Abstract
Summary
Background and objectives
Chronic kidney disease (CKD) is known to be associated with increased all-cause and cardiovascular mortality, but no large studies examined the cancer-specific mortality in non–dialysis-dependent CKD patients. Such outcome data are needed for proper allocation of resources and would help to develop better preventive services.
Design, setting, participants, & measurements
Between 1998 and 1999, 123,717 adults were recruited from four health screening centers in Taiwan. The estimated glomerular filtration rate was calculated using the four-variable Modification of Diet in Renal Disease Study equation for the Chinese. Mortality was ascertained by computer linkage to the national death registry after a median follow-up of 7.06 years. Cox proportional hazards regression models were used to estimate the impact of CKD on cancer-specific mortality.
Results
A higher risk for overall cancer mortality was found in CKD patients compared with non-CKD patients (adjusted hazard ratio, 1.2). CKD was associated with increased mortality from liver cancer, kidney cancer, and urinary tract cancer, with an adjusted hazard ratio of 1.74, 3.3, and 7.3, respectively. A graded relationship between the severity of renal impairment and cancer mortality was also found.
Conclusions
Patients with CKD had a higher mortality risk of liver cancer, kidney cancer, and urinary tract cancer. This is the first large study that showed an inverse association between renal function and liver cancer mortality. The increased mortality could be caused by higher cancer incidence or worse response to cancer treatment. Future research is warranted to clarify the mechanism.
Introduction
Taiwan has the highest incidence and prevalence of end-stage renal disease (ESRD) in the world (1), and the number of non–dialysis-dependent chronic kidney disease (CKD) patients is also increasing rapidly. According to an epidemiologic survey in 2006, the prevalence of patients with CKD, defined as an estimated glomerular filtration rate (eGFR) of <60 ml/min per 1.73 m2, was 6.9% in Taiwan (2). CKD is well recognized to be associated with increased all-cause and cardiovascular (CV) mortality (3). In the Cardiovascular Health Study, Fried et al. (4) further reported an increased risk for various non-CV mortality in CKD patients, including cancer mortality.
Thus far, very little research has addressed the incidence of cancer in non–dialysis-dependent CKD (5). Meanwhile, data regarding the impact of CKD on cancer-specific mortality are lacking, which require a much larger sample size and longer follow-up time. Such mortality data are important in proper allocation of medical resources and would help to develop better preventive services.
Thus, we investigated the impact of CKD on cause-specific mortality among a large, population-based prospective cohort, with special focus on cancer related deaths. The 123,717 study participants were recruited from four nationwide health screen centers in Taiwan. Deaths that occurred during the median follow-up of 7.06 years were ascertained by computer linkage to the national death registry. Cox proportional hazards regression models were used to estimate the effect of baseline eGFR on cancer-specific mortality while adjusting for possible confounders.
Materials and Methods
Study Population
Between 1998 and 1999, the data were collected from four private nationwide MJ Health Screening Centers in Taiwan. A total of 124,513 community-dwelling persons, 20 years of age and older, were recruited. The composition of the study cohort and briefing of this institute has been reported elsewhere (6). The MJ group is one of Asia's largest health management organizations, and its quality control was rated as excellent by the College of American Pathologists. Comprehensive health evaluation, including physical examination, blood, image tests, and a self-administered questionnaire, was performed in all participants. Patients who reported previous cancer history, were receiving long-term dialysis, or had renal transplantation were excluded at enrollment. Those with missing data on serum creatinine were also excluded. After exclusion, the sample size of this study was reduced to 123,717.
All participants provided informed consent authorizing the MJ Health Screening Center to analyze the data obtained from their health screening programs. Protocols for patient recruitment and data analyses were reviewed and approved by the Institutional Review Boards of the MJ Health Screening Center in Taiwan.
Measurement of Kidney Function
Serum creatinine level was measured using a Hitachi 7150 Automated analyzer (Hitachi, Tokyo, Japan) by applying the uncompensated Jaffé method. eGFR was calculated using the four-variable version of the Modification of Diet in Renal Disease Study equation for Chinese Patients (7). Briefly, eGFR (ml/min per 1.73 m2) = 175 × (serum creatinine−1.234) × (age−0.179) × 0.79 (if female). CKD stages were classified according to individual eGFRs as follows: ≥60 (stage 1 and 2), 45 to 59 (stage 3a), 30 to 44 (stage 3b), 15 to 29 (stage 4), and <15 ml/min per 1.73 m2 (stage 5). An eGFR of <60 ml/min per 1.73 m2 was defined as CKD.
Measurement of Covariates
Anthropometric characteristics, blood pressure (BP), body height, weight, and levels of plasma fasting glucose, total cholesterol, triglycerides, HDL cholesterol, and hepatitis B surface antigen (HBsAg) of each participant were measured as described in our previous report (8). Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). Hypertension was defined as systolic BP of ≥ 140 mmHg, diastolic BP of ≥ 90 mmHg, or history of hypertension and use of anti-hypertensive drugs. Diabetes mellitus was defined as fasting glucose of ≥126 mg/dl or history of diabetes mellitus and use of either oral hypoglycemic agents or insulin. Information regarding medical comorbidities, cigarette smoking status, and alcohol drinking habits was obtained by a self-administered questionnaire. Smoking status was classified as current, former, or never smokers. Alcohol drinking habits were classified as never drinkers or current drinkers.
Outcome Ascertainment
Deaths were ascertained by computer linkage to the national death registry using individual identification numbers. All deaths that occurred between study entry and December 31, 2005 were included. Deaths with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) 390 to 459 were classified as CV-related deaths. CV-related deaths were further categorized as stroke-related deaths (ICD-9-CM 430 to 436) and coronary heart disease (CHD)–related deaths (ICD-9-CM 410 to 414). Deaths with ICD-9-CM codes other than 390 to 459 were classified as non-CV mortality. For non-CV mortality, we further classified the events into the following five major categories: cancer, infection, pulmonary disease, diabetes mellitus, and cirrhosis. Cancer-related deaths included those with ICD-9-CM 140 to 239, whereas individual site-specific cancers were further classified by the ICD-9-CM codes provided in the figures. Infection-related deaths included pneumonia, urinary tract infection, sepsis, and other infectious and parasitic diseases (ICD-9-CM 1 to 139, 480 to 486, 507 to 510, and 599). Pulmonary-related deaths included chronic obstructive pulmonary disease, asthma, pneumoconiosis, etc., with ICD-9-CM 490 to 519 (excluding pneumonia, lung abscess, and empyema). Diabetes mellitus–related deaths included those with ICD-9-CM 250. Cirrhosis-related deaths included those with ICD-9-CM 571. Deaths other than the above main categories were not analyzed because of small individual case numbers.
Statistical Analyses
The data are presented as means and standard deviation (SD) for continuous variables. A t test was used to compare mean values of unpaired data between the two groups. Proportions and categorical variables were tested by the χ2 test. The age-standardized rates of all-cause mortality, CV mortality, and cancer mortality were calculated by applying the observed age-specific mortality rates to the World Health Organization standard population in 2000 (9).
Cox proportional hazards regression models were used to estimate the hazard ratio (HR) of CKD on cause-specific mortality while treating the other competing risks as censored (10). The time to event was defined as the time from entry into the study until the end of follow-up (December 31, 2005) or date of death if earlier. Participants with an eGFR of >60 ml/min per 1.73 m2 were regarded as the reference group. The proportional hazards assumption was tested with the Schoenfeld residual method (11).
When investigating the relationship between CKD stages and all-cause mortality, CV mortality, stroke mortality, and CHD mortality, we applied the Cox proportional hazards model adjusted for age, gender, BMI, total cholesterol, hypertension, diabetes mellitus, smoking status, and eGFR categories.
Regarding the mortality from various non-CV causes and site-specific cancers, only age, gender, and eGFR categories were adjusted in the initial Cox proportional hazards model because of smaller sample sizes. For cancers that were found to be significantly associated with CKD, we further adjusted for individual cancer-specific risk factors. Possible interactions between CKD and other covariates were examined. To test for a linear trend across eGFR categories, we used the median eGFR level for each category as a continuous variable in the multivariate model. Sensitivity analyses that excluded deaths within the first 2 years of follow-up were conducted to eliminate the possible influence from undiagnosed prevalent cancers. The statistically significant level (P value) was set at 0.05 (two-sided). These statistical analyses were performed using the PC version of SPSS statistical software (15th version; SPSS, Chicago, IL).
Results
Baseline Characteristics
The mean ± SD age of the study population was 42.8 ± 13.9 years, with 47.3% being men. The mean eGFR was 88.5 ± 18.8 ml/min per 1.73 m2, and there were 5150 (4.2%) CKD patients. The baseline characteristics of the participants are summarized in Table 1, stratified by CKD. CKD patients were older and had a higher BMI, higher levels of fasting glucose, total cholesterol, and triglycerides than their non-CKD counterparts. In addition, more CKD patients were men (66.3%), had a higher prevalence of hypertension (58.5%) and diabetes mellitus (13.1%), and were current smokers (24.3%) and alcohol drinkers (19.6%).
Table 1.
Baseline characteristics according to the presence of CKD
| Non-CKD (n = 118,567) | CKD (n = 5,150) | P | |
|---|---|---|---|
| Gender (% male) | 46.5 | 66.3 | <0.001 |
| Age (years) | 42.0 ± 13.4 | 61.5 ± 11.9 | <0.001 |
| BMI (kg/m2) | 23.0 ± 3.5 | 24.5 ± 3.3 | <0.001 |
| Fasting glucose (mg/dl) | 98 ± 23 | 107 ± 35 | <0.001 |
| TCHOL (mg/dl) | 200 ± 38 | 215 ± 42 | <0.001 |
| Triglycerides (mg/dl) | 122 ± 104 | 164 ± 126 | <0.001 |
| HDL-C (mg/dl) | 48.5 ± 15.9 | 44.4 ± 16.2 | <0.001 |
| Hypertension (%) | 19.6 | 58.5 | <0.001 |
| Diabetes mellitus (%) | 4.4 | 13.1 | <0.001 |
| Smoking status | |||
| never smoker (%) | 71.6 | 61.8 | <0.001 |
| former smoker (%) | 6.2 | 13.9 | |
| current smoker (%) | 22.1 | 24.3 | |
| Alcohol drinking (%) | 17.6 | 19.6 | <0.001 |
Data is presented as mean ± SD for continuous variables. CKD was defined as an eGFR of <60 ml/min per 1.73 m. TCHOL, total cholesterol; HDL-C, HDL cholesterol.
Main Causes of Death across CKD Stages
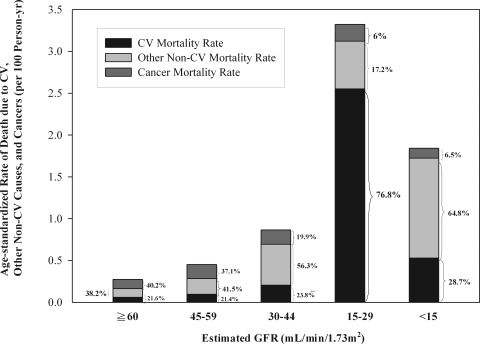
During a median follow-up of 7.06 years (862,842 person-years), 2710 deaths were ascertained. Among these, 523 deaths (19.3%) were from CV causes, and 1117 deaths (41.2%) were from cancers. The rates for age-standardized all-cause mortality, CV mortality, and cancer mortality across different CKD stages are shown in Figure 1. As eGFR declined, there was an incremental increase in age-standardized all-cause mortality, especially patients with CKD stage 4 (eGFR of 15 to 29 ml/min per 1.73 m2). Among non-CKD patients, cancer was the most common cause of death (40.2%), followed by other non-CV causes (38.2%) and CV causes (21.6%). However, the percentage of cancer mortality decreased in patients with more advanced CKD stages, whereas the percentage of CV mortality markedly increased. It is notable that the mortality data of patients with CKD stage 5 might be less representative of the real condition, because many of these patients might have already been receiving dialysis and were not included in our study.
Figure 1.
Age-standardized mortality rate due to CV causes, other non-CV causes, and cancer (per 100 person-years), according to estimated GFR. Other non-CV mortality rate refers to the non-CV mortality rate after exclusion of cancer.
CV and Non-CV Mortality in CKD Patients
By using Cox proportional hazards regression model, we found a higher risk for all-cause and CV mortality among patients with more advanced CKD stages compared with those with an eGFR >60 ml/min per 1.73 m2 (Table 2). Among those who died of CV causes, CKD was significantly associated with an increased risk for deaths caused by stroke and coronary heart disease.
Table 2.
HRs for eGFR on cause-specific mortality
| Causes of Death | eGFR (ml/min per 1.73 m2) |
P, trend | CKD Status |
|||||
|---|---|---|---|---|---|---|---|---|
| ≥60 | 45 to 59 | 30 to 44 | 15 to 29 | <15 | Non-CKD | CKD | ||
| All-cause mortalitya (n = 2,710) | 1 (Ref) | 1.35 (1.20 to 1.51)c | 2.30 (1.89 to 2.78)c | 3.50 (2.58 to 4.76)c | 6.39 (4.30 to 9.49)c | <0.001 | 1 (Ref) | 1.62 (1.46 to 1.79)c |
| CV mortalitya (n = 523) | 1 (Ref) | 1.62 (1.28 to 2.06)c | 2.81 (1.93 to 4.09)c | 2.59 (1.27 to 5.26)d | 9.14 (4.31 to 19.37)c | <0.001 | 1 (Ref) | 1.92 (1.55 to 2.36)c |
| stroke mortalitya (n = 174) | 1 (Ref) | 1.37 (0.89 to 2.12) | 1.89 (0.86 to 4.12) | 2.06 (0.50 to 8.41) | 13.92 (5.11 to 37.95)c | <0.001 | 1 (Ref) | 1.64 (1.12 to 2.40)e |
| CHD mortalitya (n = 150) | 1 (Ref) | 1.78 (1.15 to 2.73)d | 3.92 (2.13 to 7.21)c | 4.20 (1.50 to 11.73)d | 15.03 (4.73 to 47.79)c | <0.001 | 1 (Ref) | 2.31 (1.59 to 3.36)c |
| Non-CV mortalityb (n = 2,187) | 1 (Ref) | 1.25 (1.10 to 1.42)d | 2.21 (1.77 to 2.75)c | 3.83 (2.75 to 5.32)c | 6.89 (4.43 to 10.72)c | <0.001 | 1 (Ref) | 1.52 (1.36 to 1.70)c |
| cancer mortalityb (n = 1,117) | 1 (Ref) | 1.10 (0.91 to 1.32) | 1.54 (1.08 to 2.20)e | 2.12 (1.17 to 3.86)e | 1.93 (0.62 to 5.98) | 0.004 | 1 (Ref) | 1.20 (1.02 to 1.42)e |
| infection mortalityb (n = 99) | 1 (Ref) | 1.11 (0.66 to 1.86) | 1.69 (0.72 to 4.00) | 3.59 (1.11 to 11.64)e | NA | 0.135 | 1 (Ref) | 1.28 (0.81 to 2.02) |
| pulmonary mortalityb (n = 80) | 1 (Ref) | 1.57 (0.92 to 2.67) | 1.16 (0.35 to 3.78) | 1.65 (0.23 to 12.08) | NA | 0.174 | 1 (Ref) | 1.50 (0.90 to 2.48) |
| diabetes m. mortalityb (n = 164) | 1 (Ref) | 1.98 (1.30 to 3.01)d | 6.89 (4.13 to 11.49)c | 8.30 (3.58 to 19.23)c | 15.71 (4.97 to 49.64)c | <0.001 | 1 (Ref) | 3.06 (2.17 to 4.32)c |
| cirrhosis mortalityb (n = 102) | 1 (Ref) | 0.77 (0.39 to 1.52) | 1.37 (0.42 to 4.41) | 2.00 (0.28 to 14.50) | 6.84 (0.95 to 49.24) | 0.686 | 1 (Ref) | 0.95 (0.53 to 1.69) |
Values expressed as HR (95% CI). CHD, coronary heart disease; diabetes m., diabetes mellitus; ref, reference; NA, not applicable.
All-cause mortality and CV mortality were adjusted for age, gender, BMI, total cholesterol, hypertension, diabetes mellitus, smoking status, and eGFR categories.
Non-CV mortality was adjusted for age, gender, and eGFR categories.
P < 0.001.
P < 0.01.
P < 0.05.
CKD patients also had a higher risk of non-CV mortality, including cancer (HR, 1.20; 95% confidence interval [CI], 1.02 to 1.42; P = 0.031) and diabetes mellitus (HR, 3.06; 95% CI, 2.17 to 4.32; P < 0.001) compared with non-CKD patients (Table 2). A graded relationship between decreased eGFR and cancer mortality was observed (P for trend = 0.001). The adjusted HRs for infection and pulmonary disease–related deaths were slightly higher in CKD but were statistically insignificant.
Site-Specific Cancer Mortality in CKD Patients
The main objective of this study was to explore the relationship between CKD and site-specific cancers. In our cohort, lung cancer, liver cancer, and colon cancer were the three leading cancer-related deaths (by age-standardized mortality rate), which is consistent with the national survey data (12). Meanwhile, the most common cancer-related death in our CKD participants was liver cancer, followed by lung cancer and urological cancers (including kidney cancer and urinary tract cancer).
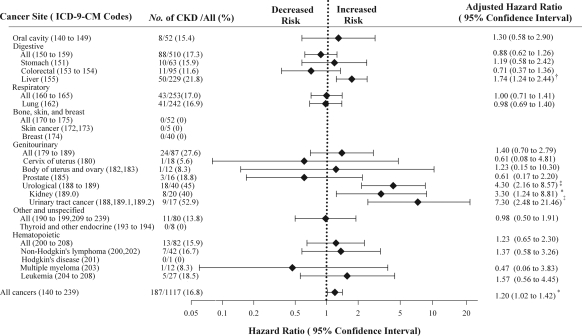
As shown in Figure 2, CKD was significantly associated with mortality caused by liver cancer, kidney cancer, and urinary tract cancer (the latter included cancers of the renal pelvis, bladder, and ureter), with the gender- and age-adjusted HRs of 1.74 (95% CI, 1.24 to 2.44; P = 0.001), 3.30 (95% CI, 1.24 to 8.81; P = 0.017), and 7.30 (95% CI, 2.48 to 21.46; P < 0.001), respectively. We further studied the relationship between renal impairment (across different CKD stages) and the mortality from these three types of cancers, with additional adjustments for individual cancer-specific risk factors (Table 3). The mortality risk of liver cancer increased steadily with the gradual decline in the eGFR, after adjustment for age, gender, HBsAg, diabetes mellitus, smoking status, and alcohol drinking habit. The adjusted HRs of liver cancer for patients with an eGFR of 45 to 59, 30 to 44, 15 to 29, and <15 ml/min per 1.73 m2 were 1.46, 2.99, 4.74, and 3.56, respectively, compared with those with an eGFR of >60 ml/min per 1.73 m2 (P for trend <0.001).
Figure 2.
Hazard ratios for CKD on site-specific cancers. Values are expressed as HR (95% CI), adjusted for age, gender, and CKD status. *P < 0.05; †P < 0.01; and ‡P < 0.001.
Table 3.
HRs for eGFR on deaths from liver cancer, kidney cancer, and urinary tract cancer
| Causes of Death | eGFR (ml/min per 1.73 m2) |
P, trend | CKD Status |
|||||
|---|---|---|---|---|---|---|---|---|
| ≥60 | 45 to 59 | 30 to 44 | 15 to 29 | <15 | Non-CKD | CKD | ||
| Liver cancera (n = 231) | 1 (Ref) | 1.46 (0.97 to 2.18) | 2.99 (1.59 to 5.64)c | 4.74 (1.73 to 12.98)c | 3.56 (0.50 to 25.5) | <0.001 | 1 (Ref) | 1.79 (1.26 to 2.54)c |
| Urological cancerb (n = 40) | 1 (Ref) | 2.89 (1.23 to 6.76)d | 5.80 (1.62 to 20.82)c | 22.61 (6.23 to 82.06)e | NA | <0.001 | 1 (Ref) | 3.83 (1.81 to 8.09)e |
| Kidney cancerb (n = 20) | 1 (Ref) | 1.60 (0.42 to 6.12) | 6.45 (1.31 to 31.89)d | 12.89 (1.52 to 108.97)d | NA | 0.02 | 1 (Ref) | 2.55 (0.86 to 7.52) |
| Urinary tract cancerb (n = 17) | 1 (Ref) | 7.32 (2.07 to 25.93)d | NA | 56.94 (9.80 to 330.84)e | NA | <0.001 | 1 (Ref) | 7.79 (2.34 to 25.98)d |
Values expressed as HR (95% CI). Ref, reference; NA, not applicable.
Deaths caused by liver cancer were adjusted for age, gender, hepatitis B surface antigen, diabetes mellitus, smoking status, drinking habits, and eGFR category.
Deaths caused by urological cancer, kidney cancer, and urinary tract cancer were adjusted for age, gender, smoking status, and eGFR category.
P < 0.05.
P < 0.01.
P < 0.001.
The mortality risk of kidney cancer and urinary tract cancer also increased dramatically with the decline of eGFR, after adjustment for age, gender, and smoking status (P for trend = 0.02 for kidney cancer; P for trend <0.001 for urinary tract cancer). Compared with patients with an eGFR of >60 ml/min per 1.73 m2, those with an eGFR of 15 to 29 ml/min per 1.73 m2 (CKD stage 4) had a 12.9- and 56.9-fold mortality risk for kidney cancer and urinary tract cancer, respectively.
Sensitivity analyses that excluded deaths within the first 2 years of follow-up showed similar results (data not shown).
Discussion
In this longitudinal follow-up of a large cohort, we showed a significant graded relationship between the severity of CKD and cancer mortality. Deaths from liver cancer, kidney cancer, and urinary tract cancer increased incrementally with the severity of renal impairment. To the best of our knowledge, this is the first large study that showed an increased mortality of liver cancer in non–dialysis-dependent CKD patients.
In the past decade, several epidemiologic surveys have reported a higher incidence of cancer in ESRD patients on long-term dialysis, which was mostly attributed to kidney cancer and urinary tract cancer (13). An increased incidence of thyroid cancer, other endocrine cancers, virus-related cancers, skin cancer, and liver cancer has also been reported in ESRD patients (14–16).
Meanwhile, the relationship between non–dialysis-dependent CKD and site-specific cancers was far less clear. A recent study by Wong et al. (5) reported an increased incidence of lung cancer and urinary tract cancer among CKD patients in Australia. In the other studies, an increased incidence and aggressiveness of upper urinary tract urothelial carcinoma was also found among CKD patients (17,18), which was considered to be associated with analgesic nephropathy or Chinese herb nephropathy (19,20). Our findings regarding the mortality of kidney cancer and urinary tract cancer confirmed and extended the previous observations.
The new finding of this study is the increased mortality caused by liver cancer in non–dialysis-dependent CKD patients. With a high prevalence of viral hepatitis in the Far East, we were able to examine the effect of CKD on liver cancer, which was not reported in previous studies because of small case numbers. We propose some possible explanations for this observation. First, there may be an increased incidence of liver cancer in CKD patients. The most important risk factors for liver cancer are viral hepatitis B and C, and an increased prevalence of hepatitis C virus (HCV) infection in advanced CKD stages was reported recently (21). We have adjusted for seropositivity of HBsAg in the multivariate analysis, but the data of HCV infection are lacking. Assuming a 3.8 times risk of liver cancer mortality for HCV carriers versus non-HCV carriers (22) and a similar prevalence of HCV carrier as in a recent local study (21), the estimated risk of liver cancer mortality in CKD versus non-CKD would be around 1.1 to 1.2 (23) (refer to Supplementary Information for details). Such estimated risk seems insufficient to explain our finding (HR of CKD on liver cancer mortality = 1.79, P = 0.001), implying that other factors may also be involved. Besides HCV infection, it is also possible that accumulated environmental carcinogens in CKD patients play a certain role in the pathogenesis of liver cancer. Several animal and epidemiology studies have reported that aristolochic acid, mycotoxins, and arsenic are associated with both renal impairment and liver cancer (24–28). Among those, arsenic contamination of the underground water used to be a health problem in parts of southern Taiwan, where arsenic exposure was found to associated with increased incidence of liver cancer, urological cancers, and also worse renal function (29,30). It is also notable that CKD is associated with increased liver cancer mortality in women (adjusted HR = 3.68; 95% CI, 2.01 to 6.75) but is less significant in men (HR = 1.35; 95% CI, 0.88 to 2.07). One possible explanation is that CKD is associated with hypogonadism (31) and thus reduced the protective effect of estrogen on female liver cancer (32).
On the other hand, the increased liver cancer mortality could also be caused by a worse response to cancer treatment in CKD patients. It is known that CKD patients have a higher risk of developing contrast nephropathy after transarterial chemoembolization (TACE) (33). A recent prospective study further showed that pre-existing renal insufficiency independently predicted increased long-term mortality after TACE (34). Because of a lack of evidence-based guidelines, CKD has been historically regarded as a relative contraindication for TACE by many clinicians (35). Therefore, it is also likely that there is underuse of necessary diagnostic imaging or intervention in CKD patients for fear of contrast nephropathy (36).
Another issue of concern is that some advanced CKD patients might have progressed to ESRD during the follow-up period. According to a recent hospital-based study in Taiwan, the authors reported a mean annual decline in eGFR of 2.24 ml/min per 1.73 m2 for CKD stages 3 (37). Meanwhile, 94.6% of the CKD patients were classified as CKD stage 3a or 3b (eGFR 30 to 59 ml/min per 1.73 m2) at the entry of our study. Thus, only a small proportion of CKD patients were expected to develop ESRD during the 7-year follow-up, which was unlikely to bias the results significantly. The strong and graded inverse association between eGFR and cancer mortality also adds to confidence in the robustness of our conclusion.
There are some limitations that need to be addressed in this study. First, the definition of CKD in our cohort relied on a single parameter, serum creatinine, which might lead to misclassification of the CKD stages in some participants. Second, we were unable to differentiate whether the increased cancer mortality was because of increased cancer incidence or worse cancer prognosis.
In conclusion, besides the increased risk for CV mortality, this study showed that CKD is an important risk factor for cancer mortality. Screening for liver cancer, kidney cancer, and urinary tract cancer in CKD patients might offer clinical benefits. It is worthwhile to conduct studies to evaluate the efficacy and safety of anti-cancer treatment in cancer patients with concomitant renal insufficiency and to assess whether the medical utilization differs in CKD patients. More future studies are warranted to better understand the associated risks and the underlying mechanisms.
Disclosures
None.
Acknowledgments
We thank Dr. Kun-Pei Lin for assistance in data analysis and the registered health practitioners of the MJ Health Screening Center in Taiwan for assistance.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. U.S. Renal Data System: USRDS 2009 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2009 [Google Scholar]
- 2. Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, Horng SS, Chang YK, Yang WC: High prevalence and low awareness of CKD in Taiwan: A study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis 48: 727–738, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Fried LF, Katz R, Sarnak MJ, Shlipak MG, Chaves PH, Jenny NS, Stehman-Breen C, Gillen D, Bleyer AJ, Hirsch C, Siscovick D, Newman AB: Kidney function as a predictor of noncardiovascular mortality. J Am Soc Nephrol 16: 3728–3735, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Wong G, Hayen A, Chapman JR, Webster AC, Wang JJ, Mitchell P, Craig JC: Association of CKD and cancer risk in older people. J Am Soc Nephrol 20: 1341–1350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF: All-cause mortality attributable to chronic kidney disease: A prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY: Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 17: 2937–2944, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Huang KC, Lin WY, Lee LT, Chen CY, Lo H, Hsia HH, Liu IL, Shau WY, Lin RS: Four anthropometric indices and cardiovascular risk factors in Taiwan. Int J Obes Relat Metab Disord 26: 1060–1068, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Ahmad OB, Boschi-Pinto C, Lopez AD, Murray CJ, Lozano R, Inoue M: Age Standardization of Rates: A New WHO Standard, Geneva, World Health Organization, 2001 [Google Scholar]
- 10. Pintilie M: Analyzing and interpreting competing risk data. Stat Med 26: 1360–1367, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Schoenfeld D: Partial residuals for the proportional hazards regression-model. Biometrika 69: 239–241, 1982 [Google Scholar]
- 12. Cancer Registry Annual Report, China, Department of Health, The Executive Yuan, Taipei, Taiwan: 2005 [Google Scholar]
- 13. Stewart JH, Buccianti G, Agodoa L, Gellert R, McCredie MR, Lowenfels AB, Disney AP, Wolfe RA, Boyle P, Maisonneuve P: Cancers of the kidney and urinary tract in patients on dialysis for end-stage renal disease: Analysis of data from the United States, Europe, and Australia and New Zealand. J Am Soc Nephrol 14: 197–207, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Birkeland SA, Lokkegaard H, Storm HH: Cancer risk in patients on dialysis and after renal transplantation. Lancet 355: 1886–1887, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, Wolfe RA, Jones E, Disney AP, Briggs D, McCredie M, Boyle P: Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 354: 93–99, 1999 [DOI] [PubMed] [Google Scholar]
- 16. Buccianti G, Maisonneuve P, Ravasi B, Cresseri D, Locatelli F, Boyle P: Cancer among patients on renal replacement therapy: A population-based survey in Lombardy, Italy. Int J Cancer 66: 591–593, 1996 [DOI] [PubMed] [Google Scholar]
- 17. Chen CY, Liao YM, Tsai WM, Kuo HC: Upper urinary tract urothelial carcinoma in eastern Taiwan: High proportion among all urothelial carcinomas and correlation with chronic kidney disease. J Formos Med Assoc 106: 992–998, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hung PH, Shen CH, Chiu YL, Jong IC, Chiang PC, Lin CT, Hung KY, Tsai TJ: The aggressiveness of urinary tract urothelial carcinoma increases with the severity of chronic kidney disease. BJU Int 104: 1471–1474, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Bengtsson U, Johansson S, Angervall L: Malignancies of the urinary tract and their relation to analgesic abuse. Kidney Int 13: 107–113, 1978 [DOI] [PubMed] [Google Scholar]
- 20. Nortier JL, Martinez MC, Schmeiser HH, Arlt VM, Bieler CA, Petein M, Depierreux MF, De Pauw L, Abramowicz D, Vereerstraeten P, Vanherweghem JL: Urothelial carcinoma associated with the use of a Chinese herb (Aristolochia fangchi). N Engl J Med 342: 1686–1692, 2000 [DOI] [PubMed] [Google Scholar]
- 21. Lee JJ, Lin MY, Yang YH, Lu SN, Chen HC, Hwang SJ: Association of hepatitis C and B virus infection with CKD in an endemic area in Taiwan: A cross-sectional study. Am J Kidney Dis 56: 23–31, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Chang KC, Tsai PS, Hsu MC, Hung SF, Tsai CC, Lu SN: Chronic hepatitis C increased the mortality rates of patients with hepatocellular carcinoma and diabetes mellitus in a triple hepatitis virus endemic community. J Gastroenterol 45: 636–645, 2010 [DOI] [PubMed] [Google Scholar]
- 23. Wang J-D: Basic Principles and Practical Applications in Epidemiological Research, Singapore, World Scientific Publishing, 2002 [Google Scholar]
- 24. Rossiello MR, Laconi E, Rao PM, Rajalakshmi S, Sarma DS: Induction of hepatic nodules in the rat by aristolochic acid. Cancer Lett 71: 83–87, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Debelle FD, Vanherweghem JL, Nortier JL: Aristolochic acid nephropathy: A worldwide problem. Kidney Int 74: 158–169, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Wild CP, Gong YY: Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 31: 71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu J, Waalkes MP: Liver is a target of arsenic carcinogenesis. Toxicol Sci 105: 24–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Glahn RP, Beers KW, Bottje WG, Wideman RF, Jr, Huff WE, Thomas W: Aflatoxicosis alters avian renal function, calcium, and vitamin D metabolism. J Toxicol Environ Health 34: 309–321, 1991 [DOI] [PubMed] [Google Scholar]
- 29. Chen CJ, Chen CW, Wu MM, Kuo TL: Cancer potential in liver, lung, bladder and kidney due to ingested inorganic arsenic in drinking water. Br J Cancer 66: 888–892, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsueh YM, Chung CJ, Shiue HS, Chen JB, Chiang SS, Yang MH, Tai CW, Su CT: Urinary arsenic species and CKD in a Taiwanese population: A case-control study. Am J Kidney Dis 54: 859–870, 2009 [DOI] [PubMed] [Google Scholar]
- 31. Lim VS: Reproductive function in patients with renal insufficiency. Am J Kidney Dis 9: 363–367, 1987 [DOI] [PubMed] [Google Scholar]
- 32. Yeh SH, Chen PJ: Gender disparity of hepatocellular carcinoma: The roles of sex hormones. Oncology 78[Suppl 1]: 172–179, 2010 [DOI] [PubMed] [Google Scholar]
- 33. McCullough PA, Adam A, Becker CR, Davidson C, Lameire N, Stacul F, Tumlin J: Risk prediction of contrast-induced nephropathy. Am J Cardiol 98: 27K–36K, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Hsu CY, Huang YH, Su CW, Chiang JH, Lin HC, Lee PC, Lee FY, Huo TI, Lee SD: Transarterial chemoembolization in patients with hepatocellular carcinoma and renal insufficiency. J Clin Gastroenterol 44: e171–e177, 2010 [DOI] [PubMed] [Google Scholar]
- 35. Jansen MC, van Hillegersberg R, Chamuleau RA, van Delden OM, Gouma DJ, van Gulik TM: Outcome of regional and local ablative therapies for hepatocellular carcinoma: A collective review. Eur J Surg Oncol 31: 331–347, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Chertow GM, Normand SL, McNeil BJ: “Renalism”: inappropriately low rates of coronary angiography in elderly individuals with renal insufficiency. J Am Soc Nephrol 15: 2462–2468, 2004 [DOI] [PubMed] [Google Scholar]
- 37. Chiu YL, Chien KL, Lin SL, Chen YM, Tsai TJ, Wu KD: Outcomes of stage 3–5 chronic kidney disease before end-stage renal disease at a single center in Taiwan. Nephron Clin Pract 109: c109–c118, 2008 [DOI] [PubMed] [Google Scholar]