Abstract
Summary
Background and objectives
Restless legs syndrome (RLS) and sleep disturbances are common among in-center hemodialysis patients and are associated with increased morbidity/mortality.
Design, setting, participants, & measurements
The FREEDOM study is an ongoing prospective cohort study investigating the benefits of home short daily hemodialysis (SDHD) (6 times/week). In this interim report, we examine the long-term effect of SDHD on the prevalence and severity of RLS, as measured by the International Restless Legs Syndrome (IRLS) Study Group rating scale, and sleep disturbances, as measured by the Medical Outcomes Study sleep survey.
Results
235 participants were included in this report (intention-to-treat cohort), of which 127 completed the 12-month follow-up (per-protocol cohort). Mean age was 52 years, 55% had an arteriovenous fistula, and 40% suffered from RLS. In the per-protocol analysis, among patients with RLS, the mean IRLS score improved significantly at month 12, after adjustment for use of RLS-related medications (18 versus 11). Among patients with moderate-to-severe RLS (IRLS score ≥15), there was an even greater improvement in the IRLS score (23 versus 13). The intention-to-treat analysis yielded similar results. Over 12 months, there was decline in the percentage of patients reporting RLS (35% versus 26%) and those reporting moderate-to-severe RLS (59% versus 43%). There was a similar and sustained 12-month improvement in several scales of the sleep survey, after adjustment for presence of RLS and use of anxiolytics and hypnotics.
Conclusions
Home SDHD is associated with long-term improvement in the prevalence and severity of RLS and sleep disturbances.
Introduction
Restless legs symptoms are relatively common in the hemodialysis (HD) population, ranging from 6 to 62% (1–4). These distressing symptoms are characterized by achy or crawling paresthesias, typically in the lower extremities, which are relieved by movement of the affected limb. As a consequence, most patients with restless legs symptoms suffer from sleep disturbances resulting in sleep fragmentation and sleep deprivation (3), anxiety, and depressive symptoms (5). Observational studies have linked restless legs syndrome (RLS) to premature discontinuation of dialysis (2), impaired quality of life (QoL) (4), increased risk of cardiovascular events (6), and increased risk of death (4,6). Pharmacologic treatment of RLS in patients with kidney failure is challenging because novel agents are often not approved for use in this population.
Poor sleep quality is also common among patients on conventional HD, ranging from 41 to 83% (7–9). The most frequently reported symptoms include insomnia, sleep-disordered breathing, and excessive daytime sleepiness. Poor sleep quality in the HD population has been linked to lower QoL measures and increased mortality risk (8).
Frequent HD has been shown to improve a number of QoL indicators (10–13). The FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study is an ongoing United States-based observational study examining the potential clinical and economic benefits of home short daily HD (SDHD) (14). We have previously demonstrated that SDHD is associated with a sustained improvement in depressive symptoms and postdialysis recovery time, a measure of postdialysis fatigue (15). In this interim report, as part of an a priori planned analysis, we examine the long-term effect of SDHD on the prevalence and severity of RLS, as well as on sleep disturbances. We hypothesized that SDHD would result in sustained improvement in the severity of restless legs symptoms and sleep disturbances.
Patients and Methods
Study Design and Setting
This was a multi-center, prospective, cohort study of SDHD with a planned 12-month follow-up (ClinicalTrials.gov identifier, NCT00288613) (14). Eligible patients were adults (age, ≥18 years) with end-stage renal disease requiring dialysis who were being initiated on SDHD (prescribed six times per week) at home and who had Medicare for their primary insurance payer. Exclusion criteria included current use of the device, prior enrollment in the study, current enrollment in an investigational drug or device trial that might affect the outcome measures, and low likelihood of surviving the first 4 to 6 weeks encompassing the training period. Written informed consent was obtained from all study participants. A central or local institutional review board approved the study protocol.
Data Collection
At enrollment, demographic information was collected, as well as clinical information on comorbid conditions, duration of dialysis, vascular access type, prior renal replacement therapy, dialysis prescription, and laboratory data (average of three recordings). A 24-hour timed urine collection was obtained at enrollment to assess residual urine volume.
Prescribed medications related to RLS and sleep disturbances were also collected at enrollment and at 4 and 12 months and included use of ropinirole/pramipexole (two drugs approved for the treatment of moderate to severe RLS); anxiolytics/muscle relaxants (alprazolam, buspirone, chlordiazepoxide, clonazepam, diazepam, and lorazepam); hypnotics (estazolam, eszopiclone, ramelteon, temazepam, trazodone, triazolam zaleplon, and zolpidem); carbidopa-levodopa; and gabapentin/pregabalin.
Short Daily HD Prescription
SDHD was delivered using the NxStage System OneTM cycler (NxStage Medical, Inc.). This device incorporates a high-flux polyethersulfone dialyzer and relies on an inverse blood-to-dialysate flow-rate ratio of 3 to 1 compared with a conventional dialysis machine. Lactate-based dialysate was delivered either in premixed, sterile, nonpyrogenic dialysate fluid bags or through an ultrapure water purification system. The initial SDHD prescription targeted a single pool Kt/Vurea of 0.45 to 0.50 on the basis of six treatments per week, using the Daugirdas second generation equation (16). This corresponded to a weekly standard Kt/Vurea of 2.0 to 2.2, meeting or exceeding the recommended threshold of 2.0 (17).
Assessment of RLS and Symptoms Severity
The presence of RLS was assessed at enrollment, and at 4 and 12 months, by administering the following question: “Do you have abnormal sensations in your legs or arms such as pins, needles, or ants crawling, and do you have an irresistible desire to move your limbs to relieve these abnormal sensations?” If RLS was present, study participants were invited to complete the International Restless Legs Syndrome (IRLS) Study Group Rating Scale (version 2.2) (18,19). The IRLS questionnaire is composed of 10 items, each consisting of a five-point Likert scale, which assesses the frequency and severity of RLS over the preceding week. Nine of the 10 items investigate two dimensions of RLS severity, symptoms (six items), and symptoms effect (three items). This self-administered instrument was developed and validated by the IRLS Study Group (18,20) and has been used to test the efficacy of drugs developed for treatment of RLS (21,22). Responses are graded from 0 to 4, with a higher score reflecting higher severity. The global score is obtained by summing the scores of all 10 items, with a maximum score of 40 used to assess the overall severity. For the symptoms and symptoms effect subscale, six and three items are completed to calculate the respective subscale score, yielding a scoring scale of 0 to 24 and 0 to 12, respectively.
During the study, the reduction in the severity of RLS was assessed by measuring mean change in the IRLS global score. For the purpose of our study, subjects with RLS at enrollment who completed the IRLS questionnaire and whose symptoms subsequently fully resolved were scored as 0 on the subsequent IRLS survey. Moderate-to-severe RLS was defined by an IRLS global score of ≥15 as previously reported (23).
Assessment of Sleep Disturbances
To assess for sleep disturbances, all of the study participants completed the sleep survey of the Medical Outcomes Study (MOS) (24,25). This self-administered instrument identifies various components of sleep habits. When taking the MOS sleep survey, study participants were instructed to relate responses to sleep habits over the preceding 4 weeks.
The sleep survey results were scored using the MOS Sleep Scale Manual (version 1.0) (26), which by combining select items calculated two separate sleep indices (sleep problems index 1 and 2) and five sleep scales (sleep adequacy, sleep disturbances, snoring, awaken short of breath or with a headache, and daytime somnolence). Each item was then converted to a 0 to 100 possible range, with a higher score reflecting more of the attribute implied by the scale name. The sleep survey also inquired about the number of hours of sleep and optimal sleep, as defined by 7 to 8 hours of sleep.
Sample Size Calculation
For this analysis, an a priori sample size calculation was performed to assess the change in the IRLS score over the study course (14). Sample size calculations were performed for the 12-month time point to achieve 80% power for a two-sided test with a type I error of 0.05, assuming a drop out rate of 30%. A minimum sample size of 43 was calculated on the basis of a mean IRLS global score improvement of 6 ± 9 (SD) units. This hypothesized mean score change was on the basis of pooled estimates observed in trials examining efficacy of pramipexole in the treatment of RLS compared with placebo (22).
Statistical Analyses
Continuous variables are presented as means ± SD or median with interquartile range, and categorical variables are presented as numbers with percentages. Baseline characteristics were compared using the unpaired t test or chi-squared test. For variables measured over two time points, comparisons were made by paired t test or Signed rank test, as appropriate, and the McNemar test (test of symmetry).
We performed a per-protocol and an intention-to-treat (ITT) analysis examining the effect of SDHD on the IRLS score and sleep disturbances. The per-protocol analysis was restricted to study participants who completed 12 months of follow-up. The ITT included all participants irrespective of early study discontinuation. For this conservative analysis, the missing values were imputed using the last observation carried forward. For both analyses, we used repeated measures analysis of covariance for changes in IRLS scores, using time as a covariate. For changes in sleep scales, we also used analysis of covariance, using baseline presence of RLS and usage of anxiolytics and hypnotics as fixed factors, and the interaction of time with medication usage as variable factors. A sensitivity analysis added the use of RLS-related medications (i.e. ropinirole/pramipexole, carbidopa-levodopa, and gabapentin/pregabalin) as a covariate to check for robustness of the findings. The results are displayed as adjusted means with 95% confidence interval. The percentage of patients experiencing optimal sleep was determined by generalized estimating equations with a binomial logit link function. All of the statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC). The differences were considered statistically significant at P < 0.05.
Results
Characteristics of the Cohort
Between January 23, 2006 and December 31, 2008, a total of 248 study participants were enrolled from 28 sites, of which nine were excluded because of consent withdrawal or study ineligibility. Of the remaining 239 participants, 235 completed the RLS survey and constituted the ITT cohort. Of the 235 participants included in this analysis, 108 discontinued before 12 months, of which 64 occurred before 4 months. Reasons for study discontinuation included kidney transplantation (n = 14), transfer out of participating dialysis center (n = 8), death (n = 11), modality change/return to in-center dialysis (n = 52), off-device for >6 weeks (n = 10), recovery of kidney function (n = 2), and other (n = 11). The remaining 127 participants completed 12 months of follow-up and constituted the per-protocol cohort.
Table 1 displays the enrollment characteristics of the 235 participants included in this report. Mean age was 52 years, 65% were men, 66% were white, 43% were diabetic, and 55% had a fistula. Mean duration of dialysis was 3.6 years, and residual urine volume was 387 ml/day. 4% of participants required assistance with daily activities, and 3% were unable to ambulate. There were no significant differences in the baseline characteristics among patients who completed 12 months of follow-up and those who did not (data not shown).
Table 1.
Baseline characteristics of the study cohort stratified by the presence or absence of RLS
| Characteristic | All Participants (n = 235) | Absence of RLS (n = 141) | Presence of RLS (n = 94) | P |
|---|---|---|---|---|
| Age, years | 52 ± 15 | 52 ± 16 | 53 ± 14 | 0.7 |
| Men | 150 (65) | 85 (62) | 65 (70) | 0.2 |
| Race | 0.002 | |||
| white | 153 (66) | 84 (61) | 69 (74) | |
| black | 70 (30) | 52 (38) | 18 (19) | |
| other | 8 (4) | 2 (1) | 6 (7) | |
| Prior renal replacement therapy | 0.04 | |||
| hemodialysis | 206 (90) | 127 (93) | 79 (85) | |
| peritoneal dialysis | 7 (3) | 3 (2) | 4 (4) | |
| kidney transplant | 5 (2) | 4 (3) | 1 (1) | |
| new to dialysis | 12 (5) | 3 (2) | 9 (10) | |
| Duration of dialysis, years | 3.6 ± 4.1 | 3.5 ± 3.7 | 3.8 ± 4.6 | 0.7 |
| Estimated dry weight, kg | 86 ± 23 | 85 ± 23 | 87 ± 24 | 0.6 |
| Body mass index, kg/m2 | 29 ± 7 | 29 ± 8 | 29 ± 7 | 0.9 |
| Residual urine volume, ml/day | 387 ± 631 | 360 ± 607 | 425 ± 667 | 0.5 |
| Vascular access type, % | 0.05 | |||
| arteriovenous fistula | 128 (55) | 68 (49) | 60 (64) | |
| arteriovenous graft | 37 (16) | 27 (20) | 10 (11) | |
| central venous catheter | 66 (29) | 43 (31) | 23 (25) | |
| Home hemodialysis training time, days | 27 ± 38 | 24 ± 12 | 31 ± 57 | 0.2 |
| Comorbid conditions | ||||
| hypertension | 203 (86) | 120 (85) | 83 (88) | 0.5 |
| diabetes mellitus | 100 (43) | 55 (39) | 45 (48) | 0.2 |
| congestive heart failure | 58 (25) | 34 (24) | 24 (26) | 0.8 |
| other cardiovascular disease | 55 (23) | 30 (21) | 25 (27) | 0.3 |
| cerebrovascular disease | 22 (9) | 14 (10) | 8 (9) | 0.7 |
| atherosclerotic heart disease | 44 (19) | 27 (19) | 17 (18) | 0.8 |
| peripheral vascular disease | 26 (11) | 17 (12) | 9 (10) | 0.6 |
| chronic obstructive lung disease | 16 (7) | 8 (6) | 8 (9) | 0.4 |
| cancer | 26 (11) | 14 (10) | 12 (13) | 0.5 |
| Current smoker | 29 (12) | 14 (10) | 15 (16) | 0.2 |
| Assistance with daily activities | 10 (4) | 4 (3) | 6 (6) | 0.2 |
| Inability to ambulate | 7 (3) | 6 (4) | 1 (1) | 0.2 |
| Selected prescribed medications | ||||
| pramipexole/ropinirole | 7 (3) | 1 (1) | 6 (7) | 0.02 |
| anxiolytics/muscle relaxants | 43 (19) | 21 (15) | 22 (24) | 0.09 |
| hypnotics/sleep medications | 35 (15) | 20 (15) | 15 (17) | 0.7 |
| carbidopa-levodopa | 3 (1) | 0 (0) | 3 (3) | 0.06 |
| gabapentin/pregabalin | 34 (15) | 15 (11) | 19 (21) | 0.06 |
| Selected laboratory values | ||||
| hemoglobin, g/dl | 11.7 ± 1.3 | 11.8 ± 1.2 | 11.6 ± 1.4 | 0.3 |
| serum ferritin, ng/ml | 517 ± 313 | 537 ± 349 | 490 ± 253 | 0.3 |
| transferrin saturation, % | 29 ± 12 | 29 ± 11 | 28 ± 13 | 0.6 |
| serum calcium, mg/dl | 8.9 ± 0.7 | 8.9 ± 0.6 | 8.9 ± 0.8 | 0.4 |
| serum phosphorus, mg/dl | 5.5 ± 1.4 | 5.3 ± 1.3 | 5.9 ± 1.5 | 0.002 |
| serum calcium-phosphorus product, mg2/dl2 | 52 ± 13 | 50 ± 12 | 56 ± 14 | 0.001 |
| serum intact parathyroid hormone, pg/ml | 465 ± 422 | 473 ± 449 | 453 ± 383 | 0.8 |
| serum urea nitrogen, mg/dl | 58 ± 17 | 57 ± 16 | 60 ± 18 | 0.2 |
| serum creatinine, mg/dl | 9.2 ± 3.9 | 9.1 ± 4.2 | 9.3 ± 3.4 | 0.7 |
| serum albumin, g/dl | 3.8 ± 0.5 | 3.8 ± 0.5 | 3.8 ± 0.5 | 0.9 |
| serum potassium, mEq/L | 4.8 ± 0.6 | 4.8 ± 0.6 | 4.9 ± 0.7 | 0.1 |
The data are presented as means ± standard deviation or numbers (percentages). The P values were calculated using unpaired t test or chi-squared test.
At enrollment, RLS was present among 94 (40%) of 235 participants. As shown in Table 1, there were no significant differences in most of the baseline characteristics among participants with and without RLS with the exception of white subjects, patients who were new to dialysis, and those with higher serum phosphorus and calcium-phosphorus product, who were more likely to self-report restless legs symptoms. In addition, participants with RLS were more likely to be prescribed ropinirole/pramipexole (P = 0.02), with a nonsignificant trend toward higher use of anxiolytics (P = 0.09), gabapentin/pregabalin (P = 0.06), and carbidopa-levodopa (P = 0.06).
Table 2 displays the individual components of the baseline sleep survey stratified by presence or absence of RLS. In brief, compared with those without RLS, participants with RLS had significantly higher baseline scores on several sleep scales including the sleep problems index 1 and 2 (P < 0.001 for both), sleep disturbances (P < 0.001), and daytime somnolence (P = 0.002). Participants with RLS also had nonsignificant trends toward higher baseline scores for snoring (P = 0.09), and waking up short of breath or with a headache (P = 0.08), all reflecting worse sleep quality and respiratory disturbances. Similarly, participants with RLS had lower sleep adequacy scores (P = 0.002) and reported slightly shorter sleep quantity (P = 0.07) and less optimal sleep (P = 0.06).
Table 2.
Baseline component scores of the MOS sleep survey stratified by the presence or absence of RLS
| Sleep Scale | All Participants (n = 235) | Absence of RLS (n = 141) | Presence of RLS (n = 94) | P |
|---|---|---|---|---|
| Sleep problems index 1 | 40 ± 22 | 36 ± 21 | 47 ± 21 | <0.001 |
| Sleep problems index 2 | 42 ± 22 | 38 ± 21 | 50 ± 21 | <0.001 |
| Sleep adequacy | 47 ± 28 | 51 ± 29 | 40 ± 25 | 0.002 |
| Sleep disturbances | 44 ± 29 | 38 ± 27 | 53 ± 29 | <0.001 |
| Snoring | 43 ± 35 | 40 ± 36 | 48 ± 34 | 0.09 |
| Awaken short of breath or with a headache | 17 ± 25 | 14 ± 23 | 20 ± 26 | 0.06 |
| Daytime somnolence | 43 ± 24 | 39 ± 24 | 49 ± 23 | 0.002 |
| Sleep quantity (hours) | 6.4 ± 1.9 | 6.6 ± 1.8 | 6.1 ± 2.0 | 0.07 |
| Optimal sleep (%) | 38 | 43 | 31 | 0.06 |
The data are presented as means ± standard deviation or percentages. The P values were calculated using unpaired t test or chi-squared test.
Effect of SDHD on RLS
At enrollment, the mean IRLS score was 19 (17,21) among participants with RLS, and 65% had an IRLS score ≥15, suggestive of moderate-to-severe RLS. The baseline IRLS scores were no different according to patient characteristics with the exception of those prescribed hypnotics, who had significantly higher scores compared with those who were not (26 ± 8 versus 17 ± 8; P = 0.001).
As shown in Table 3, in both the per-protocol (n = 46) and ITT (n = 94) analysis, SDHD resulted in a sustained improvement in the IRLS score at 4 and 12 months (P < 0.001). Indeed, in the per-protocol cohort, compared with baseline values, the mean IRLS score decreased by 5 to 7 points at month 4 (P = 0.002) and month 12 (P = 0.0001), respectively. These findings were attenuated but remained robust in the ITT analysis (Table 3; P < 0.0001). In a sensitivity analysis restricted to the 25 participants from the per-protocol cohort with baseline moderate-to-severe RLS, SDHD resulted in an even larger 8- to 9-point decrease in the mean IRLS score at 4 and 12 months (23 (21,25) versus 14 (9,20) versus 13 (8,18); P < 0.001). The ITT analysis yielded similar results among the 57 participants with baseline moderate-to-severe RLS, with a significant 6- to 7-point improvement over the same time period (P < 0.001). Of note, only two participants with a baseline IRLS score <15 witnessed an increase in their score to ≥15 at month 12.
Table 3.
Effect of short daily hemodialysis on the IRLS score
| IRLS Scale | Baseline | Month 4 | Month 12 | Global P |
|---|---|---|---|---|
| Global score | ||||
| per-protocol subcohort | 18 (16, 21) | 13 (10, 16) | 11 (8, 15) | 0.001 |
| ITT subcohort | 19 (17, 21) | 14 (12, 17) | 14 (11, 17) | <0.0001 |
| Symptoms severity subscale | ||||
| per-protocol subcohort | 12 (11, 14) | 8 (6, 11) | 8 (6, 10) | <0.001 |
| ITT subcohort | 13 (11, 14) | 9 (8, 11) | 9 (8, 11) | <0.0001 |
| Impact on daily living subscale | ||||
| per-protocol subcohort | 3 (3, 4) | 3 (2, 4) | 2 (1, 3) | 0.05 |
| ITT subcohort | 4 (3, 5) | 3 (3, 4) | 3 (2, 4) | 0.003 |
The data are presented as adjusted means (95% confidence interval). Adjustment variables include use of RLS medications (at any time for per-protocol analysis and at baseline for ITT analysis), time, and interaction of these two variables. The per-protocol subcohort includes 46 participants, and the ITT subcohort includes 94 participants who had RLS at baseline. The global P values are based on testing time (baseline, month 4, and month 12) as a fixed effect from repeated measures analysis of covariance. P < 0.02 for pair-wise comparisons of baseline versus month 4 and month 12 for the IRLS global score and its two subscales (in both per-protocol and ITT subcohort), with the exception of the impact on daily living subscale comparison of baseline versus month 4.
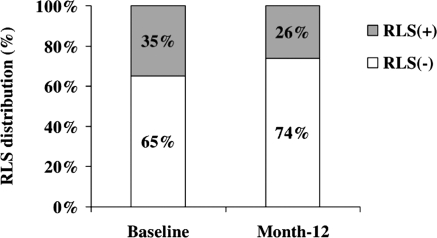
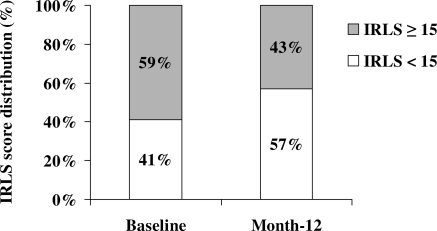
In the per-protocol cohort, the percentage of participants reporting restless legs symptoms decreased significantly from 35% at baseline to 26% at month 12 (P = 0.05; Figure 1) and the percentage of those suffering from moderate-to-severe RLS (IRLS score ≥15) decreased from 59% to 43% over the same time period (P = 0.06; Figure 2). Of note, the percentage of participants prescribed RLS-associated medications did not significantly change over the 12-month period (14% versus 18%; P = 0.2).
Figure 1.
Percentage of study participants with RLS at baseline and after 12 months of short daily hemodialysis (per-protocol cohort, n = 127). P = 0.05 by the McNemar test.
Figure 2.
Percentage of study participants with moderate-to-severe restless legs symptoms (defined by IRLS score, ≥15) at baseline and after 12 months of short daily hemodialysis (per-protocol subcohort, n = 46). P = 0.06 by the McNemar test.
Effect of SDHD on Sleep Disturbances
Although patients with RLS had a higher prevalence of sleep disturbances at baseline (Table 2), in both the per-protocol and ITT analysis, after adjustment for presence of RLS and use of hypnotics and anxiolytics, SDHD resulted in a significant improvement in the majority of the individual components of the sleep survey at 4 and 12 months, with the exception of a few domains (Table 4). In particular, in the per-protocol cohort, both the sleep problems index 1 and 2 scores decreased by 6 to 7 points at 4 and 12 months (P = 0.001). These findings were similar in the ITT analysis (P < 0.0001). Sleep adequacy improved significantly by 5 to 6 points in the ITT cohort at 4 and 12 months; however, the increase was not significant in the per-protocol cohort. Finally, SDHD had no effect on sleep quantity or gains of percent achieving optimal sleep.
Table 4.
Effect of short daily hemodialysis on components of the MOS sleep survey
| Sleep Scale | Baseline | Month 4 | Month 12 | Global P |
|---|---|---|---|---|
| Sleep problems index 1 | ||||
| per-protocol cohort | 39 (35, 43) | 33 (29, 37) | 33 (29, 36) | 0.001 |
| ITT cohort | 43 (40, 46) | 37 (34, 40) | 38 (35, 41) | <0.0001 |
| Sleep problems index 2 | ||||
| per-protocol cohort | 41 (37, 45) | 35 (31, 39) | 34 (31, 38) | <0.001 |
| ITT cohort | 45 (42, 48) | 39 (36, 42) | 39 (36, 42) | <0.0001 |
| Sleep adequacy | ||||
| per-protocol cohort | 49 (44, 54) | 52 (47, 57) | 53 (49, 58) | 0.23 |
| ITT cohort | 44 (41, 48) | 50 (46, 54) | 49 (46, 53) | 0.007 |
| Sleep disturbances | ||||
| per-protocol cohort | 43 (38, 48) | 38 (33, 43) | 36 (30, 41) | 0.004 |
| ITT cohort | 47 (43, 51) | 42 (37, 46) | 40 (36, 44) | <0.0001 |
| Snoring | ||||
| per-protocol cohort | 44 (37, 51) | 39 (32, 46) | 37 (30, 44) | 0.08 |
| ITT cohort | 46 (41, 51) | 44 (39, 49) | 44 (38, 49) | 0.4 |
| Awaken short of breath or with a headache | ||||
| per-protocol cohort | 17 (12, 21) | 9 (5, 13) | 12 (8, 16) | 0.002 |
| ITT cohort | 18 (14, 21) | 12 (9, 15) | 14 (11, 17) | <0.001 |
| Daytime somnolence | ||||
| per-protocol cohort | 39 (35, 43) | 30 (26, 34) | 32 (27, 36) | 0.0001 |
| ITT cohort | 44 (41, 47) | 38 (35, 42) | 39 (35, 43) | <0.0001 |
| Sleep quantity (hours) | ||||
| per-protocol cohort | 6.5 (6.2, 6.9) | 6.6 (6.3, 6.9) | 6.6 (6.2, 7.0) | 0.9 |
| ITT cohort | 6.3 (6.0, 6.6) | 6.5 (6.2, 6.7) | 6.4 (6.2, 6.7) | 0.3 |
| Optimal sleep (%) | ||||
| per-protocol cohort | 42 (34, 52) | 37 (29, 47) | 38 (29, 48) | 0.7 |
| ITT cohort | 37 (30, 44) | 35 (28, 43) | 35 (28, 42) | 0.9 |
The data are presented as adjusted means (95% confidence interval). The adjustment variables include use of anxiolytics and hypnotics, baseline presence of RLS, time, and interaction of time and use of medications. The per-protocol analysis includes 127 participants, and the ITT analysis includes 235 participants. The global P values are based on testing time (baseline, month 4, and month 12) as a fixed effect from repeated measures analysis of covariance. P ≤ 0.05 for pair-wise comparisons of baseline versus month 4 and month 12 for individual components of the MOS sleep survey (both per-protocol and ITT cohort), with the exception of the snoring scale, sleep quantity, and optimal sleep comparisons of baseline versus month 4 and month 12.
Of note, in the per-protocol cohort, the percentage of participants prescribed hypnotics or anxiolytics did not significantly change over the 12-month period (35% versus 37%; P = 0.7). Finally, in a sensitivity analysis, after adjustment for the presence of RLS and the use of RLS-related medications, anxiolytics, and hypnotics, SDHD remained associated with a significant improvement in the majority of the individual components of the sleep survey at 4 and 12 months in both the per-protocol and ITT analysis (data not shown).
Discussion
In the present interim report from the FREEDOM study, we demonstrate that over a 12-month period, the initiation of SDHD in the home setting, primarily in a prevalent dialysis population, results in sustained and clinically meaningful improvement in restless legs symptoms and sleep disturbances. Among patients with moderate-to-severe RLS (IRLS score, ≥15), a switch to SDHD resulted in an impressive IRLS score improvement of 8 to 9 points, exceeding the 6-point improvement observed in clinical trials examining the efficacy of RLS-related medications (22). In addition, after adjustment for the presence of RLS at baseline and the use of several psychotropic drugs, SDHD remained associated with a significant improvement in the majority of the sleep survey scales.
RLS is a distressing neurologic disorder characterized by an uncomfortable sensation in the legs, with an irresistible urge to move them. RLS can adversely affect sleep quality, often prompting patients to wake up during the night and have difficulty returning to sleep. Up to 80% of patients with RLS will also manifest the associated movement disorder periodic limb movement disorder, in which involuntary leg movements occur during sleep, thereby disturbing sleep quality (2).
RLS is common among patients undergoing maintenance dialysis, with a prevalence rate as high as 62% (1,2,4). Although its exact pathogenesis remains unclear, underdialysis, vitamin deficiencies, iron deficiency, and hyperparathyroidism have all been incriminated (4). However, in our study, we did not find a difference in the baseline serum ferritin or parathyroid hormone level among patients with and without RLS.
Treatment options for RLS are limited, and most are not successful at eliminating the symptoms. Although mild RLS may be treated with massage, relaxation methods, exercise, and changes to sleep environment, moderate-to-severe symptoms often require medications, which are challenging to use in patients on dialysis. Hypnotics, anxiolytics, carbidopa-levodopa, gabapentin, and pregabalin have also been used to medicate RLS, although more recently, ropinirole and pramipexole were approved specifically for the treatment of moderate-to-severe RLS.
In dialysis patients, RLS and sleep disturbances have been associated with increased morbidity and mortality. Indeed, in a small cohort of prevalent dialysis patients, the presence of RLS was associated with poor sleep measures including lengthening of sleep onset, increased number of nocturnal awakenings, and total sleep reduction, as well as a significantly increased mortality risk (2). In a large incident dialysis cohort, patients with severe RLS had lower QoL indicators, including lower physical and mental component summary scores, lower vitality, higher bodily pain, and lower sleep quality, and had a 39% risk increase in all-cause mortality (4). RLS has been linked to higher serum phosphorus levels, higher anxiety levels, and a greater degree of emotion-oriented coping (27), and more recently, RLS severity has been linked to de novo cardiovascular events and higher short-term mortality (6). Finally, in a large cohort of prevalent dialysis patients, poor sleep quality was associated with lower mental and physical component summary scores and a higher mortality risk (8).
To our knowledge, the FREEDOM study is the largest prospective cohort study of home SDHD. The strengths of our interim analysis include a large number of participants recruited from 28 sites, the use of two validated surveys to assess the QoL measures of interest, and the 12-month follow-up. The results are internally valid, because they remained robust on both the per-protocol and ITT analysis. In a recent randomized controlled trial, as compared with conventional thrice-weekly in-center HD, in-center SDHD (prescribed six times per week, with an achieved standard Kt/Vurea of 3.6) was associated with favorable changes in the composite coprimary outcomes of death or 12-month change in left ventricular mass and death or 12-month change in the RAND-36 physical-health composite score (13). It is worth noting that in our observational cohort study, home SDHD targeting a weekly standard Kt/Vurea of only 2.0 to 2.2 was associated with a significant improvement in several QoL indicators, including depressive symptoms, dialysis recovery time, RLS severity, and sleep disturbances. These favorable changes might be due in part to the location of the therapy in the home setting and self-care dialysis.
Study limitations include selection biases that are evident by the recruitment of a relatively younger patient population. However, 29% had a central venous catheter for vascular access, 43% were diabetic, and 25% had a history of heart failure, suggesting that the cohort was not necessarily healthier. Although the study experienced a high attrition rate, the characteristics of the participants who dropped out did not differ from those who completed 12 months of follow-up. In addition, the ITT analysis attempted to circumvent this attrition rate. Finally, the absence of a control group is an important limitation, although the observed improvement persisted beyond 4 months, arguing against the potential for regression to the mean.
In conclusion, the present interim report of the FREEDOM study demonstrates that initiation of SDHD at home is associated with an improvement in restless legs symptoms and sleep disturbances. These observations support previous knowledge that SDHD improves other QoL measures including depressive symptoms and postdialysis recovery time (15).
Disclosures.
None.
Acknowledgments
The FREEDOM study is sponsored and funded by NxStage Medical, Inc. (Lawrence, MA). The following authors are members of the NxStage Scientific Advisory Board that oversees the FREEDOM study: John M. Burkart, Fredric O. Finkelstein; Bertrand L. Jaber; Michael A. Kraus; Brent W. Miller; and Brigitte Schiller. This work was presented in part at the 30th Annual Dialysis Conference, Seattle, Washington, March 07 to 09, 2010.
The following FREEDOM Study investigators enrolled at least one subject as of December 31, 2008: Mirel Abramovici (Renal Center of Westwood, Westwood, New Jersey), Sujatha Addagatla (Apollo Healthcare, Niagara Falls, New York), George Aronoff (University of Louisville, Louisville, Kentucky), Rachid Daoui (Hortense & Louis Rubin Dialysis Center, Clifton Park, New York), William Elliott (DaVita Bluemound Dialysis, Wauwatosa, Wisconsin), Claude Galphin (Nephrology Associates, Chattanooga, Tennessee), Todd Gehr (Virginia Commonwealth University/Renal Advantage Inc., Richmond, Virginia), Martin Gelman (Personal Dialysis, Inc., Brighton, Massachusetts), Tim Govaerts (Dialysis Center of Lincoln, Lincoln, Nebraska), Gerald Groggel (University of Nebraska/Renal Advantage Inc., Omaha, Nebraska), Andrea Iannuzzelli (Silver Care Dialysis, Cherry Hill, New Jersey), Heidi Joist (Renal Advantage Inc., Frontenac, Missouri), Michael A. Kraus (Indiana University, Indianapolis, Indiana), Pius Kurian (DaVita Midwest, Fairborn, Ohio), Janice Lea (Emory University/Renal Care Partners, Sandy Springs, Georgia), Brent W. Miller (Washington University/Barnes Jewish Dialysis Center, Street Louis, Missouri), Chandra Mohan (Wellspan Dialysis, York, Pennsylvania), James Novak (Henry Ford Hospital/Greenfield Health, Detroit, Michigan), James Porile (Nephrology Inc., Mishawaka, Indiana), Dana Rabideau (Fort Smith Regional Dialysis, Fort Smith, Arizona), Victor Rozas (Great Lakes Renal Network, Alma, Michigan), Ahmet Sevimli (Munson Dialysis, Traverse City, Michigan), Marvin Sinsakul (Circle Medical Management, Chicago, Illinois), Brigitte Schiller (Satellite Healthcare, San Jose, California), Scott Solcher (Kansas Dialysis Services, Topeka, Kansas), Robert Szewc (Barlite SW Kidney, San Antonio, Texas), Amy Williams (Mayo Clinic, Rochester, Minnesota), and Bessie Young (Northwest Kidney Centers, Seattle, Washington).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Walker S, Fine A, Kryger MH: Sleep complaints are common in a dialysis unit. Am J Kidney Dis 26: 751–756, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Winkelman JW, Chertow GM, Lazarus JM: Restless legs syndrome in end-stage renal disease. Am J Kidney Dis 28: 372–378, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Gigli GL, Adorati M, Dolso P, Piani A, Valente M, Brotini S, Budai R: Restless legs syndrome in end-stage renal disease. Sleep Med 5: 309–315, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Unruh ML, Levey AS, D'Ambrosio C, Fink NE, Powe NR, Meyer KB: Restless legs symptoms among incident dialysis patients: Association with lower quality of life and shorter survival. Am J Kidney Dis 43: 900–909, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Tuncel D, Orhan FO, Sayarlioglu H, Isik IO, Utku U, Dinc A: Restless legs syndrome in hemodialysis patients: Association with depression and quality of life. Sleep Breath 2010, July 1 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6. La Manna G, Pizza F, Persici E, Baraldi O, Comai G, Cappuccilli ML, Centofanti F, Carretta E, Plazzi G, Coli L, Montagna P, Stefoni S: Restless legs syndrome enhances cardiovascular risk and mortality in patients with end-stage kidney disease undergoing long-term haemodialysis treatment. Nephrol Dial Transplant 2010, November 5 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7. Unruh ML, Hartunian MG, Chapman MM, Jaber BL: Sleep quality and clinical correlates in patients on maintenance dialysis. Clin Nephrol 59: 280–288, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, Kurokawa K, Rayner HC, Furniss AL, Port FK, Saran R: Sleep quality predicts quality of life and mortality risk in haemodialysis patients: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 23: 998–1004, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Merlino G, Gigli GL, Valente M: Sleep disturbances in dialysis patients. J Nephrol 21[Suppl 13]: S66–S70, 2008 [PubMed] [Google Scholar]
- 10. Reynolds JT, Homel P, Cantey L, Evans E, Harding P, Gotch F, Wuerth D, Finkelstein S, Levin N, Kliger A, Simon DB, Finkelstein FO: A one-year trial of in-center daily hemodialysis with an emphasis on quality of life. Blood Purif 22: 320–328, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Walsh M, Culleton B, Tonelli M, Manns B: A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int 67: 1500–1508, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, Tonelli M, Donnelly S, Friedrich MG, Kumar A, Mahallati H, Hemmelgarn BR, Manns BJ: Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: A randomized controlled trial. JAMA 298: 1291–1299, 2007 [DOI] [PubMed] [Google Scholar]
- 13. The FHN Trial Group: In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jaber BL, Finkelstein FO, Glickman JD, Hull AR, Kraus MA, Leypoldt JK, Liu J, Gilbertson D, McCarthy J, Miller BW, Moran J, Collins AJ: Scope and design of the Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements (FREEDOM) Study. Am J Kidney Dis 53: 310–320, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Jaber BL, Lee Y, Collins AJ, Hull AR, Kraus MA, McCarthy J, Miller BW, Spry L, Finkelstein FO: Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: Interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis 56: 531–539, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Daugirdas JT: Second generation logarithmic estimates of single-pool variable volume Kt/V: An analysis of error. J Am Soc Nephrol 4: 1205–1213, 1993 [DOI] [PubMed] [Google Scholar]
- 17. Foundation NK: KDOQI Clinical Practice Guidelines and Clinical Practice Recommendations for 2006 Updates: Hemodialysis Adequacy, Peritoneal Dialysis Adequacy and Vascular Access. Am J Kidney Dis 48[Suppl 1]: S1–S322, 2006. 17045862 [Google Scholar]
- 18. Walters AS, LeBrocq C, Dhar A, Hening W, Rosen R, Allen RP, Trenkwalder C: Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med 4: 121–132, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisi J: Restless legs syndrome: Diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med 4: 101–119, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Abetz L, Arbuckle R, Allen RP, Garcia-Borreguero D, Hening W, Walters AS, Mavraki E, Kirsch JM: The reliability, validity and responsiveness of the International Restless Legs Syndrome Study Group rating scale and subscales in a clinical-trial setting. Sleep Med 7: 340–349, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Trenkwalder C, Hundemer HP, Lledo A, Swieca J, Polo O, Wetter TC, Ferini-Strambi L, de Groen H, Quail D, Brandenburg U: Efficacy of pergolide in treatment of restless legs syndrome: The PEARLS Study. Neurology 62: 1391–1397, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Quilici S, Abrams KR, Nicolas A, Martin M, Petit C, Lleu PL, Finnern HW: Meta-analysis of the efficacy and tolerability of pramipexole versus ropinirole in the treatment of restless legs syndrome. Sleep Med 9: 715–726, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hening WA, Allen RP, Ondo WG, Walters AS, Winkelman JW, Becker P, Bogan R, Fry JM, Kudrow DB, Lesh KW, Fichtner A, Schollmayer E: Rotigotine improves restless legs syndrome: A 6-month randomized, double-blind, placebo-controlled trial in the United States. Mov Disord 25: 1675–1683 [DOI] [PubMed] [Google Scholar]
- 24. Hays R, Stewart A: Sleep Measures. In: Measuring Functioning and Well Being: The Medical Outcomes Study Approach, edited by Stewart A, Ware JJ. Durham, Duke University Press, 1992, pp 235–259 [Google Scholar]
- 25. Hays RD, Martin SA, Sesti AM, Spritzer KL: Psychometric properties of the Medical Outcomes Study Sleep measure. Sleep Med 6: 41–44, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Spritzer K, Hays R: MOS Sleep Scale: A Manual for Use and Scoring. Version 1.0, Los Angeles, CA, 2003 [Google Scholar]
- 27. Takaki J, Nishi T, Shimoyama H, Inada T, Matsuyama N, Kumano H, Kuboki T: Interactions among a stressor, self-efficacy, coping with stress, depression, and anxiety in maintenance hemodialysis patients. Behav Med 29: 107–112, 2003 [DOI] [PubMed] [Google Scholar]