Abstract
Summary
Background and objectives
The choice of induction agent in the elderly kidney transplant recipient is unclear.
Design, setting, participants, & measurements
The risks of rejection at 1 year, functional graft loss, and death by induction agent (IL2 receptor antibodies [IL2RA], alemtuzumab, and rabbit antithymocyte globulin [rATG]) were compared among five groups of elderly (≥60 years) deceased-donor kidney transplant recipients on the basis of recipient risk and donor risk using United Network of Organ Sharing data from 2003 to 2008.
Results
In high-risk recipients with high-risk donors there was a higher risk of rejection and functional graft loss with IL2RA versus rATG. Among low-risk recipients with low-risk donors there was no difference in outcomes between IL2RA and rATG. In the two groups in which donor or recipient was high risk, there was a higher risk of rejection but not functional graft loss with IL2RA. Among low-risk recipients with high-risk donors, there was a trend toward a higher risk of death with IL2RA.
Conclusions
rATG may be preferable in high-risk recipients with high-risk donors and possibly low-risk recipients with high-risk donors. In the remaining groups, although rATG is associated with a lower risk of acute rejection, long-term outcomes do not appear to differ. Prospective comparison of these agents in an elderly cohort is warranted to compare the efficacy and adverse consequences of these agents to refine the use of induction immunosuppressive therapy in the elderly population.
Introduction
The elderly comprise the largest growing segment of patients with End Stage Renal Disease (ESRD) and are the fasting growing segment of transplant recipients (1–3).
Immunosuppressive management in the elderly transplant recipient is increasingly complex, with higher risks of infection posttransplantation (4–6) and decreased immunogenicity (4,7,8) with increasing age. It is further complicated by the preferential allocation of higher risk donor organs to the elderly (9), modifying the baseline risks for posttransplant outcomes in this group of patients. In particular, the role and choice of induction agent remains unclear in the elderly because there are few data to inform the risks and outcomes associated with induction use in this population (3).
In this paper we outline the use of induction immunosuppressive agents in elderly transplant recipients in the United States and examine the association between choice of induction therapy and outcomes after kidney transplantation in an attempt to inform a risk-stratified approach to induction therapy in the elderly kidney transplant recipient.
Materials and Methods
Study Design
All recipients of deceased-donor kidney-only transplants who were transplanted between January 1, 2003 and December 31, 2008 (with follow-up until March 1, 2009) and were aged ≥60 years at the time of transplant were identified using data from the Organ Procurement Transplantation Network/United Network of Organ Sharing (OPTN/UNOS). Patients in whom no immunosuppressive therapy was reported or those who reportedly received multiple induction agents were excluded. The cohort was then restricted to those who received an Interleukin 2 receptor antibody (IL2RA), rabbit antithymocyte globulin (rATG), or alemtuzumab induction. Those who received other induction agents comprised a very small proportion and were excluded, leaving a total of 14,820 patients.
This cohort was then divided into four subgroups using clinical characteristics selected a priori on the basis of donor and recipient risk factors as outlined in Figure 1. The four subgroups included (1) high-immunologic-risk recipients with a kidney from a low-risk donor, (2) high-immunologic-risk recipients with a kidney from a high-risk donor, (3) low-immunologic-risk recipients with a kidney from a high-risk donor, and (4) low-immunologic-risk recipients with a kidney from a low-risk donor (Figure 1). High-immunologic-risk recipients were defined as those with a peak Panel Reactive Antibodies (PRA) > 20%, prior kidney transplantation, or black race. The cutoff of peak PRA > 20% was based on cutoffs used in prospective studies (10). High-risk donors were defined as those meeting the criteria of expanded criteria donor (ECD), donors after cardiac death (DCD), or having a cold ischemic time (CIT) > 24 hours. Within each subgroup, acute rejection at 1 year, death-censored graft survival, and patient survival were compared by induction agent. Acute rejection rates were determined as reported acute rejection episodes by individual centers to UNOS and were not restricted to biopsy-proven acute rejection episodes. Functional graft survival was determined from the date of transplantation until retransplantation or return to dialysis therapy; patients were censored at the time of death or at the end of the study period.
Figure 1.
Cohort of all deceased-donor kidney transplant recipients aged ≥60 years between 2000 and 2008 using data from OPTN/UNOS.
The definition of “elderly” has varied in prior studies and reports. The cohort age cutoff of ≥60 years was chosen in this study because this is consistent with prior reports (11–14) and allowed sufficient power for multivariate analyses in each strata. To account for the age range in this elderly cohort, recipient age was stratified and included in each multivariate model. In addition, more parsimonious multivariate models were repeated excluding patients <65 years of age and yielded similar results where power was sufficient (data not shown).
Statistical Methods
Donor, recipient, and transplant characteristics were described using means (±SD) or frequency. Continuous variables, including age, CIT, duration of dialysis, and peak PRA were categorized because these effects on outcome were not linear. Comparisons between groups were made using the Wilcoxon rank-sum test for continuous variables and the Kruskall–Wallis or χ2 test for categorical variables.
Patient and graft survival were estimated using the Kaplan–Meier product-limit method and comparisons between groups were made using the log-rank test. Logistic and Cox regression models were fitted to determine the risk of acute rejection at 1 year, death-censored graft loss, and patient death for recipients given IL2RA and alemtuzumab compared with rATG. In addition, 1-year patient and death-censored graft survival were examined as outcomes to account for variable follow-up times in the study groups. Unadjusted cause-specific hazards of death were also examined using competing risk Cox models.
All donor, recipient, and transplant factors significantly (P < 0.10) associated with the outcome on univariate analyses or deemed clinically relevant were included as covariates in the multivariate models. The need for dialysis within the first week after transplantation was included in each model to account for potential confounding by indication in the study design. The proportional hazards assumption was tested for variables in the models using time-varying covariates. Visual inspection of log(−log S[t]) versus log t plots across primary categorical variables did not indicate serious violations to the proportional hazards assumptions. To adjust for variation in clinical practice by transplant year, this factor was included in all multivariate models. For all variables, missing data were categorized as such and entered in multivariate models. All analyses were performed using Stata Statistical Software, release 9.1 (StataCorp LP, College Station, TX).
Results
Induction Use Over Time
Figure 2 outlines the use and choice of induction therapy over time among recipients aged ≥60 years, demonstrating an increase in the use of induction immunosuppression. Although the use of T lymphocyte depleting agents has increased, the use of IL2RA appears to be decreasing over time. Similar trends for induction use were seen in patients aged 18 to 60 years, suggesting these trends are not age specific (data not shown).
Figure 2.
Induction use over time among deceased-donor kidney-only transplant recipients aged ≥60 years.
Baseline Characteristics (Table 1)
Table 1.
Characteristics of deceased-donor kidney transplant recipients aged ≥60 years reported to receive induction immunosuppression
| Factor | rATG (n = 7140) | Alemtuzumab (n = 1465) | IL2RA (n = 6215) | P |
|---|---|---|---|---|
| Mean donor age (years ± SD) | 43.7 ± 17.4 | 44.6 ± 17.8 | 42.0 ± 17.5 | <0.001 |
| Donor age categories (%) | <0.001 | |||
| <60 years | 80.1 | 78.0 | 83.1 | |
| 60 to 64 years | 10.4 | 10.4 | 9.1 | |
| 65 to 69 years | 6.1 | 7.8 | 5.0 | |
| ≥70 years | 3.5 | 3.9 | 2.9 | |
| Male donor (%) | 57.3 | 55.5 | 57.8 | 0.29 |
| AA donor (%) | 11.6 | 12.8 | 9.5 | <0.001 |
| Donor death due to stroke (%) | 47.7 | 47.4 | 45.7 | 0.07 |
| Mean donor serum creatinine (mg/dl) | 1.15 ± 0.93 | 1.12 ± 0.76 | 1.10 ± 1.02 | <0.001 |
| Donor diabetes (%) | 8.7 | 9.2 | 6.5 | <0.001 |
| ECD (%) | 32.5 | 35.0 | 27.3 | <0.001 |
| DCD (%) | 9.4 | 11.5 | 6.4 | <0.001 |
| Mean recipient age (years ± SD) | 65.8 ± 4.5 | 66.1 ± 4.8 | 66.2 ± 4.8 | <0.001 |
| Recipient age categories (%) | ||||
| 60 to 69 years | 79.7 | 78.2 | 76.6 | <0.001 |
| ≥70 years | 20.3 | 21.8 | 23.4 | |
| Male recipient (%) | 60.3 | 61.6 | 63.8 | <0.001 |
| AA recipient (%) | 25.7 | 21.6 | 19.1 | <0.001 |
| Prior transplant (%) | 6.4 | 5.9 | 3.7 | <0.001 |
| Mean CIT (hours) | 18.4 ± 8.4 | 21.2 ± 9.4 | 18.2 ± 8.0 | <0.001 |
| missing (%) | 9.9 | 10.6 | 8.2 | |
| Pretransplant dialysis duration (%) | 0.006 | |||
| pre-emptive | 11.3 | 14.2 | 11.0 | |
| 0–1 year | 9.1 | 10.6 | 9.7 | |
| 1 to 3 years | 31.7 | 31.4 | 35.0 | |
| >3 years | 44.1 | 39.5 | 41.3 | |
| Peak PRA (%) | ||||
| 0 | 53.0 | 41.7 | 59.2 | <0.001 |
| <20 | 22.4 | 31.8 | 23.8 | |
| 20 to 50 | 7.5 | 6.4 | 6.2 | |
| >50 | 14.2 | 12.5 | 7.1 | |
| missing | 2.8 | 7.7 | 3.7 | |
| Recipient hypertension (%) | 76.9 | 72.4 | 78.7 | <0.001 |
| missing | 12.4 | 14.5 | 8.8 | |
| Recipient diabetes mellitus (%) | 30.1 | 29.4 | 29.8 | <0.001 |
| missing | 15.8 | 20.4 | 13.9 | |
| Cause of ESRD (%) | <0.001 | |||
| glomerulonephritis | 10.1 | 9.4 | 11.3 | |
| diabetes | 7.4 | 2.9 | 14.7 | |
| hypertension | 28.1 | 27.0 | 24.0 | |
| other | 54.4 | 60.8 | 49.9 | |
| Zero ABDR HLA mismatches (%) | 13.0 | 13.7 | 15.6 | <0.001 |
| Pulsatile perfusion (%) | 26.0 | 31.9 | 20.2 | <0.001 |
| Immunosuppression at discharge (%) | ||||
| CNI use at discharge (%) | <0.001 | |||
| none | 6.3 | 9.1 | 7.9 | |
| cyclosporine | 12.2 | 6.1 | 31.6 | |
| tacrolimus | 82.5 | 83.8 | 60.1 | |
| AZA/MPA/mTOR at discharge (%) | <0.001 | |||
| none | 5.0 | 19.9 | 3.9 | |
| AZA | 1.0 | 0.2 | 1.9 | |
| MPA | 84.7 | 78.1 | 84.8 | |
| sirolimus/everolimus | 5.7 | 1.4 | 4.7 | |
| Steroid at discharge (%) | 68.1 | 29.6 | 89.2 | <0.001 |
| missing | 2.9 | 11.5 | 7.8 | |
| Dialysis in first week (%) | 27.0 | 20.6 | 22.7 | <0.001 |
Proportion of missing not provided if <5%. AA, African American; AZA, azathioprine; CNI, calcineurin inhibitor; MPA, mycophenolicacid; mTOR, mammalian target of rapamycin.
Compared with patients who received rATG induction, those who received induction with an IL2RA appeared to have a lower immunologic risk because they included fewer African American patients, fewer patients with a prior transplant, more patients with a PRA < 20%, and more patients with zero HLA mismatches. Recipients in the IL2RA group received more kidneys from lower risk donors who were younger and had a lower mean terminal serum creatinine level, with fewer donors that were African American, died of a stroke, had a history of diabetes mellitus or hypertension, and qualified as ECD or DCD.
Compared with patients that received rATG, those who received alemtuzumab included fewer African American patients, fewer patients with a prior transplant, more pre-emptive transplant recipients, and more patients with zero HLA mismatches. In addition, alemtuzumab recipients included fewer patients with a history of hypertension but had longer mean CIT. Donors in the alemtuzumab group included more donors that were African American, had diabetes, and were DCD. However, donor death due to cerebrovascular accident was more common in the rATG group.
Maintenance Immunosuppression at Discharge
In the rATG group, 94% were discharged on a calcineurin inhibitor (CNI), 85% on a mycophenolic acid agent (MPA), and 68% on steroids at discharge. Similarly in the IL2RA group, 92% of patients were discharged on a CNI, but cyclosporine use was more common (32% with IL2RA versus 12% with rATG). Also, 85% were discharged on an MPA, but steroid use was more common (89%). In the alemtuzumab group CNI use remained common (91%); however, MPA use was less common (78%) and only 30% of patients were discharged on steroid therapy.
Donor/Recipient Risk Groups
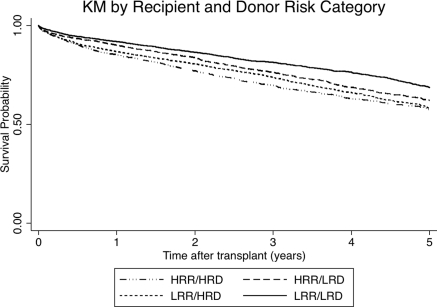
Recipient, donor, and transplant characteristics for each risk strata are outlined in Table 2, demonstrating reasonable stratification of donor and recipient risk factors for graft loss. Importantly maintenance immunosuppressive protocols appear relatively similar in the different risk categories. Figure 3 demonstrates Kaplan–Meier curves for overall graft survival (including death) for each risk group, with the best survival in the low-risk recipient and donor group and the worst in the high-risk recipient and donor group.
Table 2.
Baseline characteristics by prespecified recipient and donor risk strata
| Characteristics | High-Immunologic-Risk Recipient |
Low-Immunologic-Risk Recipient |
||
|---|---|---|---|---|
| High-Risk Donor (n = 2849) | Low-Risk Donor (n = 3155) | High-Risk Donor (n = 4468) | Low-Risk Donor (n = 4348) | |
| Mean recipient age (years ± SD) | 65.7 ± 4.4 | 65.1 ± 4.2 | 66.9 ± 5.1 | 65.9 ± 4.6 |
| Male recipient (%) | 54.9 | 50.5 | 68.7 | 67.9 |
| AA recipient (%) | 59.3 | 52.1 | 0 | 0 |
| Prior transplant (%) | 10.6 | 14.8 | 0 | 0 |
| Mean CIT (hours) | 22.6 ± 9.4 | 15.3 ± 5.4 | 21.3 ± 9.4 | 15.2 ± 5.5 |
| Mean pretransplant dialysis duration (months ± SD) | 47.1 ± 30.2 | 48.5 ± 31.3 | 36.5 ± 24.2 | 38.6 ± 26.4 |
| Peak PRA > 20 (%) | 49.1 | 57.2 | 0 | 0 |
| Recipient diabetes mellitus (%) | 30.3 | 28.3 | 30.8 | 30.0 |
| Zero ABDR HLA mismatches (%) | 9.4 | 15.9 | 9.7 | 20.5 |
| Mean donor age (years ± SD) | 50.7 ± 16.1 | 34.4 ± 14.4 | 52.2 ± 16.0 | 35.1 ± 14.9 |
| Male donor (%) | 53.7 | 61.7 | 53.9 | 60.0 |
| AA donor (%) | 13.0 | 16.1 | 8.2 | 8.3 |
| Donor diabetes (%) | 11.4 | 4.8 | 20.5 | 5.0 |
| ECD (%) | 58.9 | 0 | 63.7 | 0 |
| DCD (%) | 16.9 | 0 | 16.9 | 0 |
| Pulsatile perfusion (%) | 36.4 | 15.4 | 34.0 | 12.3 |
| Immunosuppression at discharge (%) | – | – | – | – |
| CNI use at discharge | ||||
| none | 7.6 | 6.7 | 8.1 | 6.6 |
| cyclosporine | 16.2 | 19.1 | 19.5 | 22.8 |
| tacrolimus | 75.9 | 73.7 | 72.1 | 70.2 |
| AZA/MPA/mTOR use at discharge (%) | ||||
| none | 6.6 | 6.0 | 6.0 | 5.6 |
| AZA | 1.1 | 0.8 | 1.5 | 1.6 |
| MPA | 85.2 | 84.0 | 83.1 | 84.4 |
| sirolimus/everolimus | 3.3 | 6.0 | 4.5 | 4.9 |
| Steroid at discharge (%) | 75.4 | 77.6 | 69.9 | 71.8 |
| Need for dialysis in first week (%) | 33.7 | 20.6 | 28.7 | 17.2 |
| Median follow-up days (25th, 75th percentile) | 613 (199, 1121) | 728 (314, 1450) | 705 (209, 1323) | 768 (354, 1483) |
P < 0.001 for all comparisons between groups. Proportion of missing not provided if <5%. AA, African American; AZA, azathioprine; CNI, calcineurin inhibitor; MPA, mycophenolicacid; mTOR, mammalian target of rapamycin.
Figure 3.
Overall graft survival in kidney transplant recipients aged ≥60 years by recipient and donor risk categories. Log rank p < 0.05.
Posttransplant Outcomes
Overall Nonstratified Cohort
In the entire cohort, elderly recipients who received rATG had the lowest cumulative rate of acute rejection within the first year after transplantation (7.3%) compared with the IL2RA (10.5%) and the alemtuzumab groups (11.4%). The adjusted odds of acute rejection at 1 year were significantly higher among recipients of IL2RA (odds ratio [OR] 1.65; 95% confidence interval [CI] 1.45 to 1.89) and alemtuzumab (OR1.35; 95% CI 1.08 to 1.69) compared with rATG. Patients who received IL2RA or rATG had no significant difference in death-censored graft survival on multivariate analysis (hazard ratio [HR] 1.09; 95% CI 0.97 to 1.21). However, there was an increased risk of death for recipients of IL2RA (adjusted HR 1.12; 95% CI 1.02 to 1.21) compared with rATG. This effect dissipated when we adjusted for acute rejection. Alemtuzumab recipients demonstrated an increased risk in death-censored graft loss (adjusted HR 1.60; 95% CI 1.34 to 1.92) and death (adjusted HR 1.32; 95% CI 1.14 to 1.53) compared with rATG.
Cohorts Stratified by Donor/Recipient Risk
Figure 4 displays Kaplan–Meier curves for death-censored graft survival and patient survival by induction type for each risk-stratified cohort. In this univariate analysis, graft and patient survival were superior with rATG compared with IL2RA and alemtuzumab in the highest risk group (high-risk recipient with high-risk donor). In addition, patient survival was superior with rATG in the low-risk recipient/high-risk donor group. Alemtuzumab was associated with inferior graft survival in low-risk recipient groups (low-risk recipient/high-risk donor and low-risk recipient/low-risk donor). Tables 3 through 5 outline the results from multivariate analyses examining the risks of acute rejection at 1 year, functional graft loss, and death for each risk-stratified cohort.
Figure 4.
Overall graft survival in kidney transplant recipients aged ≥60 years by induction agent, stratified by recipient and donor risk categories.
Table 3.
The adjusted risk of acute rejection in the first year after transplantation among recipients aged ≥60 years stratified by donor/recipient risk groups
| High-Immunologic-Risk Recipient |
Low-Immunologic-Risk Recipient |
|||
|---|---|---|---|---|
| High-Risk Donora (n = 2849) | Low-Risk Donorb (n = 3155) | High-Risk Donorc (n = 4468) | Low-Risk Donord (n = 4348) | |
| rATG, n | 1522 | 1721 | 2120 | 1777 |
| Alemtuzumab, n | 401 | 245 | 466 | 353 |
| IL2RA, n | 926 | 1189 | 1882 | 2218 |
| AR in first year | ||||
| rATG | 1.00 | 1.00 | 1.00 | 1.00 |
| alemtuzumab | 1.39 (0.93 to 2.07) | 0.98 (0.56 to 1.70) | 1.64 (1.12 to 2.38) | 0.69 (0.40 to 1.19) |
| IL2RA | 1.78 (1.34 to 2.35) | 1.45 (1.12 to 1.89) | 1.78 (1.42 to 2.23) | 1.30 (1.02 to 1.66) |
| IL2RA (not adjusted for DGF)e | 1.77 (1.34 to 2.33) | 1.48 (1.14 to 1.91) | 1.73 (1.38 to 2.16) | 1.17 (0.91 to 1.51) |
| Recipient age <70 years | NS | 1.67 (1.12 to 2.48) | NS | NS |
| Black recipient race | 1.51 (1.34 to 2.34) | NS | – | – |
| PRA > 20 | 1.86 (1.34 to 2.58) | NS | – | – |
| Zero HLA mismatches | 0.51 (0.29 to 0.88) | 0.51 (0.33 to 0.78) | 0.49 (0.32 to 0.76) | 0.73 (0.53 to 1.01) |
| Prior transplant | 1.72 (1.14 to 2.61) | NS | – | – |
| Tacrolimus use | 0.73 (0.55 to 0.96) | NS | NS | NS |
| MPA at discharge | NS | 0.65 (0.48 to 0.88) | NS | 0.66 (0.49 to 0.89) |
| ECD | 1.56 (1.19 to 2.05) | – | 1.46 (1.16 to 1.84) | – |
| Transplant year | ||||
| 2000 to 2002 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2003 to 2004 | NS | NS | NS | NS |
| 2005 to 2006 | NS | NS | 0.73 (0.56 to 0.93) | 0.73 (0.53 to 1.00) |
| 2007 to 2008 | NS | 0.56 (0.40 to 0.79) | 0.48 (1.38 to 2.17) | NS |
| *DGF | 2.00 (1.55 to 2.59) | 2.45 (1.88 to 3.19) | 1.70 (1.37 to 2.11) | 2.37 (1.82 to 3.10) |
Data presented as OR (95% CI) unless otherwise indicated.
Steroid use at discharge, DCD, ESRD from diabetes mellitus, CIT > 24 hours, CIT unknown, PRA unknown, and pump use were all nonsignificant (P > 0.10).
Steroid use at discharge, ESRD from diabetes mellitus, PRA unknown, CIT unknown, and pump use were NS (P > 0.10).
Steroid use at discharge, DCD, pump use, ESRD from diabetes mellitus, PRA unknown, and CIT > 24 hours were NS (P > 0.10).
Steroid use at discharge, pump use, and CIT unknown were NS (P > 0.10).
Logistic regression model ran without including DGF (dialysis in the first week after transplantation) as a covariate.
DGF, dialysis in first week after transplantations.
Table 5.
The adjusted risk of death among recipients aged ≥ 60 years, stratified by donor/recipient risk groups
| High-Immunologic-Risk Recipient |
Low-Immunologic-Risk Recipient |
|||
|---|---|---|---|---|
| High-Risk Donora (n = 2849) | Low-Risk Donorb (n = 3155) | High-Risk Donorc (n = 4468) | Low-Risk Donord (n = 4348) | |
| rATG, n | 1522 | 1721 | 2120 | 1777 |
| Alemtuzumab, n | 401 | 245 | 466 | 353 |
| IL2RA, n | 926 | 1189 | 1882 | 2218 |
| Death | ||||
| rATG | 1.00 | 1.00 | 1.00 | 1.00 |
| alemtuzumab | 1.65 (1.27 to 2.16) | 1.16 (0.81 to 1.66) | 1.60 (1.23 to 2.06) | 0.83 (0.58 to 1.18) |
| IL2RA | 1.16 (0.96 to 1.41) | 1.08 (0.91 to 1.28) | 1.15 (0.99 to 1.34) | 1.03 (0.88 to 1.20) |
| Recipient age <70 years | 0.57 (0.47 to 0.70) | 0.79 (0.65 to 0.98) | 0.78 (0.67 to 0.90) | 0.63 (0.54 to 0.74) |
| Black recipient race | 0.86 (0.71 to 1.03) | 0.86 (0.73 to 1.02) | – | – |
| PRA > 20 | NS | NS | – | – |
| Dialysis duration >3 years | 1.41 (1.17 to 1.69) | 1.48 (1.08 to 1.73) | 1.34 (1.17 to 1.54) | 1.25 (1.08 to 1.45) |
| ESRD from diabetes mellitus | 1.40 (1.11 to 1.77) | 1.26 (1.03 to 1.55) | 1.20 (1.01 to 1.43) | 1.59 (1.34 to 1.87) |
| Tacrolimus use at discharge | 0.77 (0.57 to 1.04) | 0.78 (0.65 to 0.92) | 0.85 (0.74 to 0.99) | 0.81 (0.70 to 0.95) |
| MPA use at discharge | 0.75 (0.61 to 0.92) | 0.81 (0.67 to 0.98) | 0.84 (0.72 to 0.98) | 0.59 (0.46 to 0.77) |
| Steroid use at discharge | NS | NS | 1.17 (0.98 to 1.37) | NS |
| ECD | 1.37 (1.14 to 1.64) | – | 1.27 (1.11–1.47) | – |
| Transplant year | ||||
| 2000 to 2002 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2003 to 2004 | NS | NS | NS | NS |
| 2005 to 2006 | NS | NS | NS | NS |
| 2007 to 2008 | 0.77 (0.57 to 1.04) | NS | NS | NS |
| *DGF | 1.53 (1.28 to 1.82) | 1.35 (1.12 to 1.62) | 1.58 (1.37 to 1.81) | 1.66 (1.40 to 1.97) |
Data presented as HR (95% CI) unless otherwise indicated.
Steroid use at discharge, CIT >36 hours, CIT unknown, DCD, PRA > 20%, PRA unknown, zero HLA mismatches, and dialysis duration unknown were NS (P > 0.10).
Steroid use at discharge and zero HLA mismatches were NS (P > 0.10).
Steroid use at discharge, diabetes mellitus cause of ESRD, DCD, CIT > 36 hours, CIT unknown, zero HLA mismatches, and dialysis duration unknown were NS (P > 0.10).
Steroid use at discharge and zero HLA mismatches were NS (P > 0.10).
DGF, dialysis in first week after transplantations.
Table 4.
The adjusted risk of graft loss (excluding death) among recipients aged ≥60 years, stratified by donor/recipient risk groups
| High-Immunologic-Risk Recipient |
Low-Immunologic-Risk Recipient |
|||
|---|---|---|---|---|
| High-Risk Donora (n = 2849) | Low-Risk Donorb (n = 3155) | High-Risk Donorc (n = 4468) | Low-Risk Donord (n = 4348) | |
| rATG, n | 1522 | 1721 | 2120 | 1777 |
| Alemtuzumab, n | 401 | 245 | 466 | 353 |
| IL2RA, n | 926 | 1189 | 1882 | 2218 |
| Functional graft loss | ||||
| rATG | 1.00 | 1.00 | 1.00 | 1.00 |
| alemtuzumab | 1.84 (1.35 to 2.51) | 0.76 (0.43 to 1.32) | 1.67 (1.23 to 2.27) | 1.99 (1.30 to 3.03) |
| IL2RA | 1.28 (1.02 to 1.60) | 1.15 (0.91 to 1.47) | 0.92 (0.76 to 1.13) | 1.13 (0.88 to 1.45) |
| IL2RA (adjusted for AR)e | 1.09 (0.79 to 1.51) | – | – | – |
| Recipient age <70 years | 0.72 (0.56 to 0.92) | NS | NS | NS |
| AA recipient race | NS | NS | – | – |
| PRA > 20 | NS | NS | – | – |
| Zero HLA mismatches | NS | NS | NS | NS |
| Dialysis duration >3 years | 1.23 (1.00 to 1.52) | 1.37 (1.08 to 1.73) | NS | NS |
| Tacrolimus use | NS | NS | 0.84 (0.69 to 1.02) | NS |
| MPA at discharge | 0.57 (0.45 to 0.73) | 0.65 (0.50 to 0.84) | 0.72 (0.59 to 0.89) | 0.59 (0.46 to 0.77) |
| Steroid use at discharge | NS | 0.79 (0.62 to 1.01) | ||
| ECD | 2.05 (1.63 to 2.59) | – | 1.91 (1.55 to 2.34) | – |
| Transplant year | ||||
| 2000 to 2002 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2003 to 2004 | NS | NS | NS | NS |
| 2005 to 2006 | NS | NS | 0.76 (0.61 to 0.95) | NS |
| 2007 to 2008 | NS | NS | NS | NS |
| *DGF | 2.51 (2.03 to 3.09) | 2.37 (1.86 to 3.01) | 2.75 (2.30 to 3.29) | 3.21 (2.53 to 4.06) |
Data presented as HR (95% CI) unless otherwise indicated.
ESRD from diabetes, CIT > 36 hours, CIT unknown, PRA > 20%, PRA unknown, AA recipient race, and dialysis duration unknown were NS (P > 0.10).
AA recipient, PRA > 20%, PRA unknown, and ESRD from diabetes mellitus were NS (P > 0.10).
Diabetes mellitus cause of ESRD, DCD, CIT > 36 hours, CIT unknown, and dialysis duration unknown were NS (P > 0.10).
CIT unknown was NS (P > 0.10).
Cox PH model ran including acute rejection in the first year as a covariate.
DGF, dialysis in first week after transplantations.
High-Risk Recipient and High-Risk Donor
Forty-seven percent of high-immunologic-risk elderly recipients in the cohort received a kidney from a high-risk donor and >30% of these patients received IL2RA induction. The use of IL2RA in this group was associated with a higher risk of acute rejection and functional graft loss compared with rATG. After adjusting for acute rejection in the Cox models, the higher risk of graft loss was not seen. When restricted to 1 year of follow-up posttransplant, similar results were seen with a higher risk of functional graft loss with IL2RA (HR 1.27; 95% CI 1.02 to 1.60).
High-Risk Recipient and Low-Risk Donor
Fifty-three percent of high-immunologic-risk elderly recipients in the cohort received a kidney from a low-risk donor and 38% of these patients received IL2RA induction, whereas 55% received rATG. In this group, the risk of acute rejection in the first year was greater with the use of IL2RA, but there was no significant difference in the risk of functional graft loss or death. This persisted when follow-up was limited to 1 year.
Low-Risk Recipient and High-Risk Donor
Fifty-one percent of low-immunologic-risk elderly recipients in the cohort received a kidney from a high-risk donor and 42% of these patients received IL2RA induction. In this group, the risk of acute rejection in the first year was significantly higher with the use of IL2RA, but there was no difference in the risk of functional graft loss or death. However, there appeared to be a nonsignificant trend toward an increased risk of death with IL2RA in this subgroup. Again, similar results were seen when restricted to 1 year of follow-up.
Low-Risk Recipient and Low-Risk Donor
Nearly 50% of low-immunologic-risk elderly recipients in the cohort received kidneys from low-risk donors and 41% of these patients received rATG induction, whereas 51% received IL2RA induction. In this group there was a higher risk of acute rejection with IL2RA; however, when the need for dialysis in the first week was excluded from the model, there was no significant difference in the risk of acute rejection between IL2RA and rATG. In addition, there was no significant difference in the risk of functional graft loss or death between IL2RA and rATG.
The unadjusted relative hazards of cause-specific death were determined with the use of IL2RA versus rATG, censoring for other causes of death. There was no difference in the risk of infectious (HR 1.14 [0.94 to 1.38]) or malignant deaths (HR 0.89 [95% CI 0.68 to 1.16]); however, the risk of cardiovascular deaths was greater in the IL2RA group (HR 1.27 [95% CI 1.08 to 1.50]).
Alemtuzumab
Alemtuzumab use was most common in the high-risk donor/high-risk recipient group, in which it was used in 14% of patients. Its use in this group was associated with a greater risk of functional graft loss and death compared with rATG. In high-risk recipients with low-risk donors, alemtuzumab use was low (8%), and no differences in graft loss or death were seen compared with rATG. Among low-immunologic-risk recipients, alemtuzumab was associated with a higher risk of functional graft loss, but an increased risk of death was only seen when there were high-risk donors.
The use of alemtuzumab was associated with an increased risk of acute rejection in the overall analysis, but the risk of acute rejection was higher with alemtuzumab only in low-risk recipients who had high-risk donor organs.
Discussion
In the last decade, the proportion of transplant recipients reported to UNOS >60 years of age has grown from 14% in 1999 to 25% in 2009, changing the face of the transplant recipient. The use and choice of induction immunosuppressive agent in elderly transplant recipients remains unresolved. Elderly patients are believed to generate a less potent immune response, which may allow for less intense immunosuppression (3,5,7). Furthermore, studies from individual centers and registry analyses have documented an increased incidence of opportunistic infections as the age of transplant recipients advances (5,6). Therefore, it has been suggested by some that less aggressive induction immunosuppressive regimens may be warranted in the elderly transplant recipient (3). However, the increased utilization of organs from ECDs has introduced an added layer of complexity in the early posttransplant management of elderly transplant recipients because most kidney allocation strategies preferentially allocate organs from higher risk donors to elderly recipients (9,15). Ultimately, a risk-stratified approach to induction is likely warranted that considers issues specific to the elderly transplant recipient, but there are few data to inform such a strategy in this population. This retrospective examination of induction utilization and associated outcomes in the United States is a first attempt to inform an evidence-based risk-stratified approach to induction in the elderly transplant recipient.
We found that a risk-stratified strategy does not appear be standard practice because >30% of elderly patients with high immunologic risk who had high-risk donor organs did not receive a T lymphocyte depleting agent. Conversely, nearly half of elderly recipients with low immunologic risk who received organs from low-risk donors were treated with rATG or alemtuzumab induction.
In our analysis, we found a higher risk of acute rejection within the first year after transplantation with IL2RA use in all groups. This is in keeping with the results from two randomized trials comparing rATG with IL2RA in a high immunologic risk, albeit younger population (10,16). Both of these trials demonstrated higher rates of biopsy-proven rejection with IL2RA use. Our results suggest that despite decreased immunogenicity in the elderly, the risk of acute rejection remains significantly higher with IL2RA.
Although the increased risk of rejection in the high-immunologic-risk recipient is not surprising, it is interesting that this is also seen in the low-immunologic-risk groups. The risk of acute rejection increases with DGF (17), and this may, in part, explain the higher risk of rejection in these groups. We were unable to adequately examine this because we found that the incidence of DGF was consistently higher in the rATG group across all strata, likely reflecting confounding by indication and making it difficult to examine the effect of DGF on graft outcomes. Indeed adjusting for DGF in the lowest risk strata strengthened the association between IL2RA use and acute rejection, highlighting the confounding by indication in this group. However, in the remaining three study groups, the risk of rejection with IL2RA persisted whether or not DGF was included in the multivariate model, suggesting that DGF is likely not the sole contributor to rejection. Another important consideration is the degree and type of CNI exposure. The use of tacrolimus was substantially lower in the IL2RA group, but it was included as a covariate in all multivariate models.
Furthermore, in the high-risk donor groups, we may speculate that the primary purpose of induction was to minimize early CNI exposure. Although we found no significant difference in the proportion of CNI use at the time of discharge, we do not have data on dosage or drug levels. It is possible that CNI exposure may have been lower in these cohorts and that the use of IL2RA with low-dose CNI may increase the risk of acute rejection relative to rATG.
Long-Term Outcomes
Although we demonstrated a higher risk of acute rejection with IL2RA, the effect of this on long-term outcomes appeared most significant in the highest risk group. In the setting in which donors and recipients have high-risk characteristics, our results suggest that rATG reduces the risk of functional graft loss. When we adjusted for acute rejection in the Cox model, the increased risk of graft loss dissipated, which suggests that this may, at least in part, be mediated by the higher risk of acute rejection with IL2RA.
There are few data comparing different induction agents in an elderly population, but our results are not in keeping with large, multicenter, prospective, randomized trials performed to date comparing rATG and IL2RA among recipients of all ages with high-risk donor and high-risk recipient factors. Brennan et al. compared rATG and basiliximab induction in a population at higher risk for rejection or DGF and did not demonstrate a significant difference in the composite endpoint of acute rejection, delayed graft function (DGF), graft loss, and death between these two agents, despite a difference in acute rejection rates (10). Similarly Noel et al. (16) compared daclizumab and rATG induction use in a high-risk, HLA-sensitized renal transplant population and found no significant difference in 1-year graft or patient survival. However, the mean age of recipients in the Brennan and Noel trials was only 50 and 45 years, respectively, which limits the generalizability of these trials for elderly recipients.
Acute rejection is associated with graft loss (18,19), and it is possible that this association may be stronger in the elderly. This was suggested in an analysis by Meier-Kriesche et al., who reported that the annual adjusted death-censored graft loss per 1000 patients over a 5-year period for patients who suffered an episode of acute rejection was higher for recipients aged >65 years of age versus those aged 18 to 55 years (20). Although these mechanisms require further study, our results suggest that the use of rATG in high-immunologic-risk elderly recipients who receive kidneys from high-risk donors may be advantageous.
The only other group of elderly patients in which rATG use may be associated with better outcomes was among low-risk recipients who received high-risk donor organs. In this group, although the risk of functional graft loss did not differ in the IL2RA and rATG groups, there was a trend toward a higher risk of death with IL2RA. Patient survival is inferior with ECD kidney transplants (15,22). It is possible that this effect is stronger in an elderly population and may potentiate the severity and long-term implications of acute rejection episodes.
Therefore, we hypothesize that although the risk of acute rejection is higher with IL2RA use in the elderly, its effect on long-term outcomes may only be realized when elderly patients are transplanted with kidneys from high-risk donors. The potential interaction between donor risk and recipient immune risk in the elderly population may be more significant and warrants further exploration.
In the remaining two subgroups, we found no significant difference in the risk of graft loss or death despite an increased risk of acute rejection with IL2RA. These data suggest that regardless of recipient immunologic risk, the relative benefit of rATG over IL2RA in the setting of low-risk donors is questionable and identifies a group in which further study is clearly warranted.
The most appropriate dosage of rATG in elderly patients is also an important consideration because lower doses may minimize the concern for toxicity with these agents. Unfortunately we were unable to examine this issue using these data, but this strategy warrants further evaluation.
Alemtuzumab in the Elderly
Alemtuzumab use as an induction agent has steadily increased over the last 7 years in the United States. In our overall analysis, alemtuzumab use was associated with a higher risk of acute rejection, all-cause graft loss, and death. Prior retrospective analyses of alemtuzumab use in younger living- and deceased-donor kidney transplant recipients have reported mixed results (23–25). Recently, a single-center randomized study of 222 kidney or pancreas transplant recipients who received alemtuzumab or rATG reported no significant difference in death or graft outcomes and in fact reported a decreased rate of biopsy-proven acute rejection episodes with alemtuzumab (26).
Although our overall results demonstrated a higher risk of acute rejection with alemtuzumab, this was not a consistent finding in the stratified analysis, with no significant difference in acute rejection seen in all but one strata. Interestingly, the risk of functional graft loss and death was greater with alemtuzumab in most groups, particularly among low-immunologic-risk recipients. This association may be explained by differences in maintenance immunosupression. For instance, steroid-free protocols were much more common in the alemtuzumab group, particularly among lower risk recipients. Steroid use at discharge was included in Cox models, but small sample sizes and insufficient longitudinal data on immunosuppressive protocols did not allow for a more thorough evaluation of this association. Regardless, we believe that the suggestion of inferior outcomes in the elderly in our analysis and the lack of data on the effect of alemtuzumab on infectious and other complications in the elderly transplant recipient argue against widespread use of this agent in the elderly transplant recipient without further study.
The results of this analysis need to be interpreted in the context of the limitations inherent to a retrospective registry analysis. Induction therapy is documented for UNOS at the time of discharge, making it difficult to ascertain whether its use was planned or whether there was a clinical indication (e.g., DGF) that may have prompted use of a particular agent. The immunosuppressive forms do specifically ask whether agents were given for the purposes of induction or rejection treatment, allowing us to exclude those treated for early rejection. In an attempt to adjust for the potential selection bias associated with DGF and induction agent use, we chose to include the need for dialysis in the first week after transplantation within each adjusted model. We also repeated the stratified analysis using dialysis in the first week as a marker of donor quality instead of predetermined donor characteristics and found similar outcomes as reported here (data not shown). Despite this, unmeasured confounding likely remains an important limitation of this analysis.
The definitions of high- and low-risk recipient and donor groups were based on prior studies, but the granularity of these data sets did not allow for more robust definitions. For instance, patients with a low PRA may still have donor-specific antibodies and therefore be misclassified in this analysis as lower immune risk. In the stratified analysis, the sample size may have limited the power of the analyses, particular when examining the use of alemtuzumab. Multivariate models were parsimonious to minimize the effect of this.
In conclusion, choice of immunosuppressive therapy in the elderly transplant recipient is complicated by their increased risk of infectious death, decreased immunogenicity, and greater likelihood of receiving a high-risk donor organ. Our findings highlight the variable use of induction therapies and suggest that rATG use may be preferable among high-risk recipients with high-risk donors and possibly low-risk recipients with high-risk donors. In the remaining groups, although there appears to be a higher risk of acute rejection, long-term outcomes do not appear to significantly differ. Ultimately, prospective comparisons of these agents in an elderly cohort are warranted to further compare the efficacy and adverse consequences of these agents to refine the use of induction immunosuppressive therapy in the elderly population.
Disclosures
None.
Acknowledgments
Jagbir Gill is funded by the St. Paul's Hospital Physician Scholar Program (Chan Foundation). John Gill is funded by the Michael Smith Foundation for Health Research. This work was presented in abstract form at the 23rd International Congress of the Transplantation Society, August 2010 in Vancouver, Canada.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Cohen DJ, St Martin L, Christensen LL, Bloom RD, Sung RS: Kidney and pancreas transplantation in the United States, 1995–2004. Am J Transplant 5: 1153–1169, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Danovitch GM, Cohen DJ, Weir MR, Stock PG, Bennett WM, Christensen LL, Sung RS: Current status of kidney and pancreas transplantation in the United States, 1994–2003. Am J Transplant 4: 904–915, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Danovitch GM, Gill J, Bunnapradist S: Immunosuppression of the elderly kidney transplant recipient. Transplantation 3: 285–291, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Meier-Kriesche HU, Ojo A, Hanson J, Cibrik D, Lake K, Agodoa LY, Leichtman A, Kaplan B: Increased immunosuppressive vulnerability in elderly renal transplant recipients. Transplantation 5: 885–889, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Meier-Kriesche HU, Ojo AO, Hanson JA, Kaplan B: Exponentially increased risk of infectious death in older renal transplant recipients. Kidney Int 4: 1539–1543, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Trouillhet I, Benito N, Cervera C, Rivas P, Cofan F, Almela M, Angeles Marcos M, Puig de la Bellacasa J, Pumarola T, Oppenheimer F, Moreno-Camacho A: Influence of age in renal transplant infections: Cases and controls study. Transplantation 7: 989–992, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Martins PN, Pratschke J, Pascher A, Fritsche L, Frei U, Neuhaus P, Tullius SG: Age and immune response in organ transplantation. Transplantation 2: 127–132, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Cecka JM: The OPTN/UNOS renal transplant registry. Clin Transpl 1–16, 2004 [PubMed] [Google Scholar]
- 9. Schold JD, Meier-Kriesche HU: Which renal transplant candidates should accept marginal kidneys in exchange for a shorter waiting time on dialysis? Clin J Am Soc Nephrol 3: 532–538, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D: Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med 19: 1967–1977, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Cardinal H, Hebert MJ, Rahme E, Houde I, Baran D, Masse M, Boucher A, Le Lorier J; Elderly Recipients Transplant Group: Modifiable factors predicting patient survival in elderly kidney transplant recipients. Kidney Int 1: 345–351, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Frei U, Noeldeke J, Machold-Fabrizii V, Arbogast H, Margreiter R, Fricke L, Voiculescu A, Kliem V, Ebel H, Albert U, Lopau K, Schnuelle P, Nonnast-Daniel B, Pietruck F, Offerman R, Persijn G, Bernasconi C: Prospective age-matching in elderly kidney transplant recipients—A 5-year analysis of the Eurotransplant Senior Program. Am J Transplant 1: 50–57, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Heldal K, Hartmann A, Leivestad T, Svendsen MV, Foss A, Lien B, Midtvedt K: Clinical outcomes in elderly kidney transplant recipients are related to acute rejection episodes rather than pretransplant comorbidity. Transplantation 7: 1045–1051, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Gill JS, Gill J, Rose C, Zalunardo N, Landsberg D: The older living kidney donor: Part of the solution to the organ shortage. Transplantation 12: 1662–1666, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK: Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA 21: 2726–2733, 2005 [DOI] [PubMed] [Google Scholar]
- 16. Noel C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, Charpentier B, Touchard G, Berthoux F, Merville P, Ouali N, Squiffelet JP, Bayle F, Wissing KM, Hazzan M: Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol 6: 1385–1392, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR: Association between delayed graft function and allograft and patient survival: A systematic review and meta-analysis. Nephrol Dial Transplant 3: 1039–1047, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Ojo AO, Hanson JA, Wolfe RA, Leichtman AB, Agodoa LY, Port FK: Long-term survival in renal transplant recipients with graft function. Kidney Int 1: 307–313, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Heaf JG, Ladefoged J: The effect of acute rejection on long-term renal graft survival is mainly related to initial renal damage. Transpl Int S26–S31, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Meier-Kriesche HU, Srinivas TR, Kaplan B: Interaction between acute rejection and recipient age on long-term renal allograft survival. Transplant Proc 33: 3425–3426, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Schold JD, Kaplan B, Chumbler NR, Howard RJ, Srinivas TR, Ma L, Meier-Kriesche HU: Access to quality: Evaluation of the allocation of deceased donor kidneys for transplantation. J Am Soc Nephrol 10: 3121–3127, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Sung RS, Guidinger MK, Lake CD, McBride MA, Greenstein SM, Delmonico FL, Port FK, Merion RM, Leichtman AB: Impact of the expanded criteria donor allocation system on the use of expanded criteria donor kidneys. Transplantation 9: 1257–1261, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Huang E, Cho YW, Hayashi R, Bunnapradist S: Alemtuzumab induction in deceased donor kidney transplantation. Transplantation 7: 821–828, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Huang E, Cho YW, Shah T, Peng A, Hayashi R, Bunnapradist S: Alemtuzumab induction in kidney transplantation. Clin Transpl 343–354, 2005 [PubMed] [Google Scholar]
- 25. Sampaio MS, Kadiyala A, Gill J, Bunnapradist S: Alemtuzumab versus interleukin-2 receptor antibodies induction in living donor kidney transplantation. Transplantation 7: 904–910, 2009 [DOI] [PubMed] [Google Scholar]
- 26. Farney AC, Doares W, Rogers J, Singh R, Hartmann E, Hart L, Ashcraft E, Reeves-Daniels A, Gautreaux M, Iskandar SS, Moore P, Adams PL, Stratta RJ: A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation 6: 810–819, 2009 [DOI] [PubMed] [Google Scholar]