Abstract
Summary
Background and objectives
Hypervolemia is an important and modifiable cause of hypertension. Hypertension improves with probing dry weight, but its effect on echocardiographic measures of volume is unknown.
Design, setting, participants, & measurements
Shortly after dialysis, echocardiograms were obtained at baseline and longitudinally every 4 weeks on two occasions. Among 100 patients in the additional ultrafiltration group, 198 echocardiograms were performed; among 50 patients in the control group, 104 echocardiograms were performed.
Results
Baseline inferior vena cava (IVC)insp diameter was approximately 5.1 mm/m2; with ultrafiltration, change in IVCinsp diameter was −0.95 mm/m2 more compared with the control group at 4 weeks and −1.18 mm/m2 more compared with the control group at 8 weeks. From baseline IVCexp diameter of approximately 8.2 mm/m2, ultrafiltration-induced change at 4 weeks was −1.06 mm/m2 more and at 8 weeks was −1.07 mm/m2 more (P = 0.044). From a baseline left atrial diameter of 2.1 cm/m2, ultrafiltration-induced change at 4 weeks was −0.14 cm/m2 more and at 8 weeks was −0.15 cm/m2 more. At baseline, there was no relationship between interdialytic ambulatory BP and echocardiographic parameters of volume. The reduction in interdialytic ambulatory BP was also independent of change in the echocardiographic volume parameters.
Conclusions
The inferior vena cava and left atrial diameters are echocardiographic parameters that are responsive to probing dry weight; thus, they reflect excess volume. However, echocardiographic volume parameters are poor determinants of interdialytic BP, and their change does not predict the BP response to probing dry weight.
Introduction
Although the assessment of volume among hemodialysis continues to pose a challenge, it is an independent marker of mortality (1). The assessment of volume is made poorly by physical examination (2,3). To judge excess volume, there are no gold standards, but its assessment has been done using a variety of tools (4). Among these tools is echocardiography. The assessment of inferior vena cava (IVC) diameter and its collapse with inspiration have been the most commonly used echocardiographic techniques to assess intravascular volume (5–8). However, two other echocardiographic methods, hepatic vein Doppler to assess right atrial pressure and the left atrial diameter, have not been assessed (9,10).
Whereas there is no gold standard, most studies have used the clinical assessment of dry weight to compare these echocardiographic parameters of intravascular volume (6–8). Some have used cardiac catheterization to compare the right atrial pressure with these indices (5). However, none have deliberately probed the dry weight in a randomized trial to assess responsiveness of these echocardiographic markers of volume to clinical changes in dry weight.
Excess volume among dialysis patients is most frequently manifested as interdialytic hypertension (11). We previously showed that probing dry weight can improve interdialytic hypertension within 4 weeks (12). The reduction in BP persists for at least 8 weeks. However, it is unclear whether putative echocardiographic parameters of volume excess are associated with interdialytic hypertension. It is also unclear whether baseline excess fluid volume assessed echocardiographically can predict the anti-hypertensive response of probing dry weight.
The purpose of this study was to evaluate the effect of probing dry weight on echocardiogaphic markers of volume excess. A further aim was whether echocardiographic markers of volume excess are associated with interdialytic BP and whether changes in echocardiographic volume parameters are associated with improvement in BP.
Materials and Methods
This is a prespecified substudy of the Dry-weight Reduction In hypertensive hemodialysis Patients trial. Detailed protocol and methods of this study has previously been published (12). Briefly, we recruited patients 18 years of age or older on long-term hemodialysis for at least 3 months, who were hypertensive based on a mean interdialytic ambulatory BP of 135/85 mmHg or more. Patients found to have well-controlled hypertension had anti-hypertensive medications withdrawn until they become hypertensive. Patients with stroke, myocardial infarction, or limb ischemia in the previous 6 months, ambulatory BP of >170/100 mmHg, who missed more than one dialysis in the prior month, had chronic atrial fibrillation, or morbid obesity (body mass index > 40 kg/m2) were excluded.
After a six hemodialysis run-in phase, during which baseline data were collected, patients were randomized in 1:2 proportion into a control group versus an ultrafiltration trial group for 8 weeks. During this 24 dialysis treatment phase, patients were seen at each dialysis visit and had evaluation of dry weight and symptoms and signs related to hypovolemia by study personnel.
Randomization to treatment or control groups was carried out in permuted blocks with 2:1 ultrafiltration:control ratio. Opaque sealed envelopes were used for treatment allocation by study personnel after assuring that the inclusion–exclusion criteria were met.
The study protocol was approved by the Institutional Review Boards and the VA Research and Development Committee, and all patients provided written informed consent. The trial was registered at ClinicalTrials.gov (NCT00067665).
Ambulatory BP Monitoring
Ambulatory BP monitoring was performed after the mid-week hemodialysis session for 44 hours. BPs were recorded every 20 minutes during the day (6:00 am to 10:00 pm) and every 30 minutes during the night (10:00 pm to 6:00 am) using a Spacelab 90207 ABP monitor (SpaceLabs Medical, Redmond, WA) in the nonaccess arm. Recordings began immediately after hemodialysis and terminated immediately before the subsequent dialysis. Accuracy of ambulatory BP recordings was confirmed against auscultated BP at baseline. Hourly means were calculated. These means were averaged over the entire course of recording to provide systolic and diastolic interdialytic ambulatory BP.
Echocardiograms
Two-dimensional guided M-mode echocardiograms were performed by dedicated technicians, 30 to 60 minutes after dialysis, in the dialysis unit with a digital cardiac ultrasound machine (Cypress Acuson; Siemens Medical). The postdialysis period was selected for echocardiography because it allows control over volume state of the patient because it is associated with the least intravascular volume. The day after dialysis would be associated with a variable change in the dimension of IVC and left atrium depending on the state of volume expansion and was not chosen for echocardiography.
The protocol specified recording of at least six cycles of two-dimensional parasternal long- and short-axis left atrial views with optimal orientation of the cursor beam used to derive additional M-mode recordings. Each patient underwent six M-mode measurements of IVC in inspiration and expiration and left atrial diameter in end systole using standards of the American Society of Echocardiography (13). All measurements were made over six cardiac cycles by a highly skilled echocardiographer and were confirmed by an experienced cardiologist.
IVC was imaged at the level just below the diaphragm in the hepatic segment by two-dimensional guided, M-mode echocardiography. IVC diameter was measured just before the P wave of the electrocardiogram during end expiration and end inspiration while avoiding Valsalva-like maneuvers. Collapse index was defined as (maximal diameter on expiration – minimal diameter on deep inspiration)/maximal diameter on expiration × 100.
The hepatic vein was identified, and pulse wave Doppler was used to obtain peak systolic and diastolic velocities. From the hepatic vein flow velocity, systolic filling fraction was derived from peak velocities as peak systolic wave velocity divided by the sum of peak systolic and diastolic velocities (9). Systolic filling fraction of <55% has been reported to have 86% sensitivity and 90% specificity in predicting mean right atrial pressure of >8 mmHg (9).
Statistical Analysis
Data were first analyzed by graphical methods. A mixed model accounting for repeated measurements was fitted for several outcome echocardiographic parameter of interest such as IVC diameter in inspiration and expiration, collapse index, systolic filling fraction, and left atrial diameter. The effect of intervention (ultrafiltration versus control), time (baseline, 4 weeks, and 8 weeks), and their interaction was tested, and 95% confidence intervals calculated using maximal likelihood estimates. The random part of the equation used subject and visits modeled using an unstructured covariance matrix. In addition, a random effect was used for the echocardiographer.
To analyze the effect of baseline echocardiographic parameters on interdialytic BP, we first dichotomized the baseline echocardiographic parameter (e.g., IVC diameter) at the median. The median of the parameter was calculated using all of the echocardiograms performed at the baseline visit. We carried this assignment forward to the week 4 and week 8 visits. A similar mixed effects model as reported above was used. The fixed part of the model had interdialytic systolic BP as an outcome variable. The predictors were the echocardiographic variable (indicator variable dichotomized about the median), intervention, and time, as well as all possible interactions of these three indicator variables. The three-way interaction indicated whether the echocardiographic variable predicted the BP response.
To analyze the effect of time-varying echocardiographic parameters on interdialytic BP, first we dichotomized the baseline echocardiographic parameter (e.g., IVC diameter) at the median. The median of the parameter was calculated using all of the echocardiograms performed at the baseline visit. We assigned all echocardiograms to a dichotomous category at week 4 and week 8 visits. A similar mixed effects model as reported above was used. The fixed part of the model had interdialytic systolic BP as an outcome variable. The predictors were the echocardiographic variable (indicator variable dichotomized about the median), intervention, and time, as well as all possible interactions of these three indicator variables. We next calculated the transitional change from low to high and high to low categories of the echocardiographic varialbe in the ultrafiltration group and control groups. We calculated the differences between these changes. Finally, we tested the significance of the differences using the Wald test.
The nominal level of significance was set at two-sided P < 0.05, and all statistical analyses were performed with Stata version 11 (StataCorp, College Station, TX).
Results
Between March 2004 and April 2008, we randomized 100 patients to the ultrafiltration group and 50 patients to the control group. Among 100 patients in the ultrafiltration group, 198 echocardiograms were performed (74 at baseline); among 50 patients in the control group, 104 echocardiograms were performed (39 at baseline). The trial flow of these participants has previously been described (12). The two treatment groups of patients who had echocardiograms were well balanced with respect to the baseline characteristics (Table 1).
Table 1.
Subject characteristics
| Clinical Characteristic | Control | UF | Total | P |
|---|---|---|---|---|
| n | 39 (35%) | 74 (65%) | 113 (100%) | |
| Age (years) | 55.5 ± 11.5 | 54.3 ± 12.6 | 54.7 ± 12.2 | 0.6 |
| Male | 31 (79%) | 48 (65%) | 79 (70%) | 0.1 |
| Race | 0.8 | |||
| white | 3 (8%) | 9 (12%) | 12 (11%) | |
| black | 35 (90%) | 63 (85%) | 98 (87%) | |
| other | 1 (3%) | 2 (3%) | 3 (3%) | |
| Pre-HD seated BP | 157.8 ± 15.8/87.0 ± 12.8 | 158.5 ± 16.2/85.5 ± 10.5 | 158.3 ± 16.0/86.0 ± 11.3 | 0.8/0.5 |
| Post-HD seated SBP | 141.5 ± 19.9/77.1 ± 13.2 | 142.9 ± 17.6/78.0 ± 10.1 | 142.4 ± 18.3/77.7 ± 11.2 | 0.7/0.7 |
| Pre-HD weight (kg) | 82.8 ± 16.7 | 82.2 ± 20.0 | 82.4 ± 18.8 | 0.9 |
| Post-HD weight (kg) | 80.0 ± 16.1 | 79.2 ± 19.2 | 79.5 ± 18.1 | 0.8 |
| Body mass index (kg/m2) | 26.7 ± 5.9 | 26.8 ± 5.7 | 26.7 ± 5.7 | 0.9 |
| Years on dialysis | 4.3 ± 6.0 | 3.6 ± 4.2 | 3.9 ± 4.9 | 0.5 |
| Etiology of ESRD | 0.7 | |||
| diabetes mellitus | 15 (38%) | 31 (42%) | 46 (41%) | |
| hypertension | 18 (46%) | 31 (42%) | 49 (43%) | |
| glomerulonephritis | 2 (5%) | 3 (4%) | 5 (4%) | |
| polycystic kidney disease | 0 (0%) | 3 (4%) | 3 (3%) | |
| other | 4 (10%) | 6 (8%) | 10 (9%) | |
| Current smoker | 15 (38%) | 23 (31%) | 38 (34%) | 0.4 |
| History of | ||||
| congestive heart failure | 4 (10%) | 15 (20%) | 19 (17%) | 0.2 |
| myocardial infarction | 6 (15%) | 13 (18%) | 19 (17%) | 0.8 |
| stroke | 4 (10%) | 7 (9%) | 11 (10%) | 0.9 |
| Urea reduction ratio (%) | 73.0 ± 6.3 | 74.2 ± 7.4 | 73.8 ± 7.0 | 0.4 |
| Albumin (g/dl) | 3.8 ± 0.4 | 3.7 ± 0.5 | 3.7 ± 0.5 | 0.9 |
| Hemoglobin (g/dl) | 12.1 ± 1.4 | 12.2 ± 1.1 | 12.2 ± 1.2 | 0.7 |
| Presence of pedal edema | 7 (18%) | 16 (22%) | 23 (20%) | 0.6 |
| Number receiving anti-hypertensive drugs | 29 (74%) | 63 (85%) | 92 (81%) | 0.2 |
| Number of anti-hypertensives in users | 2.1 ± 1.7 | 2.2 ± 1.6 | 2.1 ± 1.6 | 0.8 |
| Dihydropyridine calcium channel blockers | 16 (41%) | 33 (45%) | 49 (43%) | 0.7 |
| Non-dihydropyridine calcium channel blockers | 2 (5%) | 4 (5%) | 6 (5%) | 0.9 |
| β-blockers | 25 (64%) | 50 (68%) | 75 (66%) | 0.7 |
| α-blockers | 3 (8%) | 5 (7%) | 8 (7%) | 0.9 |
| Centrally acting agents | 8 (21%) | 21 (28%) | 29 (26%) | 0.4 |
| Vasodilators | 9 (23%) | 10 (14%) | 19 (17%) | 0.2 |
| ACE inhibitors | 20 (51%) | 38 (51%) | 58 (51%) | 1 |
| Angiotension receptor blockers | 4 (10%) | 14 (19%) | 18 (16%) | 0.2 |
Table 2 shows the echocardiographic characteristics at baseline and the change at 4 and 8 weeks in the control and ultrafiltration (UF) groups. Baseline IVCinsp diameter was 4.9 mm/m2 in the control group and 5.2 mm/m2 in the UF group. In the control group, IVC diameter increased by 0.44 mm/m2at 4 weeks and 0.81 mm/m2 at 8 weeks. In the UF group, IVC diameter decreased by 0.51 mm/m2 at 4 weeks and 0.37 mm/m2 at 8 weeks. As a result, with ultrafiltration, change in IVCinsp diameter was −0.95 mm/m2 (P = 0.031) more compared with the control group at 4 weeks and −1.18 mm/m2 more compared with the control group at 8 weeks (P = 0.017). From baseline IVCexp diameter of approximately 8.2 mm/m2, ultrafiltration-induced change at 4 weeks was −1.06 mm/m2 more (P = 0.031) and at 8 weeks was −1.07 mm/m2 more (P = 0.044). From a baseline left atrial diameter of 2.1 cm/m2, ultrafiltration-induced change at 4 weeks was −0.14 cm/m2 more (P = 0.007) and at 8 weeks was −0.15 cm/m2 more (P = 0.005). The changes in hepatic vein systolic filling fraction, a marker of right atrial pressure, and IVC collapse index were not significant between groups over time.
Table 2.
Echocardiographic volume parameter at baseline and its change over time between intervention groups
| Echocardiographic Volume Parameter | Interventi on Group | Baseline |
Week 4 Change from Baseline |
Week 8 Change from Baseline |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 95% Confidence Interval | P | Mean | 95% Confidence Interval | P | Mean | 95% Confidence Interval | P | ||
| IVC inspiration index (mm/m2) | Control | 4.94 | (3.97, 5.90) | 0.44 | (−0.26, 1.14) | 0.22 | 0.81 | (0.01, 1.61) | 0.046a | |
| UF | 5.24 | (4.45, 6.03) | −0.51 | (−1.02, −0.01) | 0.047a | −0.37 | (−0.91, 0.18) | 0.191 | ||
| UF-Control | 0.31 | (−0.65, 1.26) | 0.53 | −0.95 | (−1.81, −0.09) | 0.031a | −1.18 | (−2.15, −0.21) | 0.017a | |
| IVC expiration index (mm/m2) | Control | 8.2 | (7.26, 9.15) | 0.43 | (−0.35, 1.22) | 0.278 | 0.78 | (−0.08, 1.64) | 0.076 | |
| UF | 8.19 | (7.48, 8.90) | −0.63 | (−1.20, −0.06) | 0.029a | −0.29 | (−0.88, 0.30) | 0.332 | ||
| UF-Control | −0.01 | (−1.07, 1.05) | 0.985 | −1.06 | (−2.03, −0.10) | 0.031a | −1.07 | (−2.12, −0.03) | 0.044a | |
| Left atrial diameter index (cm/m2) | Control | 2.13 | (1.97, 2.29) | 0.07 | (−0.01, 0.16) | 0.074 | 0.06 | (−0.03, 0.14) | 0.21 | |
| UF | 2.2 | (2.07, 2.34) | −0.06 | (−0.13, −0.00) | 0.035a | −0.1 | (−0.16, −0.04) | 0.002b | ||
| UF-Control | 0.08 | (−0.06, 0.22) | 0.286 | −0.14 | (−0.24, −0.04) | 0.007b | −0.15 | (−0.26, −0.05) | 0.005b | |
| Collapse index (%) | Control | 39.86 | (32.25, 47.46) | 0.25 | (−5.73, 6.23) | 0.935 | −3.18 | (−9.83, 3.47) | 0.349 | |
| UF | 36.51 | (29.98, 43.03) | 2.22 | (−2.05, 6.50) | 0.308 | 3.12 | (−1.40, 7.64) | 0.177 | ||
| UF-Control | −3.35 | (−9.71, 3.01) | 0.302 | 1.98 | (−5.37, 9.33) | 0.598 | 6.3 | (−1.73, 14.32) | 0.124 | |
| Hepatic vein systolic filling fraction (%) | Control | 57.22 | (54.32, 60.12) | −0.22 | (−3.68, 3.24) | 0.901 | −1.08 | (−4.74, 2.58) | 0.564 | |
| UF | 56.48 | (54.60, 58.36) | 1.99 | (−0.35, 4.34) | 0.096 | 0.38 | (−2.21, 2.98) | 0.771 | ||
| UF-Control | −0.74 | (−4.19, 2.72) | 0.676 | 2.21 | (−1.97, 6.39) | 0.3 | 1.46 | (−3.03, 5.95) | 0.523 | |
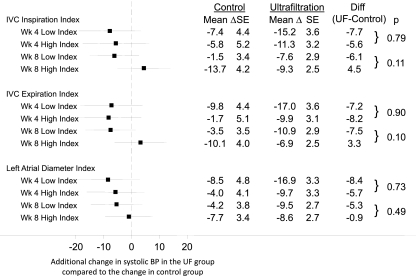
At baseline, there was no relationship between interdialytic ambulatory BP and any of the echocardiographic parameters of volume noted in Table 2 (P > 0.15 for all comparisons; data not shown). Figure 1 shows the reduction in interdialytic ambulatory BP as a function of three echocardiographic volume parameters dichotomized at a median value at the baseline visit. For example, in the case of the IVC inspiration index, at 4 weeks, the mean reduction in systolic ambulatory BP in the UF group was 7.7 mmHg greater compared with the control group in those with an IVC index below the median at the baseline visit. In comparison, the mean reduction in systolic ambulatory BP in the UF group was 5.6 mmHg greater in those with an IVC index above the median (and therefore presumable more volume overload) at the baseline visit. The difference between 7.7 mmHg reduction and 5.6 mmHg reduction was not statistically significant (P = 0.79). Although not statistically significant, at 8 weeks, the results showed greater reduction (6.1 mmHg) in the low IVC inspiration index group (which presumably is less volume overloaded at baseline) compared with the high index group (4.5 mmHg increase). The results showed that none of the echocardiographic volume parameters predicted the systolic BP change at 4 or 8 weeks. Neither at 4 weeks nor at 8 weeks did the interaction values between the high and low indices achieve the nominal value of significance.
Figure 1.
Changes in 44-hour interdialytic systolic BP as a function of echocardiographic volume parameter. The echocardiographic volume parameter was dichotomized at the median value at the baseline visit yielding a low index and a high index. Compared with the high index, low index would be expected to have less volume. The mean additional change BP (shown in the forest plot) in the ultrafiltration group at 4 and 8 weeks did not differ between low index and high index.
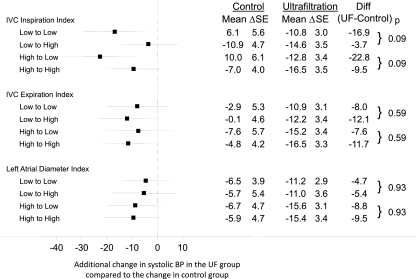
Figure 2 shows the reduction in interdialytic ambulatory BP as a function of three echocardiographic volume parameters dichotomized at a median value at the baseline visit. This dichotomized value was used to grade changes in indices at 4 and 8 weeks. Only the 8-week results are shown, because the 4-week results looked even less significant. As in the baseline model, shown in Figure 1, the results showed that none of the echocardiographic volume parameters predicted the ultrafiltration-induced change in systolic BP at 8 weeks. Neither at 4 weeks (data not shown) nor at 8 weeks (Figure 2) were the interaction values between the high and low indices significant.
Figure 2.
Changes in 44-hour interdialytic systolic BP as a function of change in echocardiographic volume parameter. As in Figure 1, the echocardiographic volume parameter was dichotomized at the median value at the baseline visit, yielding a low index and a high index. This value was used to classify patients into low or high index groups at 4 and 8 weeks. The forest plot shows the 8-week additional change from baseline in systolic BP in the ultrafiltration group compared with the control group. BP change evoked by the transition in volume from low to high was similar to that from low to low. Similarly, BP change evoked by the transition in volume from high to low was similar to that from high to high. Thus, changes in volume state were not predictive of change in BP.
Discussion
This study showed that IVC diameter in expiration and inspiration and the left atrial diameter are volume responsive. Hence, they are at least partly markers of volume. However, echocardiographic volume parameters at baseline are poor determinants of interdialytic BP, and their change does not predict the BP response to probing dry weight.
Probing dry weight led to improved ambulatory BP and some echocardiographic markers of volume overload. However, no relationship was seen between baseline echocardiographic volume markers and BP. Furthermore, echocardiographic volume markers were unable to predict the responsiveness to probing dry weight. There are several possible explanations for these apparently discrepant findings. First, echocardiographic parameters only reflect intravascular volume and not the expanded extracellular fluid volume that is more closely related to hypertension. In the postdialysis state, even when patients are volume expanded, an increased rate of ultrafiltration may shrink echocardiographic volume and falsely classify these patients as “dry” when they are truly “wet.” In fact, IVC expands because of vascular refilling; this rate of refilling is more rapid in patients who get a shorter dialysis (and therefore more aggressive ultrafiltration) (6). Second, patients may have different sensitivities to volume. For some patients, a small reduction of IVC or left atrial diameter may lead to a large change in BP, whereas for others, a large change may be needed to effect a drop in BP. Differences in sensitivities may mask the overall relationship between echocardiographic volume and change in BP. Third, venous compliance may play a role in determining the diameter of IVC and atrial diameter. In those with low venous compliance, a small change in volume may lead to large changes in diameter. Thus, venous compliance can confound the measurement of intravascular volume.
The pioneering study by Cheriex et al. (5) in 18 hemodialysis patients reported the usefulness of IVC diameter and its collapse with inspiration as a marker of volume. These authors reported a good relationship between IVC diameter and right atrial pressure and between collapse index and right atrial pressure; right atrial pressure was measured invasively. Collapse index was found not to correlate with changes in blood volume. Like Cheriex et al., we found that IVC diameter is modifiable with dry weight reduction; therefore, it reflects in part intravascular volume. We also could not discover an improvement in collapse index despite improvement in interdialytic BP. In contrast to the study of Cheriex et al., our study was much larger and was randomized, and we studied a more clinically meaningful endpoint of interdialytic ambulatory BP in a randomized controlled trial.
Although Cheriex et al. (5) have suggested a reference standard of volume overload based on IVC diameter and collapse index, the problem of using these thresholds to classify patients as hypovolemic, euvolemic, or hypervolemic is shown by the study of Brennen et al. (7). This study reported that, depending on the criterion used, before dialysis, hypovolemia was found in an astounding 39 to 47% of the patients. An additional 21 to 25% were euvolemic before dialysis, despite being above dry weight. As in our study, collapse index was found to be of limited value. Even among children on chronic hemodialysis, IVC diameter did not vary significantly with changes in dry weight in a given patient (8).
Left atrial diameter is a part of routine echocardiographic evaluation, and this study found that it is volume responsive. Thus, this measurement can be easily used among patients who have other reasons to have left atrial enlargement such as mitral regurgitation. Furthermore, left atrial volume has been reported to be a correlate of fatal and nonfatal cardiovascular events among hemodialysis patients (10). In contrast, hepatic vein Doppler was not found to be of consistent value in our study. One reason for this may be the technical difficulty associated with its measurement, especially in the postdialysis state, where the hepatic veins may be so collapsed that they are hard to visualize.
This echocardiographic study is in sharp contrast to the study on relative plasma volume monitoring in same patients (14). Relative plasma volume measures the state of vascular refilling in response to an ultrafiltration stress and may therefore better reflect the state of volume expansion. Volume expansion was indeed better detected with relative plasma volume slopes because not only did relative plasma volume slopes steepen in response to probing dry weight, but those who achieved the steepest slopes also had the greatest declines in interdialytic ambulatory BP. Thus, relative plasma volume monitoring may be a better strategy than echocardiograms in identifying volume overload.
A strength of our study is repeated echocardiograms in the dialysis unit by certified technicians using a prespecified study protocol in the context of a randomized trial. However, our study has some limitations. Although the analysis of echocardiographic parameters was prespecified, patients were not randomized based on these parameters. Although we did not discover a relationship between echocardiographic signs of volume excess and subsequent improvement in interdialytic ambulatory BP, in the absence of randomization based on the echocardiographic parameter, we cannot dismiss that a cause and effect relationship does not truly exist. Before performing echocardiograms, we could have waited longer after dialysis for fluid equilibration to occur. However, this would make our study less feasible. Finally, there were few non–African-American patients in our study. Whether the results of our study are generalizable to non–African-American patients will need to be shown in future studies.
In conclusion, among chronic hemodialysis patients, inferior cava diameter assessment in inspiration and expiration and the left atrial diameter are markers of volume. However, volume assessment based on these echocardiographic markers does not seem to be a predictor of BP reduction among hypertensive hemodialysis patients. The assessment of dry weight in patients on long-term hemodialysis has been a long-term challenge. However, echocardiographic methods used in our study do not seem to be helpful in judging dry weight.
Disclosures
None.
Acknowledgments
We are indebted to the participating hemodialysis patients who volunteered their time; staff of the dialysis units at Dialysis Clinics, Clarian Health, and the Roudebush VA Medical Center; the faculty of the Division of Nephrology for allowing us to study their patients; the research technicians research fellows; and the members of the Data and Safety Monitoring Board. This work was supported by National Institutes of Health Grant 5RO1-DK062030-07.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Agarwal R: Hypervolemia is associated with increased mortality among hemodialysis patients. Hypertension 56: 512–517, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal R, Andersen MJ, Pratt JH: On the importance of pedal edema in hemodialysis patients. Clin J Am Soc Nephrol 3: 153–158, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sinha AD, Agarwal R: Can chronic volume overload be recognized and prevented in hemodialysis patients? The pitfalls of the clinical examination in assessing volume status. Semin Dial 22: 480–482, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Jaeger JQ, Mehta RL: Assessment of dry weight in hemodialysis: An overview. J Am Soc Nephrol 10: 392–403, 1999 [DOI] [PubMed] [Google Scholar]
- 5. Cheriex EC, Leunissen KM, Janssen JH, Mooy JM, Van Hooff JP: Echography of the inferior vena cava is a simple and reliable tool for estimation of ‘dry weight’ in haemodialysis patients. Nephrol Dial Transplant 4: 563–568, 1989 [PubMed] [Google Scholar]
- 6. Katzarski KS, Nisell J, Randmaa I, Danielsson A, Freyschuss U, Bergstrom J: A critical evaluation of ultrasound measurement of inferior vena cava diameter in assessing dry weight in normotensive and hypertensive hemodialysis patients. Am J Kidney Dis 30: 459–465, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Brennan JM, Ronan A, Goonewardena S, Blair JEA, Hammes M, Shah D, Vasaiwala S, Kirkpatrick JN, Spencer KT: Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol 1: 749–753, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Krause I, Birk E, Davidovits M, Cleper R, Blieden L, Pinhas L, Gamzo Z, Eisenstein B: Inferior vena cava diameter: A useful method for estimation of fluid status in children on haemodialysis. Nephrol Dial Transplant 16: 1203–1206, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Nagueh SF, Kopelen HA, Zoghbi WA: Relation of mean right atrial pressure to echocardiographic and Doppler parameters of right atrial and right ventricular function. Circulation 93: 1160–1169, 1996 [DOI] [PubMed] [Google Scholar]
- 10. Tripepi G, Benedetto FA, Mallamaci F, Tripepi R, Malatino L, Zoccali C: Left atrial volume monitoring and cardiovascular risk in patients with end-stage renal disease: a prospective cohort study. J Am Soc Nephrol 18: 1316–1322, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Agarwal R: Volume-associated ambulatory blood pressure patterns in hemodialysis patients. Hypertension 54: 241–247, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal R, Alborzi P, Satyan S, Light RP: Dry-weight reduction in hypertensive hemodialysis patients (DRIP): A randomized, controlled trial. Hypertension 53: 500–507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sahn DJ, DeMaria A, Kisslo J, Weyman A: Recommendations regarding quantitation in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 58: 1072–1083, 1978 [DOI] [PubMed] [Google Scholar]
- 14. Sinha AD, Light RP, Agarwal R: Relative plasma volume monitoring during hemodialysis aids the assessment of dry weight. Hypertension 55: 305–311, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]