Abstract
Summary
Background and objectives
Diffuse C4d staining in peritubular capillaries (PTCs) during an acute rejection episode (ARE) is the footprint of antibody-mediated rejection. In current clinical practice, diffuse C4d+ staining during acute rejection is regarded as an inferior prognostic sign. This case-control study investigated the prognostic role of mere C4d staining for graft outcome during an ARE in a well defined cohort of similarly ARE-treated patients.
Design, setting, participants, & measurements
All kidney transplant recipients in the authors' center from January 1, 1995 to December 31, 2005 were reviewed. From these patients, 151 had a clinical ARE. Paraffin and/or frozen material was available for 128 patients showing a histologically proven ARE within the first 6 months after transplantation. All ARE patients were treated similarly with high-dose pulse steroids and in the case of steroid unresponsiveness with anti-thymocyte globulin. Biopsies were scored according to Banff criteria. Frozen and paraffin sections were stained by immunofluorescence (IF) and immunohistochemistry (IHC) for C4d, respectively, and scored for PTC positivity.
Results
Diffuse C4d+ staining in PTCs was found in 12.5% and 4.2% sections stained by IF or by IHC, respectively. Four patients showed diffuse positive staining with both methods but showed no different risk profile from other patients. No relation between C4d staining and clinical parameters at baseline was found. C4d staining was not associated with steroid responsiveness, graft, or patient survival.
Conclusions
This study shows that C4d staining is not related to clinical outcome in this cohort of histologically proven early AREs.
Introduction
In renal transplantation, long-term graft survival strongly depends on events occurring early (i.e., within 1 year) after transplantation. A major event is the occurrence of an acute rejection episode (ARE), which is a main risk factor for the development of graft loss over time (1–3). An ARE can be mediated by cellular- and antibody-mediated reactions. In antibody-mediated rejection (AMR), donor-specific antibodies (DSAs) can bind complement factors and initiate the classical pathway of complement activation. During the activation cascade, C4d, a complement split product, is formed. C4d has the capacity to covalently bind to target molecules on the endothelium of peritubular capillaries (PTCs) and is therefore regarded as a footprint of AMR (4). Sensitivity (95%) and specificity (96%) of diffuse C4d staining in PTCs for the presence of DSAs is high (5). Other studies have found a strong relation between diffuse C4d staining of PTCs and the presence of DSAs (6–8).
Acute AMR is associated with nonresponsiveness to standard rejection therapy of steroids (i.e., steroid resistance) and has a detrimental effect on graft outcome (8–12). The outcome of AMR significantly improves when promptly treated with aggressive immunosuppressive regimens (13–17). The combination of circulating DSAs, histomorphological features of AMR, and diffuse C4d deposition is currently the gold standard in the diagnosis of AMR (16). Previous studies found unfavorable graft outcome for C4d+ stained biopsies (7,18–20). This might suggest that sole C4d deposition could be used as prognostic marker and also guide more aggressive therapy.
In 1993, Feucht et al. were the first to describe C4d staining in renal transplant biopsies (21). In 93 renal allografts showing dysfunction after transplantation, an incidence of 46.2% diffuse C4d and 8.6% focal C4d staining was found. C4d staining significantly correlated to 1-year graft survivals of 57%, 63%, and 90% in diffuse, focal, and negative staining, respectively. Subsequent studies showed an unfavorable graft outcome in diffuse C4d+ stained biopsies taken on clinical indication (6–8,18–22). However, these studies have five major drawbacks. First, most studies included biopsies with a broad range of histologic diagnoses, causing heterogeneity. Second, follow-up time (average 5 years) was relatively short in most studies. Third, in some studies rejection therapy differed between C4d+ and C4d− patients. Fourth, some studies used other criteria to determine C4d positivity than the Banff criteria. Fifth, some studies included more than one biopsy per patient and used the biopsy that stained most positive for C4d as the index biopsy.
The prognostic value for graft survival of untreated C4d+ AREs has not been investigated in a cohort of patients with AREs who were not differently treated for this. We questioned whether C4d+ AREs show a difference in long-term renal function compared with C4d− AREs when treated according to the same therapy regimen.
Materials and Methods
Patients
We reviewed all 723 patients who received a renal transplant in our center from 1995 until 2006, of which 498 (68.9%) never had a rejection episode. One hundred and twenty-eight patients who had a clinically suspect and histologically proven first ARE within 6 months after transplantation (17.7% of all single renal transplant patients) were included in this study. A total of 104 frozen and 118 paraffin-embedded renal biopsies were available, with an overlap of 94 patients. Maintenance and rejection therapy were analyzed.
All patients received calcineurin inhibitor (CNI)-based maintenance immunosuppression (Neoral: cyclosporine [CsA] microemulsion [86%]; or Prograft: tacrolimus [Tac] [14%]) and corticosteroids (P) with or without mycophenolate mofetil (MMF; 62.5%). Since 2000, all patients received prophylactic therapy with an IL-2 receptor antagonist (basiliximab), and one patient received induction with anti-thymocyte globulin (ATG). Of the 128 patients with biopsy-proven acute rejection, 30% received prophylactic antibody therapy.
AREs were treated with high-dose methylprednisolone (1 g intravenously for 3 consecutive days). If serum creatinine (SCr) did not return to baseline within a 20% range, a 10-day course of ATG at a dose 5 mg/kg was given. Steroid resistance was defined as the use of ATG therapy. Biopsies were taken before steroid therapy was started. Patients who had undergone a pancreas-kidney or other combined organ transplantation were excluded from this study. All patients had a negative complement-dependent cytotoxicity crossmatch before transplantation.
Clinical Data
Donor and recipient age and sex, donor source, number of rejection episodes and re-transplantation, percentage panel reactive antibodies (PRAs) present before transplantation, time between transplantation and the occurrence of the ARE, HLA mismatches, delayed graft function, patient and graft survival, quantitative proteinuria, and steroid resistance were analyzed. Graft failure was defined as return to dialysis. Graft failure was censored for patient death.
SCr was used as a surrogate marker for renal function over time. During patient follow-up, SCr was measured at regular check-up times and on additional clinical indications. SCr at 1 year after transplantation was taken as baseline. We used time until patients reached a SCr value of 150% and 200% of their SCr at 1 year as an indicator of renal function follow-up. In a Kaplan–Meier survival analysis, time to these events was compared between the C4d+ and C4d− group.
Histology
For routine diagnostic evaluation, paraffin-embedded sections were cut and stained with silver methamine, hematoxylin and eosin, and periodic acid–Schiff. Two independent pathologists (I.B. and N.G.) blindly scored biopsies for morphologic features by light microscopy using the Banff classification (16).
C4d Staining
Staining protocols have been described previously (23–25). Immunofluorescent staining was performed on 4-μm frozen sections with monoclonal mouse anti-human C4d antibody (Quidel, San Diego, CA). Immunohistochemical staining was performed on 4-μm paraffin sections with polyclonal rabbit anti-human C4d antibody (Biomedica Gruppe, Wien, Austria). Two independent and blinded observers (IF: M.E. and K.K.; IHC: I.B. and M.vG) semiquantitatively scored all sections. C4d staining in PTCs was evaluated according to the area percentage of positive staining in the renal cortex as described in the Banff '07 criteria (16). Three C4d staining groups were made: negative (<10% of PTCs), focal (10% to 50% of PTCs), and diffuse positive (>50% of PTCs) staining. Necrotic and fibrotic areas (if present) were excluded from evaluation.
Statistical Analyses
SPSS version 16.0 was used to perform statistical analyses. For continuous variables, means with standard errors were calculated and differences were assessed by independent sample t tests. For categorical data, crosstabs were made and differences were calculated by Fisher exact test. Univariate analyses using a logistic regression model were performed, and a multivariate analysis was performed using a logistic regression model. Survival analyses were performed using Kaplan–Meier survival curves and log-rank tests to test for differences between C4d groups. The significance threshold was set at P < 0.05.
Results
Patient Characteristics
Of the 128 patients included in this study, 77% received a donor kidney from a deceased donor and 23% received a kidney from a living donor. Mean follow-up time was 7.3 years (±3.9).
Immunosuppressive maintenance therapy was comparable for all patients in the cohort, consisting mostly of a CNI (CyA or Tac) in combination with low-dose P alone or P with IL-2 receptor or MMF.
Prevalence of C4d Positivity
We investigated C4d staining patterns by IF (frozen sections) and IHC (paraffin-embedded sections) staining techniques in the same cohort and related these with clinical outcome. Thirteen of the 104 (12.5%) sections stained by IF and 5 of the 118 (4.2%) sections stained by IHC showed diffuse C4d+ staining (Table 1). Focal C4d+ staining was seen in six (5.8%) and two (1.7%) of the patients, respectively.
Table 1.
Prevalence of C4d staining categories of PTCs stained by IF or IHC
| C4d Staining Category | Prevalence IF | Prevalence IHC |
|---|---|---|
| Negative | 85 (81.7) | 111 (94.1) |
| Focal positive | 6 (5.8) | 2 (1.7) |
| Diffuse positive | 13 (12.5) | 5 (4.2) |
| Total | 104 (100) | 118 (100) |
Data presented as n (%).
Four patients showed diffuse C4d+ staining with both techniques (Table 2). Overall, these patients were not remarkably different compared with the whole cohort in age, maintenance therapy, donor age and sex, PRAs, delayed graft function, HLA mismatches, rejection episodes, timing of rejection, steroid resistance, or vascular rejection. One of these patients lost graft function 10 days after transplantation and transplantectomy was performed. The explanted organ showed severe diffuse vascular rejection with necrosis and graft ischemia. The other three patients maintained stable graft function and their grafts survived during the follow-up period (range 1.86 to 6.43 years).
Table 2.
Overlap between C4d staining categories using IF and IHC
| C4d IF → | Negative | Focal Positive | Diffuse Positive | Total |
|---|---|---|---|---|
| C4d IHC ↓ | ||||
| Negative | 77 | 5 | 7 | 89 |
| Focal Positive | 0 | 1 | 0 | 1 |
| Diffuse Positive | 0 | 0 | 4 | 4 |
| Total | 77 | 6 | 11 | 94 |
As depicted in Table 2, one biopsy was diffusely C4d+ stained by IHC, but no IF staining was performed because of a lack of frozen material. Seven diffusely C4d+ biopsies with IF staining were negative using IHC. No paraffin material was available for IHC staining of two diffusely C4d+ biopsies using IF. Of the six focal C4d+ cases using IF, only one was focal C4d+ using IHC; all others were negative. All biopsies negative for C4d staining by IF were also negative in the IHC staining.
Statistical analyses were performed on IF- and IHC-stained cases. No differences were found in the IHC-stained cases. The results hereafter are only those analyzed using the IF staining. Diffuse C4d staining was called “C4d+” and focal and negative C4d staining were combined as one group, called “C4d−.” Others categorized C4d staining in the same way (5).
C4d and Banff Histology
C4d staining (IF) only related to the absence of tubular atrophy (8.3% versus 50.6%; P = 0.006) and not to any of the other individual components of the Banff score (Table 3). After IF and IHC C4d staining and blind scoring of biopsies, the biopsies of 14 patients showing diffuse C4d+ were subsequently reassessed (I.B.) to investigate histomorphological characteristics (e.g., granulocytic infiltrate, microthrombi, peritubular capillaritis [Table 3], or necrotizing vascular rejection) indicative of a possible AMR (4). None of those biopsies showed characteristics indicative of an antibody-mediated component.
Table 3.
Banff characteristics in relation to IF C4d staining patterns
| Banff Characteristic | C4d+ (n = 12), n (%) | C4d− (n = 85), n (%) | P |
|---|---|---|---|
| Glomerulitis | 1 (8.3) | 9 (10.6) | 1.00 |
| Chronic glomerular changes | 1 (8.3) | 5 (5.9) | 0.56 |
| Tubulitis | 7 (58.3) | 49 (57.6) | 1.00 |
| Tubular atrophy | 1 (8.3) | 43 (50.6) | 0.006 |
| Interstitial infiltrate | 5 (41.7) | 48 (56.5) | 0.37 |
| Interstitial fibrosis | 2 (16.7) | 38 (44.7) | 0.12 |
| Intima arteritis (vascular rejection) | 4 (33.3) | 33 (43.4) | 0.75 |
| Peritubular capillaritis | 4 (33.3) | 22 (25.9) | 0.74 |
| Chronic vascular changes | 5 (41.7) | 41 (52.6) | 0.55 |
C4d + diffuse positive-stained slides versus C4d− focal positive- or negative-stained slides. All characteristics were split in presence (score 1 to 3) and absence (0), except for glomerulitis, tubulitis, interstitial infiltrate, and peritubular capillaritis, where we dichotomized for mild (0, 1) and severe (2, 3). Fisher exact test was used to calculate significances of differences between groups. Values are expressed as mean and number (%).
C4d and Baseline Clinical Characteristics
Baseline clinical characteristics are shown in Table 4. Only donor age was significantly lower in the C4d+ group (38 ± 15 years versus 48 ± 14 years; P = 0.02). No significant difference in number of living related donors was observed between the C4d+ and C4d− group (1 of 13 [7.7%] versus 13 of 91 [14.3%], respectively, P = 1.00; data not shown). The interquartile range for follow-up was 6.46 years (3.47 to 10.45) and 6.80 years (3.80 to 10.57) for the C4d+ and C4d− group, respectively.
Table 4.
Baseline clinical characteristics between C4d+ (diffusely stained with IF) and C4d− (focally positive or negative with IF staining) groups
| Characteristics | C4d+ | C4d− | Univariate Analyses |
Multivariate Analyses |
||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | |||
| Gender (male/female) | 9/4 (69.2/30.8) | 59/32 (64.8/35.2) | 1.22 | 0.35 to 4.28 | 0.76 | 1.03 | 0.17 to 6.25 | 0.98 |
| Age (years) | 50.0 ± 11.4 | 46.5 ± 12.5 | 1.02 | 0.98 to 1.08 | 0.34 | 1.04 | 0.96 to 1.12 | 0.37 |
| Donor sex (male/female) | 2/11 (15.4/84.6) | 36/54 (40.0/60.0) | 0.27 | 0.06 to 1.30 | 0.10 | 0.24 | 0.03 to 1.71 | 0.16 |
| Donor age (years) | 37.9 ± 14.9 | 48.0 ± 13.7 | 0.95 | 0.91 to 0.99 | 0.02 | 0.94 | 0.89 to 0.99 | 0.03 |
| Donor type (living/deceased) | 4/9 (30.8/69.2) | 20/71 (22/78) | 1.58 | 0.44 to 5.66 | 0.48 | 1.88 | 0.28 to 12.56 | 0.51 |
| HLA-A mismatches (0/1/2) (%) | 31/61/8 | 36/54/10 | 1.25 | 0.35 to 4.38 | 0.73 | 1.49 | 0.23 to 3.58 | 0.67 |
| HLA-B mismatches (0/1/2) (%) | 8/77/15 | 29/55/16 | 4.84 | 0.60 to 39.2 | 0.14 | 0.81 | 0.04 to 16.95 | 0.89 |
| HLA-DR mismatches (0/1/2) (%) | 15/70/15 | 36/60/4 | 3.10 | 0.65 to 14.9 | 0.16 | 3.48 | 0.30 to 40.55 | 0.32 |
| Number of transplantations | 1.31 ± 0.63 | 1.16 ± 0.45 | 1.66 | 0.61 to 4.55 | 0.32 | 2.99 | 0.60 to 14.83 | 0.18 |
| PRA pretransplantation (%) | 10.6 ± 22.9 | 6.5 ± 16.3 | 0.56 | 0.12 to 2.52 | 0.45 | |||
| Delayed graft function (days) | 1 (7.7) | 26 (28.6) | 0.21 | 0.03 to 1.68 | 0.14 | 0.21 | 0.01 to 3.58 | 0.28 |
| Induction therapy | 3 (23.1) | 29 (31.9) | 0.60 | 0.16 to 2.50 | 0.52 | |||
| CNI (CsA/Tac) | 12/1 (92.3/7.7) | 77/13 (85.6/14.4) | 0.50 | 0.06 to 4.12 | 0.52 | |||
| MMF | 7 (53.8) | 58 (63.7) | 0.66 | 0.21 to 2.14 | 0.49 | |||
| Steroid resistance | 7 (53.8) | 43 (47.3) | 1.30 | 0.41 to 4.18 | 0.66 | |||
| Timing of rejection (days) | 25.92 (6 to 156) | 33.42 (3 to 178) | 0.99 | 0.97 to 1.01 | 0.50 | 1.00 | 0.98 to 1.02 | 0.83 |
| Rejection episodes (n) | 1.69 ± 0.63 | 1.86 ± 0.97 | 0.80 | 0.39 to 1.65 | 0.55 | 1.02 | 0.45 to 2.32 | 0.95 |
| Proteinuria (%) | 9 (69.2) | 68 (74.7) | 0.76 | 0.21 to 2.71 | 0.74 | |||
| Interstitial infiltrate | 5 (41.7) | 48 (56.5) | 0.55 | 0.16 to 1.88 | 0.34 | 0.73 | 0.12 to 4.69 | 0.75 |
| Tubulitis | 7 (53.8) | 49 (57.6) | 1.03 | 0.30 to 3.50 | 0.96 | 1.08 | 0.12 to 9.94 | 0.95 |
| Vascular rejection | 4 (33.3) | 33 (43.4) | 0.65 | 0.18 to 2.35 | 0.51 | 0.47 | 0.09 to 2.57 | 0.39 |
Donor age was significantly different between groups. HLA-DR mismatches seem to be present more often in the C4d + group, although this does not reach statistical significance. PRA levels were divided in three groups for analysis: ≤5%, 5% to 85%, and ≥85%. Proteinuria is grams of total protein per 24 hours 1 year after transplantation with a cutoff of >0.15 g/24 h. Multivariate analysis outcome. Clinical and histological risk factors were included in a multivariate logistic model. Donor age remained significantly different between C4d + and C4d− groups. Other characteristics were not significantly different between C4d groups. No risk profile for C4d staining could be established. Values are expressed as mean ± SD, as number (%), or as mean with minimum and maximum days in parentheses. OR, odds ratio; CI = confidence interval.
In a multivariate analysis, C4d was only related to donor age and not to any other clinical parameter (Table 4). Univariate analyses for clinical characteristics were also performed separately for living and deceased donors (data not shown). Results concerning differences in graft outcome between C4d+ and C4d− groups were similar in living and deceased donor recipients (younger donor age in the C4d+ group in the living kidney donors [38 ± 8 years versus 51 ± 12 years; P = 0.049]).
Twelve of the 13 C4d+ patients were primarily treated with high-dose P; one patient primarily received ATG. Eight patients subsequently received ATG because of nonresponsiveness to steroids. These numbers were similar in the C4d− group.
C4d and Clinical Outcome
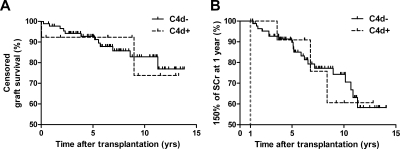
Incidence of steroid resistance was comparable between the C4d+ and the C4d− group (54%, and 47%, respectively; P = 0.770). Patient survival was similar between the two C4d staining categories (data not shown). One-year graft survival was 92% for the C4d+ group and 97% for the C4d− group, whereas 5- and 10-year graft survivals were 92% and 74% for the C4d+ group and 92% and 82% for the C4d− group, respectively (P > 0.05; Figure 1A). Results concerning differences between the C4d+ and C4d− group were similar between living and deceased donors.
Figure 1.
(A) Graft survival and (B) renal function follow-up for patients with diffuse C4d+ stained PTCs using IF (dotted line) and focal C4d stained/negative C4d stained PTCs using IF (continuous line). (A) No significant difference in graft survival was observed between groups (P = 0.969). (B) Renal function follow-up was indicated by a SCr value of 150% of SCr at 1 year posttransplantation. No significant difference in renal function over time was observed between groups (P = 0.981). Both were tested using Kaplan–Meier analysis.
No difference was found between the C4d+ and C4d− group for renal function over time, as measured by the time when SCr reached 150% or 200% of its value at 1 year after transplantation. Both showed a similar curve in the Kaplan–Meier analysis for time and the percentage of events of reaching 150% and 200% SCr values (P = 0.981 [Figure 1B] and P = 0.482, respectively). No differences were found in development of proteinuria (g/24 h) between the C4d+ and C4d− group.
Discussion
The occurrence of an early ARE is a risk factor for loss of renal allograft function over time. In this study, C4d staining patterns in a group of similarly treated patients during their first ARE were investigated. We compared C4d staining patterns in biopsies of patients with a histologically proven first ARE within 6 months after transplantation. Patients in this retrospective cohort were all treated for their ARE according to the same protocol, firstly with high-dose steroids and in the case of steroid resistance with ATG. Biopsy sections were stained by two techniques: IF on frozen and IHC on paraffin-embedded tissue sections. Diffuse C4d+ staining was seen in 12.5% of IF- and in 4.2% of IHC-stained sections. We found no clinical or histologic risk profile related to C4d+ staining. In addition, we found no difference in occurrence of steroid resistance or graft survival for patients who in retrospect showed a C4d+ or a C4d− ARE. We conclude that the C4d staining pattern during an early ARE does not predict renal allograft function or survival over time.
Since the introduction of diffuse C4d staining as a marker for antibody-mediated activity in the renal transplant biopsy, several studies concluded that C4d staining of biopsies with allograft dysfunction could be used as an adequate predictive marker for graft outcome (6–8,18,20–22,26) However, previous studies had several drawbacks, as described in the Introduction section, which could explain the discrepancies between those studies and our study.
The consistent histologic findings of an ARE and the consistent treatment of that episode makes our patient group unique. Graft survival in our cohort of patients was excellent (overall 10-year graft survival: 77.9% ± 4.7%). Our C4d+ ARE biopsies are most likely not indicative for AMR, but perhaps of a more subclinical type. The presence of C4d in biopsies might reflect some antibody being present in a T cell predominant acute rejection, rather than it being an AMR. Aside from tubular atrophy, no concurring histopathological parameters could be found. It is not known why there are significantly more biopsies showing tubular atrophy in the C4d− group. It is possible that the association of C4d− staining with tubular atrophy may reflect the younger age of donors in the C4d+ group. More research in a new patient cohort is required to elucidate this finding. Furthermore, none of the clinical characteristics related to AMR (e.g., recipient being female and/or young, high PRAs, number of successive transplantations, and number of HLA mismatches) were significantly more abundant in the C4d+ group. It is possible that our C4d+ ARE biopsies are of a more subclinical type, which might be due to better HLA matching and adequate DSA screening techniques before transplantation and our donor allocation protocol. We therefore strongly recommend adequate screening of patients pretransplantation and exclusion of any unacceptable mismatches.
Nickeleit et al. found no difference in outcome between C4d+ and C4d− AREs (27). However, in this study, C4d+ patients were treated more aggressively and the paper concluded that additionally treated C4d+ AREs may hold the same prognosis as C4d− AREs. In the study presented here, we show that patients with a C4d+ ARE who did not receive additional acute rejection treatment have the same graft outcome as a C4d− ARE. This could be because C4d deposition may indicate a state of graft accommodation that does not lead to graft failure (28). The question remains how C4d as a marker for graft accommodation can be discriminated from C4d deposition due to ongoing AMR. Furthermore, we explicitly studied whether the presence of diffuse C4d+ staining during an ARE was associated with graft survival if not additionally treated. It is not known whether C4d+ patients (or a subgroup of C4d+ patients) would have improved graft function if they would have been more aggressively treated. We therefore do not state that additional therapy in C4d+ ARE patients is not indicated, but we encourage further investigation on this subject.
The incidence of C4d staining in our cohort seems to be rather low compared with earlier studies. In various studies a range of 10% to 55% diffuse C4d+ staining is seen using either staining technique (5–8,18–21,26,27,29). Different explanations for this can be given. Inclusion criteria in this study were tightly controlled because we required all patients to have a histologically proven ARE, whereas other studies show heterogeneity with regards to biopsy inclusion criteria, resulting in a broad histomorphological spectrum. Also, the time point at which the biopsies were taken differed between studies. There could be differences in C4d+ incidence between centers, as has also been suggested by Mengel et al. (30) In a study with protocol biopsies stained for C4d, they reported that C4d incidence was related to the center the biopsy was obtained from. This could be due to differences in laboratory techniques, transplantation procedures, or therapy regimens between centers.
Until now, few studies compared C4d staining techniques with IF and IHC. The C4d staining protocol used to be applied on frozen sections by IF using a monoclonal antibody (21). Recently, an IHC method for paraffin-embedded sections has been developed using a polyclonal antibody against C4d. Seemayer et al. investigated both staining techniques on inter- and intraobserver variation and found a favorable result for IF-stained frozen sections (κ = 0.9 [IF] versus κ = 0.3 [IHC]) (31). However, in that study, C4d staining by both staining techniques was not investigated in relation to graft outcome over time. Also, in the most recent Banff criteria it was noted that C4d staining by IF showed more positivity and is possibly more sensitive, but both C4d staining techniques show strong relations with DSAs and it is still unclear which staining technique is to be preferred (14). We are the first to investigate C4d staining patterns by both staining techniques in the same cohort and relate it with clinical outcome. We found less C4d positivity in IHC-stained sections compared with IF sections and found no relation to outcome.
There are several drawbacks to our study. First, this was a single-center study; therefore, the results might be difficult to extrapolate to other studies. Furthermore, although this is a relatively large patient cohort, few C4d+ ARE patients were found. In addition, because of the retrospective nature of the study, presence of DSAs at the time of biopsy was not measured. However, earlier studies repeatedly showed that C4d staining was strongly related to DSAs (5,8).
In conclusion, clinicians might feel the urge to more aggressively treat patients with C4d positivity during a histologically proven ARE. However, this retrospective study shows that C4d staining is not related to clinical outcome in this large cohort of first histologically proven early (<6 months) rejection episodes of patients who were not additionally treated.
Disclosures
None.
Acknowledgments
We gratefully acknowledge Annemieke van der Wal for her excellent technical support. Part of this material was presented at the annual meeting of the American Society of Nephrology, October 27 through November 1, 2009, San Diego, CA; at the annual meeting of the Dutch Transplantation Society, March 26 through 28, 2008, Zeewolde, The Netherlands; and at the annual meeting of the Dutch Association for Pathology, April 22 and 23, 2008, Zeist, The Netherlands.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Matas AJ, Gillingham KJ, Payne WD, Najarian JS: The impact of an acute rejection episode on long-term renal allograft survival (t1/2). Transplantation 57: 857–859, 1994 [DOI] [PubMed] [Google Scholar]
- 2. Sijpkens YW, Doxiadis II, Mallat MJ, de Fijter JW, Bruijn JA, Claas FH, Paul LC: Early versus late acute rejection episodes in renal transplantation. Transplantation 75: 204–208, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Solez K, Vincenti F, Filo RS: Histopathologic findings from 2-year protocol biopsies from a U.S. multicenter kidney transplant trial comparing tarolimus versus cyclosporine: A report of the FK506 Kidney Transplant Study Group. Transplantation 66: 1736–1740, 1998 [DOI] [PubMed] [Google Scholar]
- 4. Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria—An addition to the Banff '97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB: Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 13: 779–787, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Kedainis RL, Koch MJ, Brennan DC, Liapis H: Focal C4d+ in renal allografts is associated with the presence of donor-specific antibodies and decreased allograft survival. Am J Transplant 9: 812–819, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lederer SR, Kluth-Pepper B, Schneeberger H, Albert E, Land W, Feucht HE: Impact of humoral alloreactivity early after transplantation on the long-term survival of renal allografts. Kidney Int 59: 334–341, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Worthington JE, McEwen A, McWilliam LJ, Picton ML, Martin S: Association between C4d staining in renal transplant biopsies: Production of donor-specific HLA antibodies, and graft outcome. Transplantation 83: 398–403, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Everly MJ, Everly JJ, Arend LJ, Brailey P, Susskind B, Govil A, Rike A, Roy-Chaudhury P, Mogilishetty G, Alloway RR, Tevar A, Woodle ES: Reducing de novo donor-specific antibody levels during acute rejection diminishes renal allograft loss. Am J Transplant 9: 1063–1071, 2009 [DOI] [PubMed] [Google Scholar]
- 10. Halloran PF, Wadgymar A, Ritchie S, Falk J, Solez K, Srinivasa NS: The significance of the anti-class I antibody response. I. Clinical and pathologic features of anti-class I-mediated rejection. Transplantation 49: 85–91, 1990 [DOI] [PubMed] [Google Scholar]
- 11. Jeannet M, Pinn VW, Flax MH, Winn HJ, Russell PS: Humoral antibodies in renal allotransplantation in man. N Engl J Med 282: 111–117, 1970 [DOI] [PubMed] [Google Scholar]
- 12. Martin S, Dyer PA, Mallick NP, Gokal R, Harris R, Johnson RW: Posttransplant antidonor lymphocytotoxic antibody production in relation to graft outcome. Transplantation 44: 50–53, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Moll S, Pascual M: Humoral rejection of organ allografts. Am J Transplant 5: 2611–2618, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Montgomery RA, Zachary AA, Racusen LC, Leffell MS, King KE, Burdick J, Maley WR, Ratner LE: Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 70: 887–895, 2000 [DOI] [PubMed] [Google Scholar]
- 15. Pascual M, Saidman S, Tolkoff-Rubin N, Williams WW, Mauiyyedi S, Duan JM, Farrell ML, Colvin RB, Cosimi AB, Delmonico FL: Plasma exchange and tacrolimus-mycophenolate rescue for acute humoral rejection in kidney transplantation. Transplantation 66: 1460–1464, 1998 [DOI] [PubMed] [Google Scholar]
- 16. Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB, Cosio F, David DS, Drachenberg C, Einecke G, Fogo AB, Gibson IW, Glotz D, Iskandar SS, Kraus E, Lerut E, Mannon RB, Mihatsch M, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Roberts I, Seron D, Smith RN, Valente M: Banff '07 classification of renal allograft pathology: Updates and future directions. Am J Transplant 8: 753–760, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Venetz JP, Pascual M: New treatments for acute humoral rejection of kidney allografts. Expert Opin Investig Drugs 16: 625–633, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Herzenberg AM, Gill JS, Djurdjev O, Magil AB: C4d deposition in acute rejection: An independent long-term prognostic factor. J Am Soc Nephrol 13: 234–241, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Lorenz M, Regele H, Schillinger M, Exner M, Rasoul-Rockenschaub S, Wahrmann M, Kletzmayr J, Silberhumer G, Horl WH, Bohmig GA: Risk factors for capillary C4d deposition in kidney allografts: Evaluation of a large study cohort. Transplantation 78: 447–452, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Regele H, Exner M, Watschinger B, Wenter C, Wahrmann M, Osterreicher C, Saemann MD, Mersich N, Horl WH, Zlabinger GJ, Bohmig GA: Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant 16: 2058–2066, 2001 [DOI] [PubMed] [Google Scholar]
- 21. Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmuller G, Land W, Albert E: Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 43: 1333–1338, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Magil AB, Tinckam KJ: Focal peritubular capillary C4d deposition in acute rejection. Nephrol Dial Transplant 21: 1382–1388, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Bakker RC, Koop K, Sijpkens YW, Eikmans M, Bajema IM, de Heer E, Bruijn JA, Paul LC: Early interstitial accumulation of collagen type I discriminates chronic rejection from chronic cyclosporine nephrotoxicity. J Am Soc Nephrol 14: 2142–2149, 2003 [DOI] [PubMed] [Google Scholar]
- 24. Eikmans M, Roos-van Groningen MC, Sijpkens YWJ, Ehrchen J, Roth J, Baelde HJ, Bajema IM, de Fijter JW, de Heer E, Bruijn JA: Expression of surfactant protein-C, S100A8, S100A9, and B cell markers in renal allografts: Investigation of the prognostic value. J Am Soc Nephrol 16: 3771–3786, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Koop K, Bakker RC, Eikmans M, Baelde HJ, de Heer E, Paul LC, Bruijn JA: Differentiation between chronic rejection and chronic cyclosporine toxicity by analysis of renal cortical mRNA. Kidney Int 66: 2038–2046, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kayler LK, Kiss L, Sharma V, Mohanka R, Zeevi A, Girnita A, Shapiro R, Randhawa PS: Acute renal allograft rejection: Diagnostic significance of focal peritubular capillary C4d. Transplantation 85: 813–820, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ: Detection of the complement degradation product C4d in renal allografts: Diagnostic and therapeutic implications. J Am Soc Nephrol 13: 242–251, 2002 [DOI] [PubMed] [Google Scholar]
- 28. Platt JL: C4d and the fate of organ allografts. J Am Soc Nephrol 13: 2417–2419, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Ranjan P, Nada R, Jha V, Sakhuja V, Joshi K: The role of C4d immunostaining in the evaluation of the causes of renal allograft dysfunction. Nephrol Dial Transplant 23: 1735–1741, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Mengel M, Bogers J, Bosmans JL, Seron D, Moreso F, Carrera M, Gwinner W, Schwarz A, De BM, Kreipe H, Haller H: Incidence of C4d stain in protocol biopsies from renal allografts: Results from a multicenter trial. Am J Transplant 5: 1050–1056, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Seemayer CA, Gaspert A, Nickeleit V, Mihatsch MJ: C4d staining of renal allograft biopsies: A comparative analysis of different staining techniques. Nephrol Dial Transplant 22: 568–576, 2007 [DOI] [PubMed] [Google Scholar]