 and 2, listing proteins according to abundance based on the number of assigned spectra. Both cases showed presence of C3, C5, and C9, indicating accumulation of AP and terminal complement complex proteins. Regulators of complement activation factor H–related protein 1 and factor H–related protein 5 were also present, as were complement regulating proteins vitronectin and clusterin. Small amount of immunoglobulins (Igγ 3 and Igγ 1) and kappa and lambda light chains were noted in case 2.
Figure 4.
Laser microdissection and mass spectrometry. (A) Glomeruli marked before dissection in case 1 (A) and case 2 (B). (B) Representative scaffold readout of proteins of interest for two cases of GN-C3 (cases 1 and 2) by Spectra. Samples are in duplicate (S1 and S2). The proteomic data show C3, FHR-1, vitronectin, FHR-5, apolipoprotein E, clusterin, and C9 in order of abundance with >95% probability. Yellow stars indicate proteins of interest, whereas red stars indicate protein ambiguity when two proteins share conserved regions.
Clinical Follow-Up
Follow-up of 6 months and 2 and 3 years was available for the three cases of GN-C3. Serum creatinine in case 1 increased from a baseline of 1.1 to 1.9 mg/dl at the time of biopsy, and after 6 months has stabilized at 1.3 mg/dl, with a 24-h urinary protein of 1.7 g/d. In case 2, the baseline serum creatinine at the time of diagnosis was 1.1 mg/dl, with proteinuria of 9.7 g/d. Two years later, the serum creatinine is 0.9 mg/dl with a proteinuria of 7 g/d. Case 3 presented with a serum creatinine of 1.1 mg/dl, which peaked at 1.6 mg/dl. Three years later, the serum creatinine is 1.3 mg/dl, with subnephrotic range proteinuria of 1.2 g/d. All three cases are being managed with maximum conservative therapy including angiotensin II blockade; none of these cases have received immunosuppressive management including steroids. Case 4 (GN-C3/DDD) is also being managed with angiotensin II blockade and has a creatinine of 1.5 mg/dl with proteinuria of 3.0 g/d. Case 5 (DDD) presented with renal failure (serum creatinine, 2.5 mg/dl), significant proteinuria (10 g/d), and anasarca and was treated with angiotensin II blockade, steroids, and mycophenolate mofetil. Nine months later, his serum creatinine is stable at 1 mg/dl, with a proteinuria of 470 mg/dl.
Discussion
Dysregulation of the AP can lead to complement debris–induced renal changes in the mesangium and glomerular capillary walls that produce an MPGN pattern of injury. DDD is the prototypical example of this type of renal disease (3–5,10). The absence of Igs by immunofluorescence and the location and character of the dense EM deposits distinguish DDD from MPGN types I and III. Some cases of MPGN, however, show a pathology that is intermediate between immune complex-mediated MPGN and DDD. In these cases of MPGN, C3 deposition is present in the absence of Ig deposition; however, EM is not one of sausage-shaped wavy dense deposits. Instead subendothelial (occasionally subepithelial) and mesangial deposits are seen that resemble MPGN type I disease. We hypothesized that because Igs are absent, these cases result from AP dysfunction, suggesting that dysregulation of the AP produces a spectrum of morphologic patterns that range from MPGN to mesangial proliferative GN or even sclerosing GN. The purpose of this study was to evaluate AP function in these unusual cases.
Activation of the AP complement cascade occurs in a sequential manner resulting in generation of effector compounds that are delivered to all surfaces indiscriminately, mandating control over progression of the cascade. Multiple complement regulators and inhibitors operate at every level to prevent self-mediated damage (14). Proteins that regulate C3 convertase assembly, activity, and half-life include factor H, factor I, decay accelerating factor, factor H–related proteins, membrane cofactor protein, and CR1. Mutations in, or antibodies against, these proteins can alter AP control and lead to the development of MPGN. Genetic background is also a risk factor for development of disease. Best studied is the Tyr402His allele variant of factor H: His402 is over-represented in DDD patients compared with Tyr402 and provides poorer factor H–mediated regulation of the C3 convertase on cell surfaces (15,16).
In the five cases we described—three of GN-C3, one of GN-C3/DDD, and one of DDD—AP dysregulation was present as evidenced by increased sMAC levels (all cases), positive APFAs (four cases), and a positive hemolytic assay (one case). However, the underlying cause of AP dysregulation varied. In cases 2 and 5, C3Nefs were detected, in case 1, FHAAs were detected, and in case 4, a CFH c.2867 C>T, p.Thr956Met mutation was identified. All cases carried at least one copy of the factor H H402 allele, a permissive genetic background associated with the development of DDD.
LMD/MS data identified complement proteins of the AP and terminal complement complex, as well as complement regulating proteins such as vitronectin, clusterin, and factor H–related proteins, in the glomeruli of the two GN-C3 cases we studied. Small amounts of Ig were detected in one case, which we believe may represent either nonspecific entrapment of small amounts of plasma proteins or C3Nefs. Proteins of the classical pathway such as C1 and C4 were not present in any abundance. These findings are consistent with AP activation and are very similar to data derived from DDD patients, suggesting a common pathogenesis for these diseases (10).
Given the complexity of the AP cascade and the similarity between DDD and GN-C3, the EM differences may reflect differences in the degree or site of AP/sMAC dysregulation. Alternatively, the response of the kidneys to complement damage may also play a role. That these diseases are part of a continuum is supported by case 4, which showed features intermediate between cases 1 to 3 and case 5. It is pertinent to note that, in cases 1 to 4, the disease course was slower and milder than is typical for DDD. In case 5 (with DDD), immunosuppressive management was included.
A clear limitation of this study is the short follow-up available. However, even within this short time frame, it is remarkable that, in cases 2 and 3, repeat kidney biopsies after 1 to 3 years showed only a slight increase in the extent of glomerular and tubulointerstitial scarring with no significant loss of renal function even with conservative management. The differential diagnosis of these cases includes postinfectious glomerulonephritis (because of the presence of subepithelial humps) and autoimmune disease (because of subepithelial, subendothelial, and mesangial deposits), and although two cases of GN-C3 were diagnosed initially as postinfectious proliferative GN, the lack of Igs on immunofluorescence studies differentiates GN-C3 from postinfectious and autoimmune proliferative GN.
Servais et al. (6) have recently described an entity they refer to as primary glomerulonephritis with C3 deposits. In their important study, the authors showed that 2 of 13 patients diagnosed as MPGN type I had alterations of AP, as did 4 of 6 patients labeled as C3 glomerulonephritis without proliferative features. Genetic testing was completed and serum protein levels were measured for several complement genes including CHF, CFI, CFB, and MCP, but the presence of autoantibodies was not assessed, and functional assays of AP activity were not performed.
Evaluating the AP in GN-C3 is important because it may have implications for treatment. For example, the presence of autoantibodies may suggest a role for rituximab, and in cases where a mutation is detected, complement-inhibiting drugs like eculizumab may be beneficial. Eculizumab (Alexion Pharmaceutics, Cheshire, CT) is a humanized recombinant antibody that targets C5 to prevent cleavage of C5 to C5b. This site of action reduces the generation of the proinflammatory peptide C5a and prevents activation of the terminal complement complex and formation of sMAC. Although its role in GN-C3 has not been studied, our data suggest that eculizumab should be tried in these patients.
MPGN is currently classified as primary/idiopathic or secondary. Primary/idiopathic MPGN includes immune complex–mediated MPGN types I and III, whereas secondary MPGN is most commonly caused by an antecedent hepatitis B or C viral infection that results in persistent antigenemia with secondary antigen–antibody immune complex deposition in the glomerulus (2,17,18). Other chronic infectious causes include shunt nephritis, abscesses, and endocarditis (19–21). Autoimmune diseases associated with MPGN include systemic lupus erythematosus and occasionally Sjögren's syndrome (22,23), and subendothelial and mesangial deposition of circulating monoclonal immunoglobulins can occur with monoclonal gammopathy of undetermined significance, lymphoproliferative disorders, and multiple myeloma (24). For these reasons, if immunoglobulins are present on immunofluorescence studies, the evaluation should be directed accordingly.
If immunofluorescence studies show predominantly C3 (C3G) and are negative for Igs, an indepth study of the AP is warranted. The AP evaluation should include sMAC levels, APFA, and hemolytic assays. If these tests are positive, mutation screening of complement genes and assays for autoantibodies to complement regulating proteins are warranted.
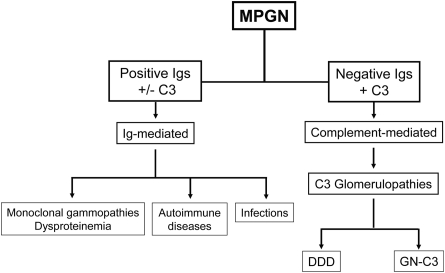
A simplified version of our interpretation of MPGN is shown in Figure 5. With respect to the terminology for this entity—primary glomerulonephritis associated with C3 deposits—the terms glomerulonephritis with C3 deposits and C3 glomerulopathy have been proposed (6–8). We use the term MPGN with C3 deposits in our renal biopsy practice to indicate a proliferative GN associated with AP dysfunction, but agree that GN-C3 is also appropriate. Because both GN-C3 and DDD are characterized by C3 deposition in the absence of Ig deposition, we propose that the term C3 glomerulopathy (C3G) be used to describe both DDD and GN-C3. We also recommend that MPGN be classified into two major groups: Ig-mediated and complement-mediated (C3G), using the term “idiopathic” MPGN (or type I or III) judiciously, because it is likely that an underlying etiology can be found in most cases. Finally, there is urgent need for standardized treatment for C3Gs that focus on the underlying etiology of the AP dysfunction.
Figure 5.
Proposed evaluation scheme for MPGN based on the presence or absence of immunoglobulins by immunofluorescence. In the absence of immunoglobulins, the genetics and functional activity of the AP should be assessed.
Disclosures
None.
Acknowledgments
This research was supported in part by NIH Grant DK074409 to S.S. and R.J.H.S. and a Fulk Family Foundation Award (Mayo Clinic) to S.S.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Rennke H: Secondary membranoproliferative glomerulonephritis. Kidney Int 47: 643–656, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Alpers CE, Smith KD: Cryoglobulinemia and renal disease. Curr Opin Nephrol Hypertens 17: 243–249, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Appel GB, Cook HT, Hageman G, Jennette JC, Kashgarian M, Kirschfink M, Lambris JD, Lanning L, Lutz HU, Meri S, Rose NR, Salant DJ, Sethi S, Smith RJ, Smoyer W, Tully HF, Tully SP, Walker P, Welsh M, Wurzner R, Zipfel PF: Membranoproliferative glomerulonephritis type II (dense deposit disease): An update. J Am Soc Nephrol 16: 1392–1403, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Nasr SH, Valeri AM, Appel GB, Sherwinter J, Stokes MB, Said SM, Markowitz GS, D'Agati VD: Dense deposit disease: Clinicopathologic study of 32 pediatric and adult patients. Clin J Am Soc Nephrol 4: 22–32, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith RJH, Alexander J, Barlow PN, Botto M, Cassavant TL, Cook HT, de Córdoba SR, Hageman GS, Jokiranta TS, Kimberling WJ, Lambris JD, Lanning LD, Levidiotis V, Licht C, Lutz HU, Meri S, Pickering MC, Quigg RJ, Rops AL, Salant DJ, Sethi S, Thurman JM, Tully HF, Tully SP, van der Vlag J, Walker PD, Würzner R, Zipfel PF; Dense Deposit Disease Focus Group: New approaches to the treatment of dense deposit disease. J Am Soc Nephrol 18: 2447–2456, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Servais A, Frémeaux-Bacchi V, Lequintrec M, Salomon R, Blouin J, Knebelmann B, Grunfeld JP, Lesavre P, Noel LH, Fakhouri F: Primary glomerulonephritis with isolated C3 deposits: A new entity which shares common genetic risk factors with haemolytic uraemic syndrome. J Med Genet 44: 193–199, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habbig S, Mihatsch MJ, Heinen S, Beck B, Emmel M, Skerka C, Kirschfink M, Hoppe B, Zipfel PF, Licht C: C3 deposition glomerulopathy due to a functional Factor H defect. Kidney Int 75: 1230–1234, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Fakhouri F, Frémeaux-Bacchi V, Noël LH, Cook HT, Pickering MC: C3 glomerulopathy: A new classification. Nat Rev Nephrol 6: 494–499, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Sethi S, Sukov W, Zhang Y, Fervenza F, Lager DJ, Miller DV, Cornell LD, Krishnan SG, Smith RJ: Dense deposit disease associated with monoclonal gammopathy of undetermined significance. Am J Kidney Dis 56: 977–982, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sethi S, Gamez JD, Vrana JA, Theis JD, Bergen HR, III, Zipfel PF, Dogan A, Smith RJ: Glomeruli of dense deposit disease contain components of the alternative and terminal complement pathway. Kidney Int 75: 952–960, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, III, Dogan A: Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood 114: 4957–4959, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Choi NH, Nakano Y, Tobe T, Mazda T, Tomita M: Incorporation of SP-40,40 into the soluble membrane attack complex (SMAC, SC5b-9) of complement. Int Immunol 2: 413–417, 1990 [DOI] [PubMed] [Google Scholar]
- 13. Nesvizhskii AI, Keller A, Kolker E, Aebersold R: A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Zipfel PF, Skerka C: Complement regulators and inhibitory proteins. Nat Rev Immunol 9: 729–740, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Abrera-Abeleda MA, Nishimura C, Smith JLH, Sethi S, McRae JL, Murphy BF, Silverstri G, Skerka C, Jozsi M, Zipfel PF, Hageman GS, Smith RJ: Variations in the complement regulatory genes factor H (CFH) and factor H related 5 (CFHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease). J Medl Genet 43: 582–589, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Skerka C, Lauer N, Weinberger AAWA, Keilhauer CN, Suhnel J, Smith R, Schlotzer-Schrehardt U, Fritsche L, Heinen S, Hartmann A, Weber BH, Zipfel PF: Defective complement control of Factor H (Y402H) and FHL-1 in age-related macular degeneration. Molec Immunol 44: 3398–3406, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Trendelenburg M, Fossati-Jimack L, Cortes-Hernandez J, Turnberg D, Lewis M, Izui S, Cook HT, Botto M: The role of complement in cryoglobulin-induced immune complex glomerulonephritis. J Immunol 175: 6909–6914, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Yamabe H, Johnson RJ, Gretch DR, Fukushi K, Osawa H, Miyata M, Inuma H, Sasaki T, Kaizuka M, Tamura N, et al. : Hepatitis C virus infection and membranoproliferative glomerulonephritis in Japan. J Am Soc Nephrol 6: 220–223, 1995 [DOI] [PubMed] [Google Scholar]
- 19. Vella J, Carmody M, Campbell E, Browne O, Doyle G, Donohoe J: Glomerulonephritis after ventriculo-atrial shunt. QJM 88: 911–918, 1995 [PubMed] [Google Scholar]
- 20. Hulton SA, Risdon RA, Dillon MJ: Mesangiocapillary glomerulonephritis associated with meningococcal meningitis, C3 nephritic factor and persistently low complement C3 and C5. Pediatr Nephrol 6: 239–243, 1992 [DOI] [PubMed] [Google Scholar]
- 21. Boseman P, Lewin M, Dillon J, Sethi S: Marfan syndrome, MPGN, and bacterial endocarditis. Am J Kidney Dis 51: 697–701, 2008 [DOI] [PubMed] [Google Scholar]
- 22. Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M; International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis: The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65: 521–530, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Adam F, Torun D, Bolat F, Zumrutdal A, Sezer S, Ozdemir FN: Acute renal failure due to mesangial proliferative glomerulonephritis in a pregnant woman with primary Sjögren's syndrome. Clin Rheumatol 25: 75–79, 2006 [DOI] [PubMed] [Google Scholar]
- 24. Sethi S, Zand L, Leung N, Smith RJH, Jevremonic D, Hermann SS, Fervenza FC: Membranoproliferative glomerulonephritis secondary to monoclonal gammopathy. Clin J Am Soc Nephrol 5: 770–782, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]