Abstract
Summary
Background and objectives
Influenza vaccination is recommended in all renal transplant recipients. However, immunosuppression in the early period post-transplant may attenuate the immunologic response to the vaccine. Additionally, it has been theorized that vaccination can induce an immune response that could trigger rejection episodes.
Design, setting, participants, & measurements
In a retrospective cohort of 51,730 adult Medicare primary patients who were first transplanted from January 2000 to July 2006 and followed through October 2006, we assessed Medicare claims for influenza vaccination and influenza infections, respectively. Outcomes included allograft loss and death.
Results
There were 9678 (18.7%) patients with claims for influenza vaccination in the first year post-transplant. Factors associated with vaccination included older age, diabetes, later year of transplant, and tacrolimus or mycophenolate at discharge. Vaccinations were less frequent among men, African Americans, highly sensitized patients, or those receiving induction immunosuppression or expanded criteria donor kidneys. Vaccination in the first year after transplant was associated with lower risk of subsequent allograft loss and death. Claims for influenza infection were reported in 310 (0.6%) patients and were not significantly associated with graft loss, although there was a trend toward death.
Conclusions
In the first year after renal transplantation, influenza vaccination was associated with a lower risk of subsequent allograft loss and death. Although this study cannot comment on formation of protective antibodies after vaccination, these data do not support withholding vaccination on the basis of concerns of adversely affecting allograft function.
Introduction
Influenza infection in transplant recipients has been associated with increased morbidity and mortality (1) and has been reported to occur at a rate of 4.3 cases per 1000 person years in recipients of renal transplants (2). Influenza vaccination is recommended in all renal transplant recipients, both before and after transplantation. There are no data regarding the timing of vaccination after transplant, but opinion-based guidelines recommend vaccinating at approximately 3 to 6 months after transplant when baseline immunosuppression levels are attained (3). Like all vaccinations, seasonal influenza vaccine administration before transplant is preferred but not always possible for deceased donor organs. Immunosuppression is most potent in the first year of transplant, and it is currently unknown whether or not immunization in the first year after transplantation is effective in preventing influenza infections or reducing their severity. There is literature to suggest that the influenza vaccine may not be effective after renal transplant on the basis of the inability to form seroprotective levels of antibodies (4,5).
Influenza infection has also been associated with allograft rejection and has been reported historically in the literature as far back as 1972. On the basis of this observation, it was postulated that infection with the influenza virus stimulated the immune system, which had adverse effects on the allograft. Because vaccination also stimulates the immune system, there have been concerns raised that influenza vaccination could also induce an immune response that could trigger acute rejection episodes (6,7). This belief questions the safety of vaccination early after transplant, especially given a presumed lack of efficacy.
Despite guidelines recommending that all transplant patients receive the influenza vaccination after transplant, there are limited data to support its efficacy or safety in the early period after transplantation. To investigate the associated risks and outcomes of influenza vaccination in the first year after renal transplantation, we utilized the United States Renal Data System (USRDS) registry, which includes data on all renal transplant recipients in the United States.
Materials and Methods
Patients and Sources
This study used the USRDS database, which incorporates extensive baseline and follow-up demographic and clinical data on all patients accessing the Medicare end-stage renal disease (ESRD) program in the United States. The variables included in the USRDS standard analysis files (SAFs), as well as methods and validation studies, are published and listed at the USRDS website, under Researcher's Guide to the USRDS Database, Section E, Contents of all of the SAFs. The demographics of the renal transplant population have been previously described (2008 USRDS report). The files SAF.TXUNOS were used as the primary data set. We used an inception cohort (on the basis of date of transplant) with patients over age 18 who underwent their first renal transplant between January 1, 2000 and July 31, 2006 and had Medicare primary insurance (parts A&B).
Outcome Variables
Influenza vaccinations and infections were defined using Institutional and Physician Supplier claims reported to Medicare from January 1, 2000 to September 30, 2006. Claims were identified by current procedure terminology and International Classification of Diseases-9th Revision diagnosis codes. Influenza vaccinations were defined using current procedure terminology codes (90655, 90656, 90657, 90658, 90659, 90660, 90724, and G0008), and influenza infections were defined using International Classification of Diseases-9th Revision diagnosis codes (487.xx). We assessed the earliest Medicare claim for influenza infection or vaccination after transplantation. Claims for vaccination were excluded if they occurred after allograft loss.
Survival Times
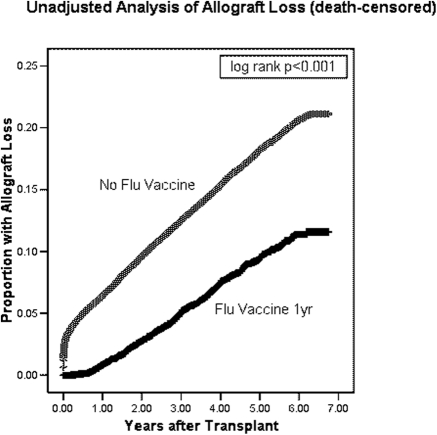
Time to allograft loss or death was calculated as the time from transplant date (tx1date) until the date of reported allograft failure (tx1fail), with recipients censored at the end of the study period (September 30, 2006) (Fig. 1).
Figure 1.
Time to allograft loss (death-censored) among adult Medicare primary renal transplant recipients who did or did not have Medicare claims for influenza vaccine in the first year post-transplantation.
Independent Variables
Patient characteristics were those at the date of transplant, with the exception of data from the Centers for Medicare and Medicaid Services Form 2728, which includes demographic and comorbidity data obtained at the first treatment for ESRD, whether dialysis or transplant. The duration of dialysis (dialysis vintage) pretransplant was defined as the time from the first recorded dialysis treatment until the date of transplantation. Acute rejection was defined using an aggregate of the variable for rejection obtained from the file TXFUUNOS and use of anti-rejection medication from the file TXIFUNOS. Other variables assessed included donor and recipient age, race, gender, induction/maintenance immunosuppressants, graft loss, delayed graft function, human leukocyte antigen (HLA) match status, panel reactive antibody (PRA), cold ischemic time, expanded criteria donor, donation after cardiac death, HIV status, and deceased-donor transplant. Data from Centers for Medicare and Medicaid Services form 2728 included information on comorbid conditions including diabetes mellitus, ischemic heart disease, congestive heart failure, peripheral vascular disease, AIDS, alcohol dependence, and tobacco use. Of note, for the purposes of this study, cyclosporine includes only the microemulsion formulation (Neoral®) to facilitate direct comparison with tacrolimus, which is also nongeneric to avoid potential unmeasured confounders that may be associated with the use of generic immunosuppressive drugs.
Statistical Analyses
All of the analyses were performed using SPSS 12.0TM (SPSS, Inc., Chicago, IL). The files were merged and converted to SPSS files using DBMS/Copy (Conceptual Software, Houston, TX). Bivariate analysis of factors associated with RM was performed with χ2 testing for categorical variables (Fisher's exact test used for violations of Cochran's assumptions) and t test for continuous variables (Mann-Whitney test used for non-normally distributed variables), respectively. Statistical significance for bivariate comparisons was defined as P < 0.05.
The independent associations between patient factors and influenza vaccination were examined using multivariate analysis with forced entry Cox regression. Variables with P < 0.10 tested in bivariate analysis for a relationship were entered into multivariate analysis as covariates, because of the possibility of negative confounding. Variables thought to have a known clinical association with outcomes were also introduced into multivariate models even if the bivariate P values were >0.10, in accordance with established principles of model development.
The association between renal allograft loss (including death) and influenza vaccination as a time-dependent variable was assessed with Cox nonproportional hazards regression. Variables found to be independently associated with influenza vaccination in the above Cox regression were included in the model, as were factors known to be independently associated with allograft loss (recipient age, black race, PRA >20%, dialysis vintage, diabetes mellitus, congestive heart failure, ischemic heart disease, tobacco use, HLA matching, donor age of >50 years, donor black race, deceased-donor transplant, expanded criteria donor, delayed graft function, cold ischemic time of >24 hours, year of transplant, and induction/discharge immunosuppression). Kaplan-Meier analysis was used to plot time to allograft loss for patients who either received or did not receive influenza vaccination. The log rank test was used for bivariate significance testing. Otherwise, patients were censored at time of death, loss to follow-up, or the end of the study period.
Results
We identified 51,730 Medicare primary first renal transplant recipients transplanted from January 1, 2000 to July 31, 2006. Mean age was 50.5 ± 13.8 years, and 29.1% were of black race. Mean dialysis vintage was 3.4 ± 2.9 years before transplant, and mean follow-up after transplant (until graft loss or death or end of study period) was 3.2 ± 1.9 years (Table 1). On univariate analysis, there was a reduced risk of allograft loss in patients who had claims for influenza vaccination within the first year after transplant (Figure 1). There were 9678 (18.7%) patients with claims for influenza vaccination in the first year after transplant. Of note, 43% of these vaccinations were given within the first 6 months.
Table 1.
Baseline characteristics, USRDS adult Medicare primary kidney recipients with Medicare claims for influenza vaccination in the first post-transplant year transplanted from January 1, 2000 to July 31, 2006 followed through September 30, 2006
| Influenza Vaccination | All Others | P | |
|---|---|---|---|
| 9678 (18.7) | 42052 (81.3) | ||
| Transplant recipient factors | |||
| recipient age (years) | 54.94 ± 13.40 | 49.45 ± 13.73 | <0.001 |
| male gender (vs. female) | 5475 (56.6) | 25679 (61.1) | <0.001 |
| African-American race (vs. all others) | 1949 (20.1) | 13103 (31.2) | <0.001 |
| follow-up time in years | 3.07 ± 1.95 | 3.23 ± 1.94 | <0.001 |
| years on dialysis prior to transplant | 3.07 ± 2.65 | 3.53 ± 2.93 | <0.001 |
| peak PRA > 20% (31.0% missing) | 1261 (19.7) | 5927 (20.2) | 0.362 |
| delayed graft function (2.7% missing)a | 1491 (15.8) | 8463 (20.7) | <0.001 |
| diabetes (10.4% missing) | 2815 (32.2) | 10314 (27.4) | <0.001 |
| hypertension (10.3% missing) | 6876 (78.7) | 29044 (77.2) | 0.001 |
| ischemic heart disease (10.4% missing) | 1049 (12.0) | 2947 (7.8) | <0.001 |
| congestive heart failure (10.4% missing) | 1098 (12.6) | 4320 (11.5) | 0.004 |
| COPD (10.4% missing) | 167 (1.9) | 589 (1.6) | 0.023 |
| tobacco use (10.4% missing) | 364 (4.2) | 1752 (4.7) | 0.054 |
| cancer (10.4% missing) | 232 (2.7) | 581 (1.5) | <0.001 |
| HLA mismatches (2.1% missing) | 3.46 ± 1.80 | 3.61 ± 1.76 | <0.001 |
| Transplant donor factors | |||
| deceased donor | 6759 (69.8) | 30185 (71.8) | 0.004 |
| donor age >50 years | 2385 (24.6) | 9866 (23.5) | 0.014 |
| expanded criteria donorb | 3783 (39.1) | 16627 (39.5) | 0.419 |
| cold ischemia time >24 h | 826 (8.5) | 3783 (9.0) | 0.155 |
| Discharge immunosuppression | |||
| tacrolimus | 6331 (65.4) | 26088 (62.0) | <0.001 |
| cyclosporine (Neoral®) | 1920 (19.8) | 8639 (20.5) | 0.124 |
| mycophenolate | 7727 (79.8) | 31745 (75.5) | <0.001 |
| rapamycin | 1023 (10.6) | 5439 (12.9) | <0.001 |
| Induction immunosuppression | 7757 (80.2) | 33886 (80.6) | 0.340 |
| Year of transplant | <0.001 | ||
| 2000 to 2001 | 2357 (24.4) | 11388 (27.1) | |
| 2002 to 2003 | 2661 (27.5) | 12296 (29.2) | |
| 2004 to 2006 | 4660 (48.2) | 18368 (43.7) |
The data are presented as the means ± standard deviation for normally distributed continuous variables and N (%) for categorical variables. COPD, chronic obstructive pulmonary disease.
Delayed graft function indicates the need for dialysis within the first week after transplant.
Expanded criteria donor indicates a donor age of >50 years with history of two of the following (stroke, hypertension, creatinine >1.5 mg/dl) or donor age of >60 years.
With multivariate analysis, factors associated with vaccination included older age, diabetes, later year of transplant, and tacrolimus or mycophenolate at discharge. Vaccinations were less frequent among men, African-American recipients, and patients with high PRA or those who received induction immunosuppression or expanded criteria donor kidneys (Table 2). Using Cox nonproportional multivariate regression analysis, vaccination in the first year after transplant was associated with lower risk of subsequent allograft loss (adjusted hazard ratio [AHR] 0.77; 95% CI 0.69 to 0.85; P < 0.001) and death (AHR 0.82; 95% CI 0.76 to 0.89; P < 0.001).
Table 2.
Cox regression analysis of factors associated with influenza vaccination in the first year after renal transplantation
| Variable | AHR | 95% CI | P |
|---|---|---|---|
| Mycophenolate | 1.27 | 1.19 to 1.35 | <0.001 |
| Tacrolimus (versus Neoral) | 1.17 | 1.11 to 1.23 | <0.001 |
| Diabetes | 1.08 | 1.03 to 1.14 | 0.003 |
| Year of transplant | 1.04 | 1.03 to 1.05 | <0.001 |
| Recipient age (per year) | 1.03 | 1.03 to 1.03 | <0.001 |
| HLA mismatch | 0.94 | 0.91 to 0.97 | <0.001 |
| Donor age of >50 years | 0.92 | 0.86 to 0.98 | 0.006 |
| Expanded criteria donora | 0.92 | 0.87 to 0.97 | 0.001 |
| Induction immunosuppression | 0.90 | 0.85 to 0.96 | 0.001 |
| Peak PRA of >20% | 0.87 | 0.81 to 0.94 | <0.001 |
| Male gender | 0.78 | 0.75 to 0.82 | <0.001 |
| African-American race | 0.60 | 0.57 to 0.64 | <0.001 |
The following values are included in the model but not significant: dialysis vintage, delayed graft function, hypertension, ischemic heart disease, congestive heart failure, chronic obstructive pulmonary disease, cancer, tobacco use, rapamycin at discharge, donor type, cold ischemia time >24 hours.
Expanded criteria donor indicates donor age of >50 years with history of two of the following (stroke, hypertension, creatinine >1.5 mg/dl) or donor age >60 years.
In the USRDS database, the exact dates of rejection are not known, so it is difficult to determine a temporal association with vaccinations. Given those limitations, acute rejection in the first year was not associated with vaccination in the first 6 (adjusted odds ratio 1.00; 95% CI 0.88 to 1.14; P = 0.965) or 12 months (adjusted odds ratio 0.97; 95% CI 0.89 to 1.07; P = 0.569] after transplant, using multivariate analysis adjusting for other factors associated with acute rejection.
Claims for influenza infection in the first year after transplant were rare, reported in 310 (0.6%) patients. Infections were not associated with allograft loss (AHR 0.94; 95% CI 0.62 to 1.42; P = 0.935) but did show a trend toward increased risk of death (AHR 1.39; 95% CI 1.00 to 1.92; P = 0.048). With the same limitations concerning temporal relationships and acute rejection episodes (described above), claims for influenza infection in the first year were associated with rejection in the first year after transplant (odds ratio 1.58; 95% CI 1.10 to 2.26); P < 0.001).
Discussion
In this study, influenza vaccination within the first year of kidney transplantation was associated with a lower risk of allograft loss and death, and there was no association with acute rejection. These findings support data from other smaller singer center studies. In the largest prospective study to date, safety of influenza vaccination was evaluated by comparing responses to vaccination in 156 renal transplant recipients. The authors reported stable serum creatinine levels at 1 week and 1 month after vaccination and observed no episodes of acute rejection during the 6-month period after vaccination. Of note, this study excluded patients within 6 months of transplant, and the median time after transplant was 6.3 years (range, 3.1 to 10.4), so the study included patients farther out from transplantation (2).
In another study of 69 renal transplant patients, serum creatinine was also unchanged after vaccination, and no acute rejection episodes were reported during 6 months of follow-up. This study also excluded patients within 6 months of transplant but did capture some patients earlier after transplant with vaccination ranging from 6 to 312 months after transplant (5,8). Two smaller studies from Poland and Greece showed similar results (9,10). The lower risk of death is consistent with other influenza vaccine studies in the chronic kidney disease and ESRD populations (11,12).
The low rate of vaccination that was noted in this study is concerning. Despite recommendations from society guidelines, only 19% of patients had claims for influenza vaccination within the first year after transplant in this cohort. This rate is lower than reported in the aforementioned chronic kidney disease and ESRD populations using similar methodology (11,12). Multiple surveys and observational studies have previously commented on the low rate of vaccination in solid-organ–transplant recipients internationally (13). In a study evaluating influenza vaccination after solid-organ transplant in 1800 patients across three health-maintenance organizations, only 51% of kidney recipients were vaccinated against influenza in the first full influenza season post-transplant (14). This is a higher proportion than was noted in our study. This may reflect better vaccine capture given that they used a database specifically designed to track vaccine safety but could also be related to regional practice variation. In a 1999 survey of United States transplant centers, 22% of responding centers indicated that they did not routinely vaccinate patients after transplant and that six centers actively discouraged vaccination. All of the respondents believed that post-transplant vaccines lacked efficacy and half reported that post-transplant vaccination increased the likelihood of inducing acute rejection. In the same survey, 54% of responding centers did advise vaccination after transplant, and among these centers, influenza vaccines were universally recommended (97%) (15).
The reasons for this low rate of vaccination are unclear but, as above, may stem from either concerns for inducing an acute rejection or the possible lack of efficacy in preventing infection. There are currently no data demonstrating that influenza vaccination after renal transplant reduces the risk of influenza infection, and the data are mixed as to whether or not the vaccine produces an adequate seroresponse in this population. One prospective study that compared seroresponse after vaccination in 165 renal transplant patients (versus 41 healthy controls) demonstrated adequate seroprotective titers but decreased seroresponse rates after vaccination. Of note, several of the patients had evidence of seroprotection before the vaccine intervention, presumably related to previous influenza vaccinations. In fact, for certain influenza strains, a greater proportion of study subjects started the study with a higher baseline level of seroprotection than the healthy controls. Another limitation is that the time interval between study enrollment and transplantation exceeded 1 year in the majority of patients, so the efficacy of vaccination early after transplant was not evaluated (2).
A separate prospective study showed inadequate antibody response after vaccination until 87 months after transplant (5). This lack of seroresponse may be related to the overall state of immunosuppression but has also been reported to be associated with specific immunosuppressive agents. In comparing different regimens, renal transplant patients on immunosuppression with mycophenolate had poorer seroprotective titers after influenza vaccination, compared with patients treated with azathioprine (5,8). Another randomized trial showed a trend toward higher seroresponse rates in patients who received sirolimus instead of calcineurin inhibitors (16).
In summary, the data are mixed with regards to seroprotective antibody titers after influenza vaccination in the renal transplant population, and there are no data correlating vaccination with actual prevention of influenza infection in this population. Even if seroprotective levels are not obtained, it is possible that any seroresponse will offer some protection or at least decrease the severity of the disease. On the basis of the above study, any research in this area will also be confounded by any previous influenza vaccines received.
Another limitation in the available data is that most studies excluded patients within 6 months after transplant. However, the available evidence supports some degree of seroresponse in the period more than 6 months after transplant, presumably as a result of the reduction of overall levels of immunosuppression. The ideal timing of vaccination with regards to effective immunoprophylaxis remains to be determined, but on the basis of our analysis, it appears safe to administer the influenza vaccine within the first year after transplantation given the absence of association of vaccination with acute rejection episodes or allograft loss.
Limitations
It is possible that some influenza vaccinations were not ascertained or misclassified. For example, if a patient was vaccinated in the workplace or paid out-of-pocket for the vaccination, claims may not have been submitted to Medicare. We feel that this is less likely in the renal transplant population, especially in the first year after transplant, when they are more likely to get the majority of their care from their transplant physicians. It is important to note that this possible misclassification would have a tendency to reduce statistical comparisons toward a null result. However, this analysis actually demonstrated improved outcomes associated with vaccination. We adjusted for the many factors previously shown to be associated with allograft survival, but because of the observational study design, we cannot rule out a role for unmeasured confounders. Limitations specific to the methods of USRDS database research have been described previously (17). Briefly, we are using an administrative database, and it is possible that data may be missing or incomplete. Although data entry is standardized and performed centrally, raw data are collected from multiple centers, and there could be a lack of standardization or misclassification of key variables. Also, as above, certain clinical information that would have been pertinent to this study is not routinely included in this database.
Conclusions
We have shown that in the first year after renal transplantation, influenza vaccination was not associated with acute rejection or allograft loss. Although this study cannot comment on formation of protective antibodies after vaccination, low vaccination rates because of concerns of precipitating allograft rejection appear to be unwarranted.
Disclosures
The views expressed in this paper are those of the authors and do not reflect the official policy of the National Institutes of Health, the Department of Army, the Department of Defense, or the United States government.
Acknowledgments
These data were the subject of an oral presentation at the 2010 American Transplant Congress meeting in San Diego, California, and were published in abstract form in a supplement to the American Journal of Transplantation (10[Suppl 4]: 127, 2010).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Nichols WG, Guthrie KA, Corey L, Boeckh M: Influenza infections after hematopoietic stem cell transplantation: risk factors, mortality, and the effect of antiviral therapy. Clin Infect Dis 39: 1300–1306, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Scharpé J, Evenepoel P, Maes B, Bammens B, Claes K, Osterhaus AD, Vanrenterghem Y, Peetermans WE: Influenza vaccination is efficacious and safe in renal transplant recipients. Am J Transplant 8: 332–337, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Danzinger-Isakov L, Kumar D: AST Infectious Diseases Community of Practice: Guidelines for vaccination of solid organ transplant candidates and recipients. Am J Transplant 9[Suppl 4]: S258–S262, 2009 [DOI] [PubMed] [Google Scholar]
- 4. Birdwell KA, Ikizler MR, Sannella EC, Wang L, Byrne DW, Ikizler TA, Wright PF: Decreased antibody response to influenza vaccination in kidney transplantrecipients: A prospective cohort study. Am J Kidney Dis 54: 112–121, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salles MJ, Sens YA, Boas LS, Machado CM: Influenza virus vaccination in kidneytransplant recipients: Serum antibody response to different immunosuppressive drugs. Clin Transplant 24: E17–E23, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Briggs JD, Timbury MC, Paton AM, Bell PRF: Viral infection and renal transplant rejection. BMJ 4: 520–522, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. David DS, Millian SJ, Whitsell JC, Schwartz GH, Riggio RR, Stenzel KH, Rubin AL: Viral syndromes and renal homograft rejection. Ann Surg 175: 257–259, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Keshtkar-Jahromi M, Argani H, Rahnavardi M, Mirchi E, Atabak S, Tara SA, Gachkar L, Noori-Froothghe A, Mokhtari-Azad T: Antibody response to influenza immunization in kidney transplant recipients receiving either azathioprine or mycophenolate: A controlled trial. Am J Nephrol 28: 654–660, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Wyzgał J, Brydak LB, Zygier D, Paczek L, Rowinski W, Grochowiecki T: Study on efficacy of influenza vaccination in renal allograft recipients. Transplant Proc 34: 572–575, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Grekas D, Alivanis P, Kiriazopoulou V, Dioudis C, Sioulis A, Derveniotis V, Tourkantonis A: Influenza vaccination on renal transplant patients is safe and serologically effective. Int J Clin Pharmacol Ther Toxicol 31: 553–556, 1993 [PubMed] [Google Scholar]
- 11. Gilbertson DT, Unruh M, McBean AM, Kausz AT, Snyder JJ, Collins AJ: Influenza vaccine delivery and effectiveness in end-stage renal disease. Kidney Int 63: 738–743, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Snyder JJ, Collins AJ: Association of preventive health care with atherosclerotic heart disease and mortality in CKD. J Am Soc Nephrol 20: 1614–1622, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berben L, Denhaerynck K, Schaub S, De Geest S: Prevalence and correlates of influenza vaccination among kidney transplant patients. Prog. Transplantation 19: 312–317, 2009 [DOI] [PubMed] [Google Scholar]
- 14. Harris K, Baggs J, Davis RL, Black S, Jackson LA, Mullooly JP, Chapman LE: Influenza vaccination coverage among adult solid organ transplant recipients at three health maintenance organizations, 1995–2005. Vaccine 27: 2335–2341, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Batiuk TD, Bodziak KA, Goldman M: Infectious disease prophylaxis in renal transplant patients: A survey of US transplant centers. Clin Transplant 16: 1–8, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Willcocks LC, Chaudhry AN, Smith JC, Ojha S, Doffinger R, Watson CJE, Smith KGC: The effect of sirolimus therapy on vaccine responses in transplant patients. Am J Transplant 7: 2006–2011, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Abbott KC, Bucci JR, Cruess D, Taylor AJ, Agodoa LY: Graft loss and acute coronary syndromes after renal transplantation in the United States. J Am Soc Nephrol 13: 2560–2569, 2002 [DOI] [PubMed] [Google Scholar]