Abstract
Summary
Background and objectives
In living-donor kidney transplantation, various donor factors, including gender, age, and baseline kidney function, predict allograft function and recipient outcomes after transplantation. Because higher phosphorus is predictive of vascular injury in healthy adults, the effect of donor phosphorus levels on recipient renal function after transplantation was investigated.
Design, setting, participants, and measurements
Phosphorus levels in 241 living donors were analyzed from a 7-year period, and recipient renal function and acute rejection at 1 year posttransplantation were examined controlling for other influencing factors, including multiple donor variables, HLA matching, and acute rejection.
Results
Female and African-American donors had significantly higher phosphorus levels predonation. By multivariable analysis, higher donor phosphorus correlated with higher recipient serum creatinine (slope = 0.087, 95% confidence interval [CI]: 0.004 to 0.169, P = 0.041) and lower recipient estimated GFR (slope = −4.321, 95% CI: −8.165 to −0.476, P = 0.028) at 12 months. Higher donor phosphorus also displayed an independent correlation with biopsy-proven acute rejection and delayed or slow graft function after transplantation.
Conclusions
In a cohort of living kidney donors, higher donor phosphorus correlated with female gender and African-American ethnicity and was an independent risk factor for early allograft dysfunction after living-donor kidney transplantation.
Introduction
Kidney transplantation is the treatment of choice for patients with end-stage renal disease (1). However, donor availability is limited, and in the United States, the wait list has grown to in excess of 100,000 patients (2). To avoid long wait times, living-donor transplantation is an attractive option for patients, and allograft outcomes are clearly superior compared with outcomes with deceased-donor transplantation (3). Knowing donor factors that can affect long-term allograft function in the recipient will help physicians in selecting the optimal donor. Living-donor factors reported to correlate with allograft outcomes in the recipient include donor kidney function, donor gender, donor BP, donor age, and donor cholesterol (4–6).
We wondered if other donor cardiovascular risk factors could also be predictive of long-term allograft outcomes. One nontraditional cardiac risk factor of increasing interest is serum phosphorus (phos). Initially, hyperphosphatemia was found to be associated with increased cardiovascular mortality in patients on hemodialysis (7). However, it has now been shown that higher phos levels are associated with increased cardiovascular mortality in nonchronic kidney disease patients (8,9). The mechanism of action is still being elucidated, but higher phos is thought to induce vascular calcification (10) and to promote vascular inflammation through secondary mediators. We therefore hypothesized that elevated phos levels in living donors may have a negative effect on the allograft function after transplantation.
Materials and Methods
Patient Population
This retrospective study performed at University Hospitals Case Medical Center analyzed living-donor kidney transplants between January 2000 and December 2006. Two hundred forty-one donors with serum phos levels drawn within a month before donation were analyzed. Donor and recipient information was retrieved from a living-donor database and chart review after institutional review board approval. Demographic data included donor and recipient age, gender, and ethnicity (African American [AA] or non-AA). In addition, donor body mass index and mean arterial pressure (MAP) were calculated from data recorded during the predonation nephrology visit. Smoking history was reviewed and considered positive in patients with >10 pack-years of smoking. Donor serum creatinine (Cr) was recorded on the day of donation before surgery, and donor GFR (estimated GFR [eGFR]) was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation (11).
Transplant data included living related versus living unrelated donor status, HLA mismatching, and immunosuppressive therapy. Thirty-six percent of recipients received induction therapy with basiliximab (27%) or anti-thymocyte globulin (9%) on the basis of protocol or perceived immunologic risk. Maintenance immunosuppression consisted of tacrolimus and mycophenolate mofetil in just over half of patients, whereas the remaining patients received other combinations of therapy. Twenty-one percent of patients underwent early steroid withdrawal per recent protocol providing there was good early function with no immediate rejection. Biopsies were undertaken for cause, and patients with Banff ‘97 acute rejection grade 1A or higher were defined as having biopsy-proven acute rejection (AR) (12). Chronic changes were analyzed from donor biopsies including global glomerulosclerosis (any), tubular atrophy and interstitial fibrosis (using Banff scoring (12)), presence of arteriosclerosis (any), and presence of calcinosis. Delayed graft function (DGF) was analyzed and defined as the need for dialysis therapy within 1 week of transplantation, and slow graft function (SGF) was defined as a recipient serum Cr ≥ 3 mg/dl on postoperative day 5 (13). Recipient serum Cr was also recorded at 12 months posttransplantation, and recipient eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Statistical Analyses
Donor phos levels were analyzed with other donor and recipient variables using unpaired t tests for dichotomous variables and Pearson correlations for continuous variables. Phos was divided into tertiles to compare means of donor variables using one-way ANOVA. Univariable and multivariable analyses of recipient Cr and eGFR were performed using linear regression analysis. All donor, recipient, and transplant variables with P ≤ 0.10 were included in the multivariable models. Donor and recipient AA ethnicity were used interchangeably in multivariate models because all AA recipients received kidneys from AA donors. Similarly, all non-AA recipients received kidneys from non-AA donors. Logistic regression analyses were performed with AR, tubular atrophy, and DGF/SGF as dependent variables using significant and relevant variables from univariable analyses. All statistics were performed using SPSS version 17.0 (Chicago, IL).
Results
Associations with donor phos and other donor variables were analyzed in all 241 kidney donors. Two hundred thirty-six patients were analyzed for DGF/SGF early after transplantation, after excluding patients deemed to have kidney injury from extrarenal sources, including vascular thrombosis (n = 2) and ureteral obstruction (n = 3). Within the first year after transplantation, patients who transferred care (n = 11), died (n = 7), or had primary allograft nonfunction (n = 3) were excluded from analysis of recipient renal function and AR. In addition, one DNA-matched identical twin transplant was excluded from recipient analysis. One final patient had early graft loss from AR and was included in the AR analysis but not in the analysis of recipient renal function. Thus, 218 patients were included in the analysis of renal function and 219 in the analysis of rejection. Baseline and posttransplantation donor and recipient variables are shown in Table 1.
Table 1.
Baseline demographic data in kidney donors and recipients
| Donor age, years | 40 ± 11 |
| Donor female | 58% |
| Donor AA | 26% |
| Donor BMI, kg/m2 | 27.6 ± 4.5 |
| Donor MAP, mmHg | 91.2 ± 9.2 |
| Donor smoking | 34% |
| Donor phos, mg/dl | 3.43 ± 0.58 |
| Donor calcium, mg/dl | 9.31 ± 0.41 |
| Donor Cr, mg/dl | 0.93 ± 0.19 |
| Donor eGFR, ml/min per 1.73 m2 | 92.9 ± 19.0 |
| Recipient age, years | 46.9 ± 13.7 |
| Recipient female | 48% |
| Recipient AA | 26% |
| Living-related transplant | 68% |
| HLA mismatch | 3.06 ± 1.66 |
| Induction therapy | 36% |
| TAC/MMF | 54% |
| Biopsy, 12 months | 25% |
| Biopsy-proven AR, 12 months | 13% |
| Recipient Cr, 12 months, mg/dl | 1.46 ± 0.41 |
| Recipient eGFR, 12 months, ml/min per 1.73 m2 | 55.7 ± 17.1 |
BMI, body mass index; TAC/MMF, tacrolimus and mycophenolate mofetil.
We examined donor variables associated with higher phos at the time of donation. Consistent with previous population-based studies (14–16), we noted higher average phos levels in women and AAs. Female donors had a mean phos level of 3.52 ± 0.53 mg/dl versus 3.31 ± 0.62 mg/dl in male donors (P = 0.006). In addition, AA donors had a mean phos level of 3.63 ± 0.64 mg/dl versus 3.37 ± 0.54 mg/dl in non-AA donors (P = 0.002). Table 2 shows the interactive effect of female gender and AA ethnicity on phos levels. There was no correlation with baseline phos level and donor age or donor renal function by serum Cr or eGFR, donor source, donor MAP, donor body mass index, or donor smoking in this analysis. Donor variables are shown after stratification by phos tertiles in Table 3.
Table 2.
Mean donor phos levels by gender and AA ethnicity
| Male | Female | |
|---|---|---|
| Non-AA | 3.23 ± 0.56 (n = 72) | 3.46 ± 0.51a (n = 111) |
| AA | 3.51 ± 0.71b (n = 29) | 3.75 ± 0.56c (n = 29) |
P = 0.005 versus non-AA male.
P = 0.04 versus non-AA male.
P = 0.008 versus non-AA female.
Table 3.
Donor demographics divided by phos tertiles
| Variable | Phos Levels (median and range) (mg/dl) |
||
|---|---|---|---|
| 2.8 (1.9 to 3.1) (n = 72) | 3.4 (3.2 to 3.6) (n = 86) | 4 (3.7 to 4.9) (n = 83) | |
| Donor age | 40 ± 11 | 41 ± 11 | 40 ± 12 |
| Donor femalea | 49% | 57% | 67% |
| Donor AAa | 15% | 24% | 31% |
| Donor Cr, mg/dl | 0.95 ± 0.21 | 0.93 ± 0.18 | 0.91 ± 0.19 |
| Donor eGFR, ml/min per 1.73 m2 | 92.1 ± 17.8 | 92.3 ± 18.4 | 94.2 ± 20.9 |
| Living related donor | 69% | 72% | 64% |
| Donor MAP, mmHg | 90.6 ± 9.1 | 92.3 ± 8.3 | 90.6 ± 10.0 |
| Donor BMI, kg/m2 | 27.3 ± 4.4 | 27.7 ± 4.5 | 27.6 ± 4.6 |
| Donor smokingb | 29% | 34% | 38% |
P < 0.05 by ANOVA.
P = NS by ANOVA.
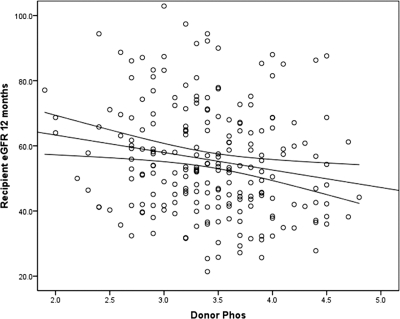
We next analyzed recipient outcomes related to donor phos levels. Despite the lack of association of phos and donor renal function, we found a negative association with donor phos and recipient renal function at 12 months posttransplantation. Using a linear regression model, we examined associations with recipient Cr at 12 months posttransplantation. By univariable analysis, higher recipient Cr was predicted by female gender (donor or recipient), older donor age, higher donor Cr, AA ethnicity (donor or recipient), nonrelated living-donor status, greater HLA mismatch, biopsy-proven AR, maintenance immunosuppression other than tacrolimus and mycophenolate mofetil, and higher donor phos (slope = 0.166, P = 0.001). Induction therapy and early steroid withdrawal were not associated with 12-month Cr. Higher donor calcium-phos product also predicted a higher recipient Cr (slope = 0.905, P < 0.001), but donor calcium alone did not. Multivariable linear regression for recipient Cr at 12 months is shown in Table 4. Higher donor phos persisted as a significant correlate with higher recipient Cr (slope = 0.087, 95% confidence interval [CI]: 0.004 to 0.169, P = 0.041). We further examined the association with donor phos and recipient eGFR at 12 months. Figure 1 shows the unadjusted negative association with donor phos and recipient eGFR (slope = −5.367, P = 0.008). We repeated the multivariable analysis above after substituting eGFR for recipient Cr as the dependent variable. In this model, donor phos remained inversely correlated with recipient eGFR at 12 months (slope = −4.321, 95% CI: −8.165 to −0.476, P = 0.028).
Table 4.
Multivariable associations with recipient Cr at 12 months
| Variable | Slope | 95% CI | P |
|---|---|---|---|
| Donor age (years) | 0.009 | (0.004 to 0.013) | <0.001 |
| Donor female | 0.167 | (0.046 to 0.288) | 0.007 |
| Donor AA | 0.117 | (0.004 to 0.230) | 0.042 |
| Donor phos | 0.087 | (0.004 to 0.169) | 0.041 |
| Donor Cr | 0.403 | (0.0098 to 0.709) | 0.010 |
| Recipient age (years) | −0.002 | (−0.006 to 0.001) | NS |
| Recipient female | −0.284 | (−0.375 to −0.194) | <0.001 |
| Living related transplant | −0.113 | (−0.238 to 0.011) | NS |
| HLA mismatch | −0.011 | (−0.045 to 0.023) | NS |
| TAC/MMF | −0.077 | (−0.170 to 0.016) | NS |
| Biopsy-proven AR, 12 months | 0.245 | (0.100 to 0.5389) | 0.001 |
R2 = 0.388.
Figure 1.
Correlation with donor phos and recipient eGFR at 1 year posttransplantation (n = 218, slope = −5.367, P = 0.008).
We next examined the relationship between donor phos and recipient AR. Surprisingly, donor phos was higher in recipients with AR (n = 30). Mean donor phos was 3.70 ± 0.49 mg/dl in patients with AR versus 3.40 ± 0.59 mg/dl in patients without AR (P = 0.008). Other correlates of AR included living related donor status (versus unrelated), with a higher rate of rejection in unrelated donors (25%) versus related donors (8%) (P < 0.001), and HLA mismatch, with a mean mismatch of 2.91 ± 1.62 in nonrejecters versus 4.43 ± 1.19 in rejecters (P < 0.001). In a logistic regression model controlling for donor status and HLA matching, donor phos remained a significant correlate with recipient AR (odds ratio 2.38, 95% CI: 1.14 to 4.97, P = 0.021). This association was unchanged after forcing donor gender and donor ethnicity into the model. Donor phos was also higher in patients biopsied for cause without AR. Twenty-seven patients had a biopsy within 12 months with no rejection, and donor phos in this cohort was 3.61 ± 0.67 versus 3.36 ± 0.57 mg/dl in patients who did not require a biopsy (P = 0.037). We reviewed biopsy results for descriptions of chronic changes including glomerulosclerosis, tubular atrophy, interstitial fibrosis, and arteriosclerosis. Analysis was limited to biopsies within a month of transplant (n = 32) to avoid the influence of recipient factors on chronic nephrosclerosis (17). Of these biopsies, 20 (63%) demonstrated evidence of AR, whereas others showed acute injury or inflammation (n = 12). The prevalence of any global glomerulosclerosis was 39%, any tubular atrophy was 32%, interstitial fibrosis >5% was 38%, and any arteriosclerosis was 38%.
Donor phos was similar in patients with and without these chronic changes, with the exception of tubular atrophy. Patients with the highest tertile of donor phos had a 54% incidence of tubular atrophy, versus 17% in the lower two tertiles (P = 0.03). Mean donor phos in patients with tubular atrophy was 3.85 ± 0.53 versus 3.45 ± 0.37 mg/dl in patients with no tubular atrophy (P = 0.02). There was a trend noted for an increase in tubular atrophy with older donor age, but controlling for donor age did not change the association with donor phos and tubular atrophy (odds ratio 25.4, P = 0.02). Tubular atrophy was mild in all patients (Banff classification = 1), and rates of atrophy were similar in patients with AR versus no AR. Finally, three patients with early biopsies were described as having renal tubular calcinosis on biopsy. Donor phos levels in these three patients were 3.6, 3.9, and 4.4 mg/dl, respectively, compared with a mean donor phos level of 3.43 ± 0.58 mg/dl in the entire cohort of living donors. Calcium levels in patients with calcinosis averaged 9.13 ± 0.35 mg/dl and were similar to the entire cohort.
We hypothesized that higher donor phos would have a negative effect early after transplantation and therefore analyzed the occurrence of DGF or SGF in relation to donor phos levels. Among the 236 patients analyzed, only two had DGF requiring dialysis after transplantation, including one patient with cortical necrosis and primary nonfunction. An additional 26 patients had SGF, with a serum Cr ≥ 3 mg/dl on postoperative day 5. Donor Cr in patients with DGF/SGF was 0.94 ± 0.18 mg/dl, versus 0.93 ± 0.20 mg/dl in patients without DGF/SGF (P = NS). Alternatively, donor phos was higher in patients with DGF/SGF with a mean level of 3.68 ± 0.47 mg/dl, versus 3.39 ± 0.57 mg/dl in patients with superior allograft function (P = 0.011). Patients with DGF/SGF also had a trend for higher donor MAP (94.3 ± 9.2 versus 90.8 ± 9.1 mmHg, P = 0.066), greater HLA mismatching (3.75 ± 1.46 versus 2.93 ± 1.66, P = 0.014), a trend for less induction therapy (21% versus 38%, P = 0.093), and a greater rate of biopsy-proven AR (29% versus 11%, P = 0.009). After controlling for these variables, donor phos remained an independent correlate with DGF/SGF (odds ratio 2.47, 95% CI: 1.11 to 5.50, P = 0.026). After forcing donor gender and AA ethnicity into the model, the correlation between donor phos and DGF/SGF remained significant.
Recipients with DGF/SGF had a significantly higher Cr at 12 months (1.76 ± 0.51 versus 1.43 ± 0.38 mg/dl, P < 0.001). We therefore added DGF/SGF to the multivariable regression analysis for 12-month Cr. In this new model, the association of donor phos and 12-month Cr was only borderline in significance, suggesting that the negative effect of donor phos on renal dysfunction was related to early kidney impairment after transplantation. DGF/SGF remained an independent correlate with 12-month Cr in this model (slope = 0.195, 95% CI: 0.048 to 0.342, P = 0.009).
Discussion
In this analysis of living kidney donors, we found that higher donor phos at baseline was associated with worse recipient renal function and a higher rate of kidney impairment early after transplantation. This finding is novel and extends our current medical knowledge regarding the risks of higher phos levels to include allograft outcomes in living-donor transplantation. Consistent with previous population-based studies (14–16), we noted higher phos levels in women and AAs. The mechanism for differences by gender and ethnicity remains unclear. Dietary phos intake has not been consistently linked to serum phos levels (15,18). Differences in sex hormones may contribute to differences between gender groups (19). There may be variability between ethnic groups in the renal response to phosphaturic hormones because AAs were recently found to have a decrease in the postprandial fractional excretion of phos despite similar levels of parathyroid hormone and fibroblast growth factor-23 (20). We did not see a correlation between donor phos and donor renal function. This is consistent with data from the National Health and Nutrition Examination Survey III, which demonstrated a lack of correlation with phos and GFR at GFR levels >30 ml/min per 1.73 m2 (15).
It is conceivable that the impaired allograft function related to higher donor phos levels was related to microvascular calcifications compromising blood flow and increasing the risk of ischemia-reperfusion injury. However, we did not observe an increase in arteriosclerosis in kidneys with higher donor phos. On the other hand, we did observe a higher rate of tubular atrophy in recipients of kidneys with higher donor phos. Phos is known to be toxic to renal tubules. Tubular atrophy was described in cases of acute phos toxicity related to oral ingestion (21), and a high-phos diet in animal models induced tubular injury and atrophy (22,23). Acute phosphate nephropathy in a deceased kidney donor led to tubular injury and poor outcomes in two recipients in a recent case report (24). Pre-existing tubular injury could predispose patients to impaired allograft function with or without rejection after kidney transplantation.
We observed an independent association between donor phos and AR in the first year after transplantation, although donor phos was also higher in recipients with no rejection on biopsy for cause. Most of these latter biopsies demonstrated acute injury and inflammation in the absence of Banff rejection. A possible mechanism linking increased phos to inflammation after transplantation is the upregulation of osteopontin (OPN) or other potentially deleterious mediators such as osteoprotegerin and fibroblast growth factor-23 in response to higher phos exposure before transplantation (25–28). OPN is a multifunctional bone regulatory protein that is upregulated by phos and acts as a potent macrophage-chemotactic stimulant (25). In an animal model, a high-phos diet was shown to upregulate OPN expression in renal tubules (29), and kidney transplant patients with rejection were found to have significantly enhanced OPN expression in tissue biopsy (30,31).
Any allograft injury related to donor phos would be expected to occur early after transplantation. This is consistent with our findings whereby donor phos levels were associated with early adverse outcomes. We observed an independent correlation with donor phos and recipient DGF/SGF after transplantation. The association with donor phos and recipient Cr at 12 months appeared to be driven by early renal injury, and DGF/SGF after transplantation correlated highly with renal function at 1 year.
We acknowledge several limitations in our study. First, our findings are based on a retrospective, single-center study. We cannot prove that higher donor phos was a direct cause of kidney functional impairment after transplantation. These data are hypothesis generating rather than conclusive, and outcomes related to donor phos levels need to be analyzed in other transplant populations. This study would have benefited from preimplantation biopsies and information about other variables such as donor or recipient parathyroid hormone and vitamin D levels. No donor had a phos level out of the normal range in this analysis. We do not advocate excluding donors with high-normal phos levels from the living-donor pool, and we strongly suspect that kidney outcomes from living donors with higher phos levels are in general superior to kidneys from deceased donors. Perhaps phos level may be considered when there are multiple potential donors available. Traditional predictors of recipient outcomes such as donor age and HLA matching also remained predictive of outcomes in this analysis.
In conclusion, donor characteristics can influence the quality of the organ from living donors. Identifying these risk factors is important to guide physicians with living-donor selection. Our study has identified donor phos as an additional donor risk factor for allograft dysfunction, perhaps related to tubular injury or inflammation in the allograft. Although donor female gender and AA ethnicity also predict outcomes, their effect may be mitigated by serum phos because they are both associated with increased phos levels. It will be important to further analyze the effects of higher donor phos levels on donor and recipient outcomes in future studies of kidney transplantation.
Disclosures
None.
Acknowledgments
We acknowledge the Leonard Rosenberg Foundation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 2. United Network For Organ Sharing. Available at: http://www.unos.org Accessed 2010
- 3. United States Renal Data System: Volume Two: Atlas of End-Stage Renal Disease. Available at: http://www.usrds.org/2009/pdf/V2_07_09.PDF Accessed 2010
- 4. Toma T, Tanabe K, Tokumoto T, Shimizu T, Shimmura H: Time-dependent risk factors influencing the long-term outcome in living renal allografts: Donor age is a crucial risk factor for long-term graft survival more than 5 years after transplantation. Transplantation 72: 940–947, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Oien CM, Reisoeter AV, Leivestad T, Dekker FW, Line PD, Os I: Living donor kidney transplantation: The effects of donor age and gender on short- and long-term outcomes. Transplantation 83: 600–606, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Issa N, Stephany B, Fatica R, Nurko S, Krishnamurthi V, Goldfarb DA, Braun WE, Dennis VW, Heeger PS, Poggio ED: Donor factors influencing graft outcomes in live donor kidney transplantation. Transplantation 83: 593–599, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Block GA, Hulbert-Shearon TE, Levin NW, Port FK: Association of serum phosphorus and calcium × phosphate product with mortality risk in chronic hemodialysis patients: A national study. Am J Kidney Dis 31: 607–617, 1998 [DOI] [PubMed] [Google Scholar]
- 8. Dhingra R, Sullivan LM, Fox CS, Wang TJ, S'Agostino RB, Sr, Gaziano JM, Vasan RS: Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med 167: 879–885, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, Sundstrom J: Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol 30: 333–339, 2010 [DOI] [PubMed] [Google Scholar]
- 10. Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM: Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15: 2857–2867, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Marcussen N, Mihatsch MJ, Nadasdy T, Nickerson P, Olsen TS, Papadimitriou JC, Randhawa PS, Rayner DC, Roberts I, Rose S, Rush D, Salinas-Madrigal L, Salomon DR, Sund S, Taskinen E, Trpkov K, Yamaguchi Y: The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Akkina SK, Connaire JJ, Israni AK, Snyder JJ, Matas AJ, Kasiske BL: Similar outcomes with different rates of delayed graft function may reflect center practice, not center performance. Am J Transplant 9: 1460–1466, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Foley RN, Collins AJ, Herzog CA, Ishani A, Kalra PA: Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 20: 397–404, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. de Boer IH, Rue TC, Kestenbaum B: Serum phosphorus concentrations in the third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 53: 399–407, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Abramowitz M, Muntner P, Coco M, Southern W, Lotwin I, Hostetter TH, Melamed ML: Serum alkaline phosphatase and phosphate and risk of mortality and hospitalization. Clin J Am Soc Nephrol 5: 1064–1071, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rush DN, Cockfield SM, Nickerson PW, Arlen DJ, Boucher A, Busque S, Girardin CE, Knoll GA, Lachance JG, Landsberg DN, Shapiro RJ, Shoker A, Yilmaz S: Factors associated with progression of interstitial fibrosis in renal transplant patients receiving tacrolimus and mycophenolate mofetil. Transplantation 88: 897–903, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Alonso A, Nettleton JA, Ix JH, de Boer IH, Folsom AR, Bidulescu A, Kestenbaum BR, Chambless LE, Jacobs DR, Jr: Dietary phosphorus, blood pressure, and incidence of hypertension in the atherosclerosis risk in communities study and the multi-ethnic study of atherosclerosis. Hypertension 55: 776–784, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burnett-Bowie SM, Mendoza N, Leder BZ: Effects of gonadal steroid withdrawal on serum phosphate and FGF-23 levels in men. Bone 40: 913–918, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gutiérrez OM, Isakova T, Smith K, Epstein M, Patel N, Wolf M: Racial differences in postprandial mineral ion handling in health and in chronic kidney disease. Nephrol Dial Transplant 25: 3970–3977, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Markowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD: Acute phosphate nephropathy following oral sodium phosphate bowel purgative: An underrecognized cause of chronic renal failure. J Am Soc Nephrol 16: 3389–3396, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Brown SA, Crowell WA, Barsanti JA, White JV, Finco DR: Beneficial effects of dietary mineral restriction in dogs with marked reduction of functional renal mass. J Am Soc Nephrol 1: 1169–1179, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Matsuzaki H, Uehara M, Suzuki K, Liu QL, Sato S, Kanke Y, Goto S: High phosphorus diet rapidly induces nephrocalcinosis and proximal tubular injury in rats. J Nutr Sci Vitaminol 43: 627–641, 1997 [DOI] [PubMed] [Google Scholar]
- 24. Agrawal N, Nair R, McChesney LP, Tuteja S, Suneja M, Thomas CP: Unrecognized acute phosphate nephropathy in a kidney donor with consequent poor allograft outcome. Am J Transplant 9: 1685–1689, 2009 [DOI] [PubMed] [Google Scholar]
- 25. Scatena M, Liaw L, Giachelli CM: Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol 27: 2302–2309, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Venuraju SM, Yerramasu A, Corder R, Lahiri A: Osteoprotegerin as a predictor of coronary artery disease and cardiovascular mortality and morbidity. J Am Coll Cardiol 55: 2049–2061, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El-Abbadi MM, Pai AS, Leaf EM, Yang HY, Bartley BA, Quan KK, Ingalls CM, Liao HW, Giachelli CM: Phosphate feeding induces arterial medial calcification in uremic mice: Role of serum phosphorus, FGF-23 and osteopontin. Kidney Int 75: 1297–1307, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuzaki H, Katsumata S, Uehara M, Suzuki K, Miwa M: High-phosphorus diet induces osteopontin expression of renal tubules in rats. J Clin Biochem Nutr 41: 179–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alchi B, Nishi S, Kondo D, Kaneko Y, Matsuki A, Imai N, Ueno M, Iguchi S, Sakatsume M, Narita I, Yamamoto T, Gejyo F: Osteopontin expression in acute renal allograft rejection. Kidney Int 67: 886–896, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Rouchop KM, Roelofs JJ, Sylva M, Rowshani AT, Ten Berge IJ, Weening JJ, Floquin S: Renal expression of CD44 correlates with acute renal allograft rejection. Kidney Int 70: 1127–1134, 2006 [DOI] [PubMed] [Google Scholar]