Abstract
Summary
Background and objectives
Elevated BP contributes to development and progression of proteinuria and decline in renal function in patients with type 2 diabetes. Our post hoc analysis assessed the baseline BP influence on the antiproteinuric effect in the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study.
Design, setting, participants, & measurements
In the AVOID study, 599 hypertensive type 2 diabetic patients with nephropathy received 6 months of aliskiren (150 mg force titrated to 300 mg daily after 3 months) or placebo added to losartan (100 mg) daily and optimal antihypertensive therapy. Changes in early morning urinary albumin:creatinine ratio and eGFR at week 24 were assessed by subgroups of baseline BP: Group A (prespecified target), <130/80 mmHg (n = 159); Group B, <140/90 mmHg but ≥130/80 mmHg (n = 189); and Group C (insufficient BP control), ≥140/90 mmHg (n = 251).
Results
Mean baseline BP (mmHg) levels for Groups A, B, and C were 120/71, 133/78, and 145/81, respectively. BP during the trial was nearly identical to baseline levels in all groups. The antiproteinuric effects of aliskiren were consistent across subgroups of baseline BP (19 to 22% reduction versus placebo). In Group C, the decline in eGFR was significantly lower with aliskiren than with placebo (P = 0.013).
Conclusions
Aliskiren (300 mg) added to losartan (100 mg) plus optimal antihypertensive therapy provides antiproteinuric effects independent of BP in patients with type 2 diabetes and nephropathy. Renal function was better preserved with aliskiren in patients with insufficient BP control.
Introduction
It is well appreciated that the antiproteinuric effect of Renin-angiotensin-aldosterone system (RAAS) blockade in part consists of lowering of systemic BP. However, indications from several studies point to an antiproteinuric effect of RAAS blockade with renal benefit independent of the initial untreated BP level (1) and the BP level achieved during treatment (2). In fact, the ADVANCE study (1) pointed to the possibility that even normotensive type 2 diabetic patients may benefit from RAAS blocking treatment delaying onset and progression of albuminuria, despite the fact that it is not current practice to treat such patients.
The concept of direct renin inhibition with aliskiren is the latest addition to drugs that block the RAAS, and the main indication of this treatment is as an antihypertensive agent (3). Aliskiren binds to renin and inhibits the conversion of angiotensinogen to angiotensin I at the first rate-limiting step in the cascade. Aliskiren has shown antiproteinuric potential in small studies (4,5), but the AVOID study (6) was the first large randomized study to investigate reduction in albuminuria with aliskiren versus placebo added on to standard recommended therapy with losartan in hypertensive patients with type 2 diabetes and nephropathy. The aim of this post hoc analysis of the AVOID study was to investigate whether the aliskiren treatment added to standard treatment exerted antiproteinuric/renoprotective effects regardless of baseline BP, i.e. whether this treatment is indicated across a range of BP levels, mirroring the diversity of patients in daily practice.
Materials and Methods
The trial enrolled hypertensive patients, ranging in age from 18 to 85 years, with type 2 diabetes and nephropathy (early morning urinary albumin creatinine ratio of >300 mg/g or >200 mg/g in patients receiving blockade of the renin-angiotensin-aldosterone system). The criteria for exclusion were known nondiabetic kidney disease, urinary albumin creatinine ratio of >3500 mg/g, estimated GFR <30 ml/min per 1.73 m2 (7), chronic urinary tract infection, >5.1 mmol/L serum potassium at the time of randomization, severe hypertension, or major cardiovascular disease within the last 6 months.
In a randomized, double-blind, placebo-controlled study conducted in 15 countries and 150 centers worldwide, we evaluated the possible renoprotective effect of aliskiren in 599 hypertensive patients with type 2 diabetes and nephropathy. The methods have been described in detail in the main publication (6). In brief, we screened 1892 potentially eligible patients at an enrollment visit. Subsequently 805 patients entered a 3-month open-label period, during which all of the previous drugs that block the renin-angiotensin-aldosterone system were discontinued, except for β-blockers, and treatment was initiated with the maximal recommended renoprotective dose of losartan (100 mg daily) plus additional antihypertensive therapy aiming at an optimal target BP, i.e. below 130/80 mmHg. During the 3-month open-label treatment period, 206 patients were excluded, leaving 599 randomized patients who were followed for a median of 6 months. The patients were randomly assigned to receive aliskiren at a dose of 150 mg once daily for 3 months, followed by 300 mg of aliskiren daily for another 3-month period or matching placebo once daily. The study protocol was in accordance with the Declaration of Helsinki (2002) and was approved by local and central review boards. All of the patients provided written informed consent.
The patients were examined 13, 12, 8, 4, and 2 weeks before randomization; at randomization; and 1, 4, 8, 11, 12, 16, and 24 weeks after randomization. BP and pulse, adverse events, concomitant medications, and adherence to medications were assessed at each visit. Three early morning spot urine samples were collected on three sequential days at: 13 and 2 weeks before randomization and 4, 8, 12, 16, and 24 weeks after randomization. The urinary albumin concentration was determined by immunoturbidimetry (8), and the serum creatinine concentration by Jaffe reaction was determined by a Roche kit (9). The old Modification of Diet in Renal Disease formula was used to estimate the GFR (7). Glycosylated hemoglobin was measured by Bio-Rad HPLC (10). All of the remaining laboratory variables were also measured centrally applying conventional laboratory techniques.
Seated BP was assessed by standard mercury sphygmomanometers with appropriate cuff size after at least 5 minutes of rest. Three measurements were obtained, two minutes apart at each time point, and the average of the three was used for calculation of the 24-hour trough level (24 hours postdose). To attain BP equality, target BP during the open-label and double-blind period was less than 130/80 mmHg for both groups. Consequently, further adjustments in BP-lowering medication were recommended after randomization. All classes of BP-lowering drugs were allowed, except for drugs blocking the renin-angiotensin-aldosterone system.
The patient population was divided in three groups (groups A, B, and C) according to their baseline BP level achieved at time of randomization, after the 3-month run-in period with a focus on optimizing antihypertensive treatment: Group A (prespecified BP target), <130/80 mmHg (n = 159); Group B (intermediate BP control), <140/90 mmHg but ≥130/80 mmHg (n = 189); and Group C (insufficient BP control), ≥140/90 mmHg (n = 251). We investigated the same efficacy measures as in the main study. The primary endpoint was urinary albumin:creatinine ratio (UACR) reduction from baseline to end of study, and secondary endpoints included proportion of patients achieving a 50% reduction in UACR, change in eGFR, and change in systolic and diastolic BP from baseline to end of study. In addition, we assessed the frequency of adverse events in the three groups.
Statistical Analysis
Baseline variables were assessed and compared between the two treatment groups according to BP subgroup. For continuous variables, the P value is from a t test for each subgroup. For categorical variables, if all of the expected cell counts were at least five across all subgroups, the P value is from a chi-square test for each subgroup. Otherwise, the P value is from a Fisher's exact test for each subgroup.
The intent-to-treat population was the analysis population for efficacy analysis at the week 24 endpoint. The intent-to-treat population consisted of all of the randomized patients who had baseline and at least one post-baseline efficacy measurement. If the value at week 24 was missing, last observation carried forward was used. The safety population was used for safety analyses. The safety population consisted of all of the randomized patients who received at least one dose of double-blind trial medication. UACR reduction from baseline was analyzed using a logistic regression model with treatment, region, and subgroup as factors, with log-transformed baseline UACR as a covariate and treatment by subgroup interaction. Change from baseline eGFR was analyzed using analysis of covariance with treatment, region, baseline proteinuria classification, and subgroup as factors; baseline eGFR as a covariate; and treatment by subgroup as an interaction. Supplementary analysis was performed using baseline systolic BP as a continuous variable investigating the relationship between BP at baseline and antiproteinuric response to aliskiren treatment. In addition, the correlation between eGFR decline and systolic BP in aliskiren- and placebo-treated patients in Group C was calculated.
Results
The baseline characteristics of the three groups can be seen in Table 1. There were no statistically significant between-treatment differences in clinical or laboratory values in any of the BP subgroups. Mean baseline BP in subgroup A (<130/80 mmHg) was 121/71 mmHg in the aliskiren group and 120/70 mmHg in the placebo group, indicating that these patients were indeed well controlled with regards to BP at baseline. In group B (<140/90 mmHg but ≥130/80 mmHg), mean baseline BP was 132/78 mmHg in the aliskiren group and 134/78 mmHg in the placebo group. In group C (≥140/90 mmHg), baseline BP in the aliskiren group was 145/81 mmHg versus 144/80 mmHg in the placebo group (Table 1).
Table 1.
Baseline characteristics of the randomized population according to baseline BP group
| Aliskiren | Placebo | P | |
|---|---|---|---|
| Group A, <130/80 mmHg (n) | 76 | 83 | |
| age (years) | 58.4 (10.1) | 60.5 (10.7) | 0.195 |
| males (%) | 51 (67.1) | 61 (73.5) | 0.378 |
| Caucasian (%) | 63 (82.9) | 73 (88.0) | 0.830 |
| systolic blood pressure (mmHg) | 120.5 (6.5) | 119.9 (8.1) | 0.601 |
| diastolic blood pressure (mmHg) | 71.0 (6.5) | 70.1 (6.6) | 0.375 |
| UACR (mg/g) | 472.9 (89.3 to 3121.4) | 495.0 (100.8 to 3102.8) | 0.677 |
| S-creatinine (μmol/L) | 113.3 (43.8) | 112.5 (43.4) | 0.905 |
| eGFR (ml/min/1.73 m2) | 66.4 (26.2) | 67.2 (25.6) | 0.846 |
| S-potassium (mmol/L) | 4.5 (0.53) | 4.5 (0.45) | 0.620 |
| glycated hemoglobin (%) | 8.0 (1.6) | 7.8 (1.6) | 0.551 |
| Group B, <140/90 mmHg ≥130/80 mmHg (n) | 94 | 95 | |
| age (years) | 59.2 (9.60) | 62.4 (9.73) | 0.023 |
| males (%) | 62 (66.0) | 72 (75.8) | 0.137 |
| Caucasian (%) | 82 (87.2) | 85 (89.5) | 0.654 |
| systolic blood pressure (mmHg) | 132.2 (6.0) | 133.5 (4.8) | 0.100 |
| diastolic blood pressure (mmHg) | 77.7 (6.7) | 77.6 (6.2) | 0.974 |
| UACR (mg/g) | 541.9 (105.2 to 2920.7) | 475.6 (102.5 to 2841.2) | 0.918 |
| S-creatinine (μmol/L) | 106.0 (36.1) | 113.7 (38.7) | 0.157 |
| eGFR (ml/min/1.73 m2) | 69.4 (26.3) | 66.3 (24.5) | 0.826 |
| S-potassium (mmol/L) | 4.5 (0.53) | 4.5 (0.50) | 0.898 |
| glycated hemoglobin (%) | 8.1 (1.2) | 8.0 (1.3) | 0.458 |
| Group C, ≥140/90 mmHg (n) | 131 | 120 | |
| age (years) | 61.0 (9.13) | 62.2 (8.74) | 0.277 |
| males (%) | 93 (71.0) | 88 (73.3) | 0.680 |
| Caucasian (%) | 114 (87.0) | 103 (85.8) | 0.113 |
| systolic blood pressure (mmHg) | 144.8 (6.2) | 144.2 (5.5) | 0.390 |
| diastolic blood pressure (mmHg) | 81.4 (7.9) | 80.4 (8.7) | 0.352 |
| UACR (mg/g) | 519.8 (100.8 to 3175.3) | 628.5 (108.7 to 3388.4) | 0.786 |
| S-creatinine (μmol/L) | 106.8 (39.7) | 110.3 (36.8) | 0.479 |
| eGFR (ml/min/1.73 m2) | 69.2 (25.0) | 67.0 (23.9) | 0.483 |
| S-potassium (mmol/L) | 4.5 (0.50) | 4.5 (0.48) | 0.441 |
| glycated hemoglobin (%) | 8.0 (1.5) | 7.8 (1.4) | 0.279 |
The values are the means ± SD, except for the UACR and eGFR values, which indicate the median (range).
Table 2 displays the difference between treatment effects in each baseline BP subgroup. The antiproteinuric effect of aliskiren treatment was consistent across baseline BP groups (between-treatment reductions of 19 to 22%), although it was statistically significant only in group C (P = 0.044). The proportion of patients achieving more than 50% reduction in UACR was consistently larger in the aliskiren-treated group (Table 2), from 23 to 26% in the aliskiren-treated patients as compared with 11 to 14% in the placebo group.
Table 2.
Endpoints
| Aliskiren | Placebo | P | |
|---|---|---|---|
| Group A, <130/80 mmHg (n) | 69 | 79 | |
| UACR change at 24 weeks (%) | −22 (−36, −5) | 0 (−17, 12) | 0.066 |
| UACR reduction ≥50 n (%) | 18 (26.1) | 10 (12.7) | 0.036 |
| decline in eGFR (ml/min/1.73 m2) per 6 months | 1.17 (1.41) | 1.75 (1.31) | 0.743 |
| blood pressure change SBP/DBP (mmHg) | 2.3/0.3 | 2.2/1.8 | 0.985/0.203 |
| Group B, <140/90 mmHg ≥130/80 mmHg (n) | 90 | 94 | |
| UACR change at 24 weeks (%) | −11 (−24, 6) | 12 (−5, 32) | 0.062 |
| UACR reduction ≥50 n (%) | 21 (23.3) | 10 (10.6) | 0.027 |
| decline in eGFR (ml/min/1.73 m2) per 6 months | 2.51 (1.22) | 2.74 (1.24) | 0.887 |
| blood pressure change SBP/DBP (mmHg) | 0.8/−0.6 | 3.7/0.8 | 0.137/0.194 |
| Group C, ≥140/90 mmHg (n) | 128 | 116 | |
| UACR change at 24 weeks (%) | −21 (−31, −9) | −3 (−16, 13) | 0.044 |
| UACR reduction ≥50 n (%) | 32 (25.0) | 16 (13.8) | 0.027 |
| decline in eGFR (ml/min/1.73 m2) per 6 months | 1.96 (1.03) | 5.47 (1.09) | 0.013 |
| blood pressure change SBP/DBP (mmHg) | 0.6/−0.5 | 3.1/−0.1 | 0.141/0.626 |
The values are the means ± SD except for the UACR change, which indicate the median (range).
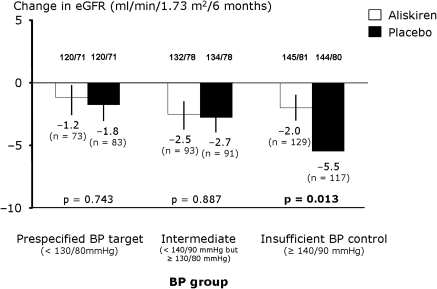
The rate of decline in eGFR over the study course of 6 months increased with increasing baseline BP in the placebo group; however, there was only a significant difference between treatment groups in subgroup C, where aliskiren-treated patients had an eGFR reduction of 2.0 ml/min per 1.73 m2 as compared with 5.5 ml/min per 1.73 m2 in the placebo group (P = 0.013) (Figure 1). There was no relationship between baseline BP, when used as a continuous variable, and antiproteinuric response to treatment. In addition, there was no correlation between eGFR decline and baseline systolic BP in aliskiren and placebo-treated patients in Group C.
Figure 1.
Change in eGFR according to baseline BP. Shown are the analyses performed on patients with available creatinine values at baseline and at the end of study (n = 586).
In all of the baseline BP subgroups, we observed an increase in SBP ranging from 0.6 to 2.3 mmHg in the aliskiren group and from 2.2 to 3.7 mmHg in the placebo group during the 6-month study duration, although the trend was toward a larger increase in the placebo groups (Table 2). Diastolic BP displayed minor reductions or was unchanged during the study (Table 2). The proportion of β-blockers and calcium channel blockers used was not significantly different between aliskiren- and placebo-treated patients in any BP group; however, information regarding the doses used was not available.
Adverse events had similar frequencies in all baseline BP subgroups, with no differences between treatment groups (Table 3). Of note, however, 9.2% of the aliskiren-treated patients in group A reported hypotension (investigator defined) as compared with none in the placebo group (P = 0.005). No other single adverse event was reported frequently (more than 2%) in any group.
Table 3.
Adverse events according to baseline BP group
| Aliskiren | Placebo | P | |
|---|---|---|---|
| Group A, <130/80 mmHg | |||
| S-potassium >6.0 mmol/L | 4 (5.3) | 1 (1.2) | 0.191 |
| S-potassium >5.5 mmol/L | 9 (12.0) | 4 (4.8) | 0.147 |
| any adverse event | 50 (65.8) | 61 (73.5) | 0.291 |
| any serious adverse event | 10 (13.2) | 9 (10.8) | 0.653 |
| discontinuations due to an adverse event | 2 (2.6) | 4 (4.8) | 0.683 |
| hypotension | 7 (9.2) | 0 | 0.005 |
| Group B, <140/90 mmHg ≥130/80 mmHg | |||
| S-potassium >6.0 mmol/L | 4 (4.3) | 0 | 0.058 |
| S-potassium >5.5 mmol/L | 11 (11.8) | 14 (14.7) | 0.557 |
| any adverse event | 65 (69.1) | 65 (68.4) | 0.914 |
| any serious adverse event | 6 (6.4) | 6 (6.3) | 0.985 |
| discontinuations due to an adverse event | 4 (4.3) | 5 (5.3) | 0.999 |
| hypotension | 2 (2.1) | 1 (1.1) | 0.621 |
| Group C, ≥140/90 mmHg | |||
| S-potassium >6.0 mmol/L | 6 (4.6) | 4 (3.4) | 0.752 |
| S-potassium >5.5 mmol/L | 21 (16.0) | 14 (11.8) | 0.332 |
| any adverse event | 86 (65.6) | 74 (61.7) | 0.512 |
| any serious adverse event | 11 (8.4) | 13 (10.8) | 0.512 |
| discontinuations due to an adverse event | 11 (8.4) | 10 (8.3) | 0.986 |
| hypotension | 3 (2.3) | 2 (1.7) | 0.999 |
The values are n (%). A patient with multiple occurrences of an adverse event (AE) under one treatment is counted only once in the AE category for that treatment.
Discussion
In our post hoc analysis of the AVOID study, a randomized double-blind placebo controlled study with aliskiren in hypertensive patients with type 2 diabetes and nephropathy, we found that the antiproteinuric effect of aliskiren added to standard treatment, including the recommended renoprotective dose of losartan (11), is equal at different levels of baseline BP control. Also consistent across the BP control groups was the statistically significant difference in number of patients with a >50% reduction in UACR from baseline to end of study, between aliskiren- and placebo-treated patients. This indicates that large reductions in UACR can be achieved by adding aliskiren treatment with 300 mg once daily to standard therapy, irrespective of pretreatment BP level. This may prove an important point in the search for therapies and drug combinations that further lower albuminuria in patients at risk for both renal and cardiovascular morbidity and mortality.
During the 6-month study, the change in eGFR was similar between treatments in the two groups with the best BP control at baseline, but in group C (≥140/90 mmHg), the placebo-treated patients had a significantly larger drop in eGFR (5.5 versus 2.0 ml/min per 1.73 m2 per 6 months, P = 0.013), suggesting that patients who need the BP-lowering treatment the most also have the greatest benefit from it.
The increase in systolic BP (SBP) in group C during the study in the placebo group was 3.1 mmHg as compared with 0.6 mmHg in the aliskiren-treated patients. Elevated BP is a risk factor for loss of kidney function (12), and the more pronounced decline in eGFR in the placebo group in patients with baseline BP ≥140/90 mmHg may partly be due to the BP difference during the study.
One important finding with clinical implications is the fact that there were significantly more reported symptoms of hypotension among the aliskiren-treated patients in Group A as compared with placebo patients, but no patient in group A experienced hypotension that led to discontinuation. This warrants special attention to hypotension by clinicians who decide to add aliskiren to well-controlled hypertensive diabetes patients with the aim of reducing albuminuria. Other adverse events were infrequent in all of the baseline BP groups.
BP reduction in our study was less than in previous studies with aliskiren used without and with an angiotensin-receptor blocker (ARB) as mono or dual therapy, but in our study many patients were treated with concomitant antihypertensive treatment aiming to reach a BP target of 130/80 mmHg. This fact may explain the minor differences in BP during the study. In type 2 diabetes, some post hoc analyses of randomized, intervention trials have studied the impact of different levels of BP control in relation to renal events.
The BENEDICT study was carried out in 1204 hypertensive patients with type 2 diabetes and normoalbuminuria for 3.6 years of follow-up (13). The primary endpoint was progression to microalbuminuria, and the post hoc analysis showed that follow-up SBP below the median of 139 mmHg resulted in slower rate of progression from normo- to microalbuminuria. Also diastolic BP (DBP), mean arterial pressure (MAP), and pulse pressure were associated with the risk of developing microalbuminuria, but follow-up SBP appeared to be the strongest predictor.
The ADVANCE trial in patients with type 2 diabetes showed renal benefits across all levels of baseline BP, most pronounced among individuals with baseline SBP levels <120 mmHg (1). The renal endpoint used was a composite of renal events defined by new-onset microalbuminuria, new-onset macroalbuminuria, doubling of serum creatinine, or end-stage kidney disease.
In a population with type 2 diabetes and overt nephropathy in a post hoc analysis of the Irbesartan Diabetic Nephropathy Trial, Pohl et al. (14) found that follow-up SBP was the strongest predictor of renal disease progression (development of doubling in s-creatinine or ESRD) as compared with DBP, mean arterial pressure (MAP), or pulse pressure. In a similar analysis of the RENAAL study, Bakris et al. (15) found that follow-up SBP above 140 mmHg had a higher rate of progression of nephropathy as compared with patients achieving SBP values below 130 mmHg.
Taken together, the abovementioned studies point to BP control as an important factor with regards to prevention of renal events. The studies do not report changes in renal function and have different endpoints compared with our study, but reduction in albuminuria is widely accepted as a surrogate renal endpoint associated with a decline in renal function and development of cardiovascular disease (16,17). Nevertheless, further hard endpoint studies investigating renal benefits of direct renin inhibition are needed (18).
From physiologic studies, it is known that different forms of RAAS blocking treatment can normalize directly measured or estimated glomerular capillary hydraulic pressure (19–21), reduce the shunt-like defects in the membrane (22), and restore the charge-selectivity properties of the glomerular membrane (23). Whether renin inhibition in combination with angiotensin II receptor blockade, as used in the AVOID study, has additive beneficial effects on these variables is not known.
However, aliskiren treatment seems to have an angiotensin II-dependent vasodilatatory effect on the efferent arteriole in the glomerulus that causes an increase in renal plasma flow and a drop in filtration fraction that exceeds the effect seen previously with angiotensin-converting enzymes and ARBs (24). The animal studies indicate that aliskiren partitions to the kidney and exhibits a prolonged renal residence, which is not seen with angiotensin-converting enzymes or ARBs (25). In addition, aliskiren inhibits the intrarenal RAAS even several days after cessation of treatment (25). We have previously demonstrated a synergistic response to combination treatment with aliskiren and irbesartan, as measured by a 12-fold increase in plasma renin concentration, pointing to increased intrarenal RAAS blockade (5).
All of the BP measurements in the study were office BP, so it can be speculated that a difference in nighttime BP levels could influence the results, but this could not be determined in our study. In stage 2 hypertension, the combination of 300 mg of aliskiren once daily and 320 mg of valsartan was significantly more effective in lowering mean 24-hour ambulatory systolic and diastolic BP than was either agent alone (26). This could be due to the long half-life of aliskiren (about 40 hours). The question of the effect of aliskiren treatment on ambulatory BP is important and will be investigated further.
Albuminuria remains the biomarker of choice when evaluating combination RAAS blockade but may have limitations. This has been debated since the publication of the ONTARGET study (27). Although the ONTARGET study included a mixed cardiovascular high-risk population with an average urinary albumin creatinine ratio of 7.2 mg/g, our study was performed in type 2 diabetes with nephropathy. Furthermore, a recent post hoc analysis of the ONTARGET data revealed that “changes in albuminuria predict cardiovascular and renal outcome and mortality independent of baseline albuminuria” (28). In addition, the residual level of albuminuria during RAAS blockade also independently of the baseline albuminuria level determines the renal outcome risk (16). Because this is a post hoc analysis, the conclusions to be made from it must be regarded as a hypothesis-generating observation that should be used to guide future research rather than clinical practice. Furthermore, whereas albuminuria remains the biomarker of choice for studies of novel agents, less evidence is available when new agents are added to standard treatments that already reduce albuminuria, such as angiotensin-receptor blockade. Nevertheless, these are data from the largest randomized study with aliskiren treatment of patients with type 2 diabetes so far, and more evidence regarding the impact of aliskiren treatment on cardiorenal endpoints will be available when the ALTITUDE study is completed (18).
Aliskiren (300 mg daily) added to losartan (100 mg daily) and recommended antihypertensive treatment reduced UACR (19 to 22% reduction versus placebo) independent of baseline BP level and BP during the trial and attenuated decline in eGFR in the group with insufficiently treated baseline BP above 140/90 mmHg. Hypotension was more frequent in the aliskiren with baseline BP <130/80 mmHg. With proper attention to this adverse event, we conclude that the use of 300 mg of aliskiren once daily in this setting can be applied safely and with clinically relevant efficacy across a broad range of BP levels in hypertensive type 2 diabetic patients with nephropathy.
Disclosures
Dr. Persson reports having received lecture fees from Novartis and having equity interest in NovoNordisk. Dr. Rossing reports having received lecture fees from Novartis and Boehringer Ingelheim and a research grant from Novartis, serving as a consultant for Merck, and having equity interest in NovoNordisk. Dr. E. Lewis reports having received grant support from Keryx Biopharmaceuticals. Dr. J. B. Lewis reports having served as a consultant for Merck and Novartis and having received grant support from Keryx Biopharmaceuticals and the National Institutes of Health. Dr. Hollenberg reports having received grant support from Novartis and Merck. Dr. Parving reports having served as a consultant for Novartis, Merck, Pfizer, and Sanofi-Aventis, having equity interest in Merck and NovoNordisk, and having received lecture fees from Novartis, Merck, Pfizer, and Sanofi-Aventis. Dr. Parving has received grant support from Novartis, AstraZeneca, and Sanofi-Aventis.
Acknowledgments
This study was funded by Novartis Pharma AG. Presented at American Society of Nephrology Renal Week 2009 and at the European Association for the Study of Diabetes Annual Meeting 2010.
Footnotes
The investigators who participated in the AVOID (Aliskiren in the Evaluation of Proteinuria in Diabetes) study are listed in the online supplemental material.
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org.
References
- 1. de Galan BE, Perkovic V, Ninomiya T, Pillai A, Patel A, Cass A, Neal B, Poulter N, Harrap S, Mogensen CE, Cooper M, Marre M, Williams B, Hamet P, Mancia G, Woodward M, Glasziou P, Grobbee DE, MacMahon S, Chalmers J: Lowering blood pressure reduces renal events in type 2 diabetes. J Am Soc Nephrol 20: 883–892, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eijkelkamp WBA, Zhang Z, Remuzzi G, Parving H-H, Cooper ME, Keane WF, Shahinfar S, Gleim GW, Weir MR, Brenner BM, de Zeeuw D: Albuminuria is a target for renoprotective therapy independent from blood pressure in patients with type 2 diabetic nephropathy: Post hoc analysis from the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) trial. J Am Soc Nephrol 18: 1540–1546, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP: Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 111: 1012–1018, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Persson F, Rossing P, Schjoedt KJ, Juhl T, Tarnow L, Stehouwer CD, Schalkwijk C, Boomsma F, Frandsen E, Parving H-H: Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int 73: 1419–1425, 2008 [DOI] [PubMed] [Google Scholar]
- 5. Persson F, Rossing P, Reinhard H, Juhl T, Stehouwer CDA, Schalkwijk C, Danser AHJ, Boomsma F, Frandsen E, Parving H-H: Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care 32: 1873–1879, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parving H-H, Persson F, Lewis JB, Lewis EJ, Hollenberg NK: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Multicenter study of Tina-quant Albumin in urine and a-N-acetyl-glucosaminidase (a-NAG) in urine. Wien klin Wschr 103: 1–64, 1991. 1673041 [Google Scholar]
- 9. Bartels H, Böhmer M, Heierli C: Serum kreatininbestimmung ohne enteiweissen. Clin Chim Acta 37: 193–197, 1972 [DOI] [PubMed] [Google Scholar]
- 10. Rohlfing CL, Little RR, Wiedmeyer HM, England JD, Madsen R, Harris MI, Flegal KM, Eberhardt MS, Goldstein DE: Use of GHb (HbA1c) in screening for undiagnosed diabetes in the U.S. population. Diabetes Care 23: 187–191, 2000 [DOI] [PubMed] [Google Scholar]
- 11. Andersen S, Rossing P, Juhl TR, Deinum J, Parving H-H: Optimal dose of losartan for renoprotection in diabetic nephropathy. Nephrol Dial Transplant 17: 1413–1418, 2002 [DOI] [PubMed] [Google Scholar]
- 12. Rossing K, Christensen PK, Hovind P, Tarnow L, Rossing P, Parving H-H: Progression of nephropathy in type 2 diabetic patients. Kidney Int 66: 1596–1605, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Ruggenenti P, Perna A, Ganeva M, Ene-Iordache B, Remuzzi G. for the BENEDICT Study Group: Impact of blood pressure control and angiotensin-converting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: A post hoc analysis of the BENEDICT trial. J Am Soc Nephrol 17: 3472–3481, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Pohl MA, Blumenthal S, Cordonnier DJ, De Alvaro F, DeFerrari G, Eisner G, Esmatjes E, Gilbert RE, Hunsicker LG, de Faria JBL, Mangili R, Moore J, Jr, Reisin E, Ritz E, Schernthaner G, Spitalewitz S, Tindall H, Rodby RA, Lewis EJ. for the Collaborative Study Group: Independent and additive impact of blood pressure control and angiotensin II receptor blockade on renal outcomes in the irbesartan diabetic nephropathy trial: Clinical implications and limitations. J Am Soc Nephrol 16: 3027–3037, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Bakris GL, Weir MR, Shanifar S, Zhang Z, Douglas J, van Dijk DJ, Brenner BM: Effects of blood pressure level on progression of diabetic nephropathy: Results from the RENAAL study. Arch Intern Med 163: 1555–1565, 2003 [DOI] [PubMed] [Google Scholar]
- 16. de Zeeuw D, Remuzzi G, Parving H-H, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: Lessons from RENAAL. Kidney Int 65: 2309–2320, 2004 [DOI] [PubMed] [Google Scholar]
- 17. de Zeeuw D, Remuzzi G, Parving H-H, Keane WF, Zhang Z, Shahinfar S, Snapinn S, Cooper ME, Mitch WE, Brenner BM: Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110: 921–927, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Parving H-H, Brenner BM, McMurray JJ, de ZD, Haffner SM, Solomon SD, Chaturvedi N, Ghadanfar M, Weissbach N, Xiang Z, Armbrecht J, Pfeffer MA: Aliskiren trial in type 2 diabetes using cardio-renal endpoints (ALTITUDE): Rationale and study design. Nephrol Dial Transplant 24: 1663–1671, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Hostetter TH, Troy JL, Brenner BM: Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int 19: 410–415, 1981 [DOI] [PubMed] [Google Scholar]
- 20. Imanishi M, Yoshioka K, Konishi Y, Okumura M, Okada N, Sato T, Tanaka S, Fujii S, Kimura G: Glomerular hypertension as one cause of albuminuria in type II diabetic patients. Diabetologia 42: 999–1005, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Trevisan R, Tiengo A: Effect of low-dose ramipril on microalbuminuria in normotensive or mild hypertensive non-insulin-dependent diabetic patients. North-East Italy Microalbuminuria Study Group. Am J Hypertens 8: 876–883, 1995 [DOI] [PubMed] [Google Scholar]
- 22. Andersen S, Blouch K, Bialek J, Deckert M, Parving H-H, Myers BD: Glomerular permselectivity in early stages of overt diabetic nephropathy. Kidney Int 58: 2129–2137, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Langham RG, Kelly DJ, Cox AJ, Thomson NM, Holthofer H, Zaoui P, Pinel N, Cordonnier DJ, Gilbert RE: Proteinuria and the expression of the podocyte slit diaphragm protein, nephrin, in diabetic nephropathy: Effects of angiotensin converting enzyme inhibition. Diabetologia 45: 1572–1576, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Fisher NDL, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK: Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 117: 3199–3205, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Feldman DL: New insights into the renoprotective actions of the renin inhibitor aliskiren in experimental renal disease. Hypertens Res 33: 279–287, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Oparil S, Yarows SA, Patel S, Fang H, Zhang J, Satlin A: Efficacy and safety of combined use of aliskiren and valsartan in patients with hypertension: A randomised, double-blind trial. Lancet 370: 221–229, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, Schumacher H, Dagenais G, Sleight P, Anderson C: Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Schmieder RE, Mann JFE, Schumacher H, Gao P, Mancia G, Weber MA, McQueen M, Teo KK, Yusuf S: Changes in albuminuria predict mortality and morbidity in patients with vascular disease. J Hypertens 28: e602, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]