Abstract
Summary
Background and objectives
Long-term follow-up data are few in children with ESRD. We sought to describe long-term survival, assess risk factors for death, and compare survival between two time periods in pediatric ESRD patients.
Design, setting, participants, & measurements
We used a population-based retrospective cohort utilizing data from a national organ failure registry and from Canada's universal healthcare system. We included 843 children (ages, 0 to 18) initiating renal replacement therapy from 1992 to 2007 and followed them until death or date of last contact (median follow-up, 6.8 years; interquartile range, 3.0 to 10.6). We assessed risk factors for death and examined cause-specific mortality.
Results
During 5991 patient-years of follow-up, 107 (12.7%) patients died. Unadjusted cumulative survival for the cohort was: 91.7% (95% CI, 89.8 to 93.7%) at 5 years and 85.8% (95% CI, 82.8 to 88.8%) at 10 years. Among patients commencing dialysis, overall adjusted survival was poorest among those who started dialysis at age <1 year. No secular trends in survival were noted for either dialysis or transplant patients. The proportion of incident patients receiving pre-emptive transplantation increased over time. Pre-emptively transplanted patients did not demonstrate superior adjusted survival compared with those who spent >2 years on dialysis before transplant (hazard ratio, 1.53; 95% CI, 0.63 to 3.67).
Conclusions
No significant improvements in survival were observed among ESRD patients over the study period. Time with transplant function had the strongest association with survival. Pre-emptive transplantation was not associated with improved survival in adjusted models.
Introduction
Age-specific mortality rates for children treated with dialysis and kidney transplantation are approximately 30 times higher than those in healthy children (1). ESRD contributes to numerous sequelae in children, including growth retardation, cognitive delay, decreased exercise capacity, poor cardiovascular health, and decreased quality of life (2–8). Substantial improvements in pediatric dialysis and transplantation have occurred over the last four decades (1). Established national and international registries document excellent short term (1 to 2 years) survival among children treated with dialysis and kidney transplantation, as well as good allograft outcomes (9–11). Longer-term patient outcomes are less well described (12), especially in North America, because of limited longitudinal data and lack of ongoing reporting to registries when patients are transferred from pediatric to adult centers.
We performed a retrospective cohort study of pediatric ESRD patients using a novel database (Canadian Pediatric End-Stage Renal Disease Database) created by linking data from the Canadian Organ Replacement Register (CORR) with data from the Canadian Institute for Health Information Discharge Abstract Database (13). CORR has prospectively collected data related to organ donation and transplantation in Canada since 1981 (14). We sought to describe long-term survival in the Canadian pediatric ESRD population and to identify risk factors for death. In addition, we aimed to assess changes in survival over calendar time in this population.
Materials and Methods
Study Design and Population
After obtaining institutional ethics approval from the University of Calgary, we established a cohort of all patients in nine Canadian provinces who initiated renal replacement therapy (RRT) (dialysis or transplant) at age ≤18 years between January 1, 1992 and December 31, 2007. Patients from the province of Quebec were not included because their data were not available for release to investigators.
Data Sources
Detailed methods on the creation of the Canadian Pediatric End-Stage Renal Disease Database for research purposes using linked data from CORR and Canadian Institute for Health Information Discharge Abstract Database are provided in the supplemental methods section (see the Appendix) and were published previously (13).
Definitions of Outcomes
The patients were followed from initiation of RRT until death, loss to follow-up, or the end of observation period (December 31, 2007). Follow-up for all incident patients included time spent in both pediatric and adult ESRD centers. The primary outcome was time to all-cause mortality, with dates of death obtained from CORR and hospitalization data. If an in-hospital death date was known and a death date was not recorded in the registry, the in-hospital death date was defined as the death date (three deaths in the cohort not recorded in CORR were recovered from hospitalization data).
Causes of death were obtained from CORR when available. The major categories were: undetermined, cardiac, infection, malignancy, social, and other (stroke, respiratory failure, gastrointestinal causes, hemorrhage, accident, and multiorgan failure). If a cause of death was not recorded in CORR and there was record of an in-hospital death for the same patient, then the “most responsible diagnosis” for admission as recorded in the hospital discharge record for that hospital admission was presumed to be the cause of death.
Study Variables
Baseline demographic data evaluated include gender, etiology of primary renal disease, and age at onset of renal replacement. Index RRT modality (hemodialysis, peritoneal dialysis, or pre-emptive transplantation), dates, and donor type for each transplant event and dates of allograft failure were obtained from the database.
Statistical Analyses
Demographic and baseline clinical characteristics were described with means (SD), medians (interquartile ranges [IQR]), or proportions. A significance level of α = 0.05 was set for all statistical testing. Time from start of renal replacement (index date) to death or date of last follow-up was analyzed using the Kaplan-Meier method and Cox proportional-hazards models. Trends in index RRT modality over calendar years were examined using a chi-squared test for trends in proportions.
We examined two overlapping but distinct groups: those who initiated dialysis and those who received a transplant using Cox proportional-hazards models. The first model determined differences in survival by age at onset of dialysis (categorized as <1 year, 1 to <10 years, and 10 to <18 years) and period of start of dialysis (1992 to 1999 and 2000 to 2007). The patients were censored at transplant, loss to follow-up, or study end (December 31, 2007). The model was adjusted for gender and primary renal disease (congenital versus other). We used interaction terms to determine whether the effect of age at onset of dialysis varied by calendar period (1992 to 1999 versus 2000 to 2007).
The second Cox proportional-hazards model focused on the interval after first transplant. We examined differences in survival by period of transplant (1992 to 1999 versus 2000 to 2007) adjusting for first transplant donor source (deceased versus living), a time-dependent variable indicating the status of transplant function (functioning versus failed with return to dialysis) and duration of dialysis before first transplant.
The proportional-hazards assumption in all models was tested on the basis of the scaled Schoenfeld residuals (15). All of the analyses were performed using R (The R Foundation for Statistical Computing, http://cran.r-project.org).
Results
Demographic Characteristics
Between January 1, 1992 and December 31, 2007, 858 pediatric patients began RRT in Canada. Fifteen multi-organ transplant recipients were excluded; therefore, the experience of 843 patients (5991 person-years) was analyzed. The median duration of follow-up was 6.83 (range, 3.00 to 10.62) years. Demographic characteristics of the study cohort are outlined in Table 1. There were more boys (52.4%) than girls in the cohort. The most common cause of ESRD was congenital anomalies of the kidney and urinary tract (29.9%). The mean age ± SD at onset of RRT was 11.3 ± 5.4 years.
Table 1.
Characteristics of pediatric end-stage renal disease patients in Canada between 1992 and 2007 (n = 843)
| Characteristic | Number (%) |
|---|---|
| Gender (male) | 442 (52.4) |
| Age at onset of RRT, in years | |
| 0 to 0.9 | 63 (7.5) |
| 1.0 to 4.9 | 84 (10.0) |
| 5.0 to 9.9 | 148 (17.6) |
| 10.0 to 14.9 | 281 (33.3) |
| 15.0 to 17.9 | 267 (31.7) |
| Index modality of RRT | |
| peritoneal dialysis | 351 (41.6) |
| hemodialysis | 327 (38.8) |
| pre-emptive transplant | 165 (19.6) |
| Etiology of ESRD | |
| congenital anomalies of the kidney and urinary tract | 242 (28.7) |
| glomerulonephritis/autoimmune | 244 (28.9) |
| genetic | 112 (13.3) |
| other/unknown | 245 (29.1) |
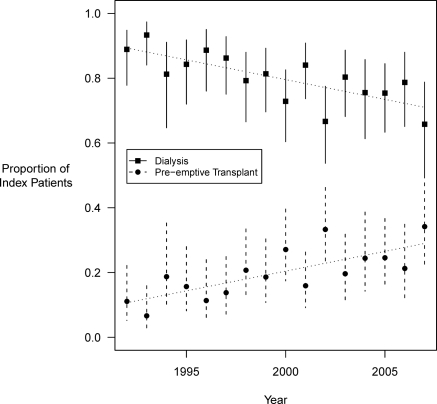
Peritoneal dialysis (41.6%) was the most common index RRT modality in the cohort. A total of 165 (19.6%) patients underwent pre-emptive transplantation without prior dialysis; of these, 50 were from deceased donors. The proportion of children undergoing pre-emptive kidney transplantation increased over the calendar years (1992 to 2007) and approached 25% at the end of observation period in year 2007 (Figure 1; chi-squared test for trend P < 0.001). The most common index RRT modality for children older than 10 years was hemodialysis (46.2%), whereas peritoneal dialysis was most common for children younger than 10 years of age (54.5%).
Figure 1.
Trends in initial renal replacement modality among pediatric ESRD patients in Canada. A chi-squared test was used to test for trends in proportions (P < 0.001).
Overall, 691 patients received a total of 747 kidney transplants (636 patients had one transplant, 54 patients had two transplants, and one patient had three transplants). The proportion of allografts from living donors was 43.7% in 1992 to 1999 and increased to 57.2% during 2000 to 2007 (P < 0.001). The median time to first deceased-donor transplant from start of dialysis was 1.34 years (IQR, 0.75 to 2.62) between 1992 and 1999 compared with 1.75 years (IQR, 1.00 to 2.77) between 2000 and 2007 (P = 0.22). The median time to first living-donor transplant dropped significantly from 1.00 year (IQR, 0.58 to 1.91) between 1992 and 1999 to 0.83 year (IQR, 0.41 to 1.58) between 2000 and 2007 (P = 0.02).
All-Cause Mortality
During 5991 person years of follow-up, there were 107 deaths, for a crude mortality rate of 17.9 per 1000 person-years (95% confidence interval [CI], 14.5 to 21.2). Of these 107 deaths, 59 were in the interval between dialysis initiation and first transplant (crude mortality rate, 45.2 per 1000 person years; 95% CI, 33.7 to 56.7). Twenty-three deaths occurred with graft function (crude mortality rate, 5.5 deaths per 1000 person years; 95% CI, 3.2 to 7.7) and 25 after graft failure and return to dialysis (crude mortality rate, 49.8 per 1000 person years; 95% CI, 30.3 to 69.3). The unadjusted 5-year cumulative survival for the cohort was 91.7% (95% CI, 89.8 to 93.7%), and the 10-year survival was 85.8% (95% CI, 82.8 to 88.8%). When patients who survived to transplant were considered separately, the unadjusted 5-year survival was 96.8% (95% CI, 95.4 to 98.3%), and unadjusted 10-year survival was 89.1% (95% CI: 85.7 to 92.7%).
The median age at death was 16.8 years (IQR, 7.0 to 21.9). The median duration of dialysis therapy among those who died without having received a transplant was 1.00 year (IQR, 0.33 to 3.66). Median time to death after first graft failure was 2.83 years (IQR, 0.91 to 5.08) among the 25 patients who died while on dialysis after first or subsequent graft failures. The demographic characteristics of those who died, according to status of renal replacement at death (dialysis only, transplant with function, and return to dialysis after graft failure), are given in Table 2. Twenty-five percent of deaths in the cohort were in-hospital deaths.
Table 2.
Characteristics of patients who died according to renal replacement modality at death
| Variable | Dialysis before Transplant (n = 59) | Functioning Graft (n = 23) | Dialysis after Graft Failure (n = 25) |
|---|---|---|---|
| Median age at onset of RRT (IQR) in years | 9.0 (0.4–15.6) | 12.0 (6.9–14.6) | 13.3 (9.9–16.3) |
| Males (n, %) | 33 (55.9) | 11 (47.8) | 11 (44.0) |
| Median age at death (IQR) in years | 11.8 (1.3–18.8) | 16.3 (14.1–22.0) | 22.9 (19.2–24.8) |
| Causes of death (n, %) | |||
| cardiac | 11 (18.6) | 2 (8.7) | 10 (40.0) |
| infection | 8 (13.6) | 4 (17.4) | 1 (4.0) |
| malignancy | 2 (3.4) | 8 (34.8) | 0 |
| social | 7 (11.9) | 0 | 2 (8.0) |
| undetermined | 16 (27.1) | 3 (13.0) | 4 (16.0) |
| other | 15 (25.4) | 6 (26.1) | 8 (32.0) |
Mortality on Dialysis.
In the adjusted model for mortality in the interval between dialysis initiation and first transplant (censor at transplant), age <1 year at the start of renal replacement was associated with poorer survival compared with age ≥10 years (hazard ratio [HR], 7.82; 95% CI, 3.97 to 15.44) (Table 3). The association between age at RRT onset and survival did not differ significantly by calendar period of dialysis. The interaction term between age at onset and period of dialysis was NS, indicating that the relationship between survival and age group at the start of dialysis did not vary by calendar period of dialysis.
Table 3.
Adjusted hazard ratios for mortality during interval between dialysis initiation and first transplant
| Risk Factor | Hazard Ratio (95% Confidence Intervals) |
|---|---|
| Age at start of dialysis | |
| <1 year | 7.82 (3.97 to 15.44) |
| 1 to <10 years | 1.47 (0.70 to 3.12) |
| 10 to <18 years | 1.00 (Reference) |
| Period of start of dialysis (2000 to 2007 versus 1992 to 1999) | 1.18 (0.69 to 2.03) |
| Etiology of renal disease) (other versus congenital anomalies)a | 1.34 (0.69 to 2.60) |
| Gender (male versus female) | 0.94 (0.54 to 1.62) |
Other causes include: glomerulonephritis, genetic, and miscellaneous other causes of ESRD in children.
Mortality after Transplantation.
In the adjusted model, patients who received their transplant between 2000 and 2007 had a 50% lower risk of death compared with those transplanted between 1992 and 1999 (Table 4); however, this difference was not statistically significant (HR, 0.50; 95% CI, 0.21 to 1.17). There was no significant difference in survival by donor source (living versus deceased donor) of first transplant (HR, 1.13; 95% CI, 0.58 to 2.21). Graft failure with return to dialysis was strongly associated with greater mortality risk in the adjusted model compared with a functioning graft (HR, 7.17; 95% CI, 3.86 to 13.34). The median time to graft failure in deceased-donor transplant recipients was 4.75 years (IQR, 1.33 to 6.96), and that in living-donor recipients was 4.87 years (IQR, 1.17 to 7.86). Repeat kidney transplantation was not associated with a change in adjusted survival, and therefore the time-dependent variable indicating a repeat transplant was not retained in the final model.
Table 4.
Adjusted hazard ratios for mortality following transplant
| Risk Factor | Hazard Ratio (95% Confidence Intervals) |
|---|---|
| Time without renal allograft function (return to dialysis) | 7.17 (3.86 to 13.34) |
| First transplant donor type (living versus deceased) | 1.13 (0.58 to 2.21) |
| Period of transplant (2000 to 2007 versus 1992 to 1999) | 0.50 (0.21 to 1.17) |
| Time spent on dialysis prior to first transplant | |
| pre-emptive transplant | 1.00 (Reference) |
| <1 year | 0.44 (0.19 to 1.05) |
| 1 to 2 years | 1.01 (0.40 to 2.54) |
| >2 years | 1.53 (0.63 to 3.67) |
Mortality after Pre-emptive Transplantation.
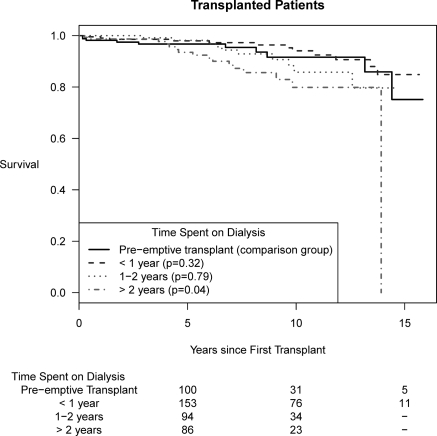
Figure 2 shows Kaplan-Meier graphs of overall survival according to time spent on dialysis before kidney transplantation. In unadjusted analyses, pre-emptive transplantation was associated with better survival compared with those who spent >2 years on dialysis before first transplant (log rank, P = 0.04). However, in the adjusted model for survival among transplant patients, no such association was observed, as shown in Table 2. Because of the strong effect of RRT modality (functioning transplant versus dialysis) noted in the transplant model, we performed additional analyses examining the association between pre-emptive transplantation and the composite outcome of time to first allograft failure or death, adjusting for period of transplant and donor type. We found no significant differences among those who were pre-emptively transplanted, as compared with those who spent >2 years on dialysis (adjusted HR, 1.19; 95% CI, 0.71 to 1.99).
Figure 2.
Kaplan-Meier graph of overall survival according to time spent on dialysis before first renal transplant.
Causes of Death
The cause of death was classified as undetermined, cardiac, infection-related, malignancy-related, social, and other. There were no cases where the cause of death was missing; 23 deaths (21.5%) were recorded as having undetermined causes in the registry; no further information on cause of death could be obtained from the data source. Cardiac deaths constituted 27.4% of all known causes of death (myocardial ischemia and infarction (n = 4), hyperkalemia (n = 4), other causes of cardiac failure (n = 4), cardiac arrest cause unknown (n = 10), and fluid overload (n = 1)). Infections (bacterial or viral) were the causes of death in 13 (15.5%) patients. Ten (11.9%) patients died of malignancy. Of these, two patients had hematopoietic malignancies according to hospital discharge diagnoses, and for the remainder, the primary site of malignancy was not recorded. Social causes of death, including death caused by drugs, alcohol, suicide, patient refusing treatment, or therapy ceased, also contributed to a significant portion of the deaths (10.7%). Other causes of death including respiratory, gastrointestinal, cerebrovascular accident, hemorrhage, and accident make up the remainder of the known causes of death (34.5%). Table 2 shows causes of death by status of renal replacement at death. Malignancy (34.8%) and infection (17.4%) were the most common causes of death among the 23 patients who died with renal allograft function.
Discussion
We studied long-term mortality among incident pediatric end-stage renal disease patients treated in Canada between 1992 and 2007 using data from a national organ failure registry within a universal health care system. Overall, 10-year survival for the entire cohort was 86%. The most important risk factors for mortality were dialysis as the RRT modality and age less than 1 year of age at the start of RRT. No significant improvements in survival after transplantation were observed in 2000 to 2007 compared with 1992 to 1999.
We considered survival in two distinct intervals: dialysis initiation to first transplant and after first transplant. Because we had no information on eligibility for transplant or wait listing, the interval between dialysis initiation and first transplant included patients who may have been ineligible for transplant for various reasons (age or comorbidity) and therefore at higher risk for mortality. The survival estimates obtained in this study provide clinicians with important information that can be used when counseling families of children starting dialysis. Consistent with reports from the Australia and New Zealand ESRD registry and Great Ormond Street Hospital (United Kingdom) (1,16), we found worse survival in children <1 year old at the start of dialysis compared with older children.
The lack of significant improvements observed in survival among pediatric renal-transplant recipients is consistent with recent United States Renal Data System and North American Pediatric Renal Transplant Cooperative Study annual reports (10,11). These findings call for further studies describing causes and mechanisms of death among pediatric transplant recipients and to address emerging threats to allograft function, such as BK virus, and to establish tailored immunosuppression strategies for best long-term allograft survival among pediatric renal-transplant recipients (17).
Peritoneal dialysis continues to be most common modality for initiating renal replacement therapy in Canada. However, when compared with Australia and New Zealand data, Canadian children were more likely to initiate renal replacement on hemodialysis (32.7% in Australia/New Zealand versus 38.8% in Canada) (18). There are numerous factors that play a role in the selection of initial modality among children including school attendance during the day, level of cognitive function, distance from a nephrology unit, family, and socioeconomic circumstances.
We found that approximately one quarter of incident patients in Canada underwent pre-emptive kidney transplantation during the latter years of observation. Although a survival advantage was observed among pre-emptively transplanted patients compared with those who spent >2 years on dialysis before first transplant in the unadjusted analysis, this benefit was not observed in adjusted models accounting for graft function. The relatively short period of time spent on dialysis before first transplant in those who were not pre-emptively transplanted likely played a role in minimizing the mortality risks associated with dialysis. Although overall survival among pediatric transplant patients is strongly associated with transplant function irrespective of first or repeat transplant status, clinicians do not have advance knowledge of transplant function in a particular patient. Therefore, the crude mortality data as demonstrated by Kaplan-Meier curves may serve as a reference to guide discussions regarding risks and benefits of time spent on dialysis before first transplant.
McDonald and Craig (1) published the largest retrospective review of 1634 children and adolescents with ESRD and found that 30% of patients with initial kidney transplant function died from cardiac disease. Because the importance of cardiovascular death among pediatric ESRD patients is well known and documented, it is critical to address modifiable cardiovascular risk factors for death such as long-term burden of hypertension, fluid overload, as well the additive effects on diabetes mellitus and hyperlipidemia (19–22). Malignancy is an important cause of death among patients with transplant function and accounted for 34.8% of mortality among patients with a functioning transplant. Therefore, assessing and mitigating the oncogenic potential of modern immunosuppression will be critical to improve survival among pediatric transplant patients.
There are a number of limitations in our study given its observational nature. The use of registry and administrative data limited our ability to collect or assess the importance of various clinical variables that may affect mortality such as time to development of diabetes and other comorbidities. Unfortunately the registry does not contain data on transplant waiting list status. We were limited by the small number of patients in certain age categories and relatively few events, and we acknowledge that analysis of dialysis and transplant intervals may be underpowered to detect a small improvement in survival over time. The determination of the cause of death from registry data is potentially problematic. Previous studies indicate that agreement between death certificates and data from dialysis registries is poor, perhaps because “kidney failure” is often listed as the cause of death on the former or because many patients die at home (where cause of death cannot readily be ascertained) (23,24). Furthermore, we identified 25% of deaths as in-hospital deaths. This percentage could be an underestimation because hospital records were available by linkage using personal health numbers for only 74.1% of patients in the cohort (13). We are also unable to capture deaths not reported to the registry or deaths out of hospital.
In conclusion, we present comprehensive long-term mortality data for Canadian pediatric ESRD patients. Survival has remained static for pediatric ESRD patients over the period of observation. The survival benefit of pre-emptive transplantation was not observed in adjusted models; nevertheless, there may be other advantages of pre-emptive transplantation in a pediatric patient. Further evaluation is necessary to elucidate factors that may be associated with increased risk of death in this population.
Disclosures
None.
Acknowledgments
This study was funded by operating grants received from the Alberta Children's Hospital Foundation and Alberta Health Services. The authors thank the director and personnel of the Canadian Organ Replacement Register (Dr. John Gill, Lilyanna Trpeski, Yingbo Na, and Robert Williams) for their assistance in creating the data set used in this study. This work submitted in abstract form was accepted for an oral presentation at the Canadian Society of Nephrology 2010 annual meeting in Montreal, Canada.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental information for this article is available online at http://www.cjasn.org/.
References
- 1. McDonald SP, Craig JC: Long-term survival of children with end-stage renal disease. N Engl J Med 350: 2654–2662, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Johnstone LM, Jones CL, Grigg LE, Wilkinson JL, Walker RG, Powell HR: Left ventricular abnormalities in children, adolescents and young adults with renal disease. Kidney Int 50: 998–1006, 1996 [DOI] [PubMed] [Google Scholar]
- 3. Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Groothoff JW, Grootenhuis M, Dommerholt A, Gruppen MP, Offringa M, Heymans HS: Impaired cognition and schooling in adults with end stage renal disease since childhood. Arch Intern Med 87: 380–385, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bartosh SM, Leverson G, Robillard D, Sollinger HW: Long-term outcomes in pediatric renal transplant recipients who survive into adulthood. Transplantation 76: 1195–1200, 2003 [DOI] [PubMed] [Google Scholar]
- 6. Mitsnefes MM: Pediatric end-stage renal disease: Heart as a target. J Pediatr 141: 162–164, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Mitsnefes MM, Daniels SR, Schwartz SM, Meyer RA, Khoury P, Strife CF: Severe left ventricular hypertrophy in pediatric dialysis: Prevalence and predictors. Pediatr Nephrol 14: 898–902, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Broyer M, Le Bihan C, Charbit M, Guest G, Tete MJ, Gagnadoux MF, Niaudet P: Long-term social outcome of children after kidney transplantation. Transplantation 77: 1033–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 9. van der Heijden BJ, van Dijk PC, Verrier-Jones K, Jager KJ, Briggs JD: Renal replacement therapy in children: Data from 12 registries in Europe. Pediatr Nephrol 19: 213–221, 2004 [DOI] [PubMed] [Google Scholar]
- 10. North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) 2009 Annual Report, Rockville, MD, The EMMES Corporation, 2009 [Google Scholar]
- 11. United States Renal Data System: USRDS 2009 Annual Data Report, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 12. Kramer A, Stel VS, Tizard J, Verrina E, Ronnholm K, Palsson R, Maxwell H, Jager KJ: Characteristics and survival of young adults who started renal replacement therapy during childhood. Nephrol Dial Transplant 24: 926–933, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Samuel SM, Tonelli MA, Foster BJ, Nettel-Aguirre A, Na Y, Williams R, Soo A, Hemmelgarn BR: Overview of the Canadian pediatric end-stage renal disease database. BMC Nephrol 11: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Canadian Institute for Health Information: Health Services Databases. Available at: http://www.cihi.ca Accessed January 25, 2011
- 15. Grambsch P, Therneau T: Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 81: 515–526, [Google Scholar]
- 16. Shroff R, Rees L, Trompeter R, Hutchinson C, Ledermann S: Long-term outcome of chronic dialysis in children. Pediatr Nephrol 21: 257–264, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Acott PD, Hirsch HH: BK virus infection, replication, and diseases in pediatric kidney transplantation. Pediatr Nephrol 22: 1243–1250, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Orr NI, McDonald SP, McTaggart S, Henning P, Craig JC: Frequency, etiology and treatment of childhood end-stage kidney disease in Australia and New Zealand. Pediatr Nephrol 24: 1719–1726, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Al-Uzri A, Stablein DM, AC R: Posttransplant diabetes mellitus in pediatric renal transplant recipients: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Transplantation 72: 1020–1024, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Baluarte HJ, Gruskin AB, Ingelfinger JR, Stablein D, Tejani A: Analysis of hypertension in children post renal transplantation: A report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS). Pediatr Nephrol 8: 570–573, 1994 [DOI] [PubMed] [Google Scholar]
- 21. Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Gronhagen-Riska C, Neumayer HH, Maes B, Ambuhl P, Hartmann A, Staffler B, Jardine AG: Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: The ALERT extension study. Am J Transplant 5: 2929–2936, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Silverstein DM: Risk factors for cardiovascular disease in pediatric renal transplant recipients. Pediatr Transplant 8: 386–393, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Perneger TV, Klag MJ, Whelton PK: Cause of death in patients with end-stage renal disease: Death certificates vs registry reports. Am J Public Health 83: 1735–1738, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sarnak MJ, Jaber BL: Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int 58: 1758–1764, 2000 [DOI] [PubMed] [Google Scholar]