Abstract
Summary
Background and objectives
Severe hyponatremia (<120 mEq/L) in hospitalized patients has a high mortality rate. We hypothesized that underlying diseases causing hyponatremia attribute to mortality rather than hyponatremia itself.
Design, setting, participants, & measurements
The relationship between mortality and serum sodium (sNa) was examined in 45,693 patients admitted to a single community teaching hospital between January 1996 and December 2007. We conducted a comprehensive retrospective review of the medical records of 53 patients who died after developing sNa <120 mEq/L before or after admission and of 32 patients who survived after developing sNa <110 mEq/L.
Results
Mortality rates tended to increase as the sNa fell from 134 to 120 mEq/L, rising above 10% for patients with sNa of 120 to 124 mEq/L. However, below sNa of 120 mEq/L, the trend reversed, such that the mortality rate progressively decreased as sNa fell. More than two thirds of patients who died after sNa <120mEq/L had at least two additional acute severe progressive illnesses, most commonly sepsis and multiorgan failure. Three deaths (5.6%) in 12 years could plausibly be related to adverse consequences of hyponatremia, and one (1.8% of the fatal cases and 0.15% of all patients with sNa <120 mEq/L) was from cerebral edema. Most patients who survived with sNa <110 mEq/L had medication-induced hyponatremia. Severe underlying illnesses were uncommon in this group.
Conclusions
The nature of underlying illness rather than the severity of hyponatremia best explains mortality associated with hyponatremia. Neurologic complications from hyponatremia are uncommon among patients who die with hyponatremia.
Introduction
Hyponatremia is the most common electrolyte abnormality in hospitalized patients, and it increases the likelihood of a hospital death (1–5). Inpatient mortality rates as high as 50% or more have been reported for patients with serum sodium concentrations (sNa) <120 mEq/L (6–9).
Some series report higher mortality rates as hyponatremia worsens (3–6,9), and others report higher mortality with uncorrected hyponatremia (10,11). It is difficult to reconcile such a rising mortality with the findings of a case series from our medical center published in 1987 (12); the reported mortality was 8% among patients with an sNa ≤110 mEq/L and 5% in a subset with an sNa ≤105 mEq/L (12,13). However, the previous case series from our center was not designed to compare mortality rates at various levels of sNa and other series, making this comparison have included very few patients with sNa <110 mEq/L (3–5).
Hyponatremia, a marker for severe heart and liver disease, is also often associated with malignancies, acute kidney injury, brain tumors, and intracerebral hemorrhage (1,11). Thus, the high mortality in hospitalized patients with hyponatremia could simply reflect the severity of the underlying diseases causing hyponatremia rather than an effect of the electrolyte disturbance itself; i.e., patients may die “with” hyponatremia rather than “from” hyponatremia. A lower mortality among patients with extremely low sNa in the absence of severe acute illnesses and a low incidence of deaths from neurologic complications from hyponatremia would support this conclusion.
Materials and Methods
Using computer retrieval of archived laboratory data, we identified the lowest sNa recorded for all patients admitted from January 1996 to December 2007 at Rochester General Hospital, a 523-bed community teaching hospital. The 45,693 patients with sNa <135 mEq/L (uncorrected for the effect of hyperglycemia) were subdivided by sNa into the following brackets: 130 to 134 (n = 35,604), 125 to 129 (n = 7601), 120 to 124 (n = 1824), 115 to 119 (n = 462), 110 to 114 (n = 152), and <110 mEq/L (n = 50). Mortality rates for each bracket were compared with the mortality rate for hospitalized patients with sNa ≥135 mEq/L. Using the laboratory computer, it was possible to correct for the effect of hyperglycemia (assuming a 1.6 mEq/L decrease in sNa for every 100 mg/dl increase in blood glucose) for those patients admitted between 2004 and 2007. Excluding cases of hyponatremia caused by hyperglycemia, spurious laboratory results, or absorption of glycine irrigant, we examined two cohorts of patients in greater detail: those who died in the hospital with their lowest sNa <120 mEq/L (including those whose sNa was <110 mEq/L) and those who survived with their lowest sNa <110 mEq/L.
A comprehensive chart review of these cases was undertaken to determine the clinical course, including cause of hyponatremia and symptoms associated with it, rate of correction, and sNa at the time of death. Comorbidities (as described in physicians' notes or as documented in laboratory or medical imaging results) were quantified using the Charlson Comorbidity Score, which is not impacted by sNa (14,15) (Table 1).
Table 1.
Charlson comorbidity score
| Weight | Clinical Conditions |
|---|---|
| 1 | Myocardial infarct; congestive heart failure; peripheral vascular disease; dementia; cerebrovascular disease; chronic lung disease; connective tissue disease; ulcer; chronic liver disease |
| 2 | Hemiplegia; moderate or severe kidney disease; diabetes; diabetes with complication; tumor; leukemia; lymphoma |
| 3 | Moderate or severe liver disease |
| 6 | Malignant tumor; metastasis; AIDS |
Results
Mortality and sNa
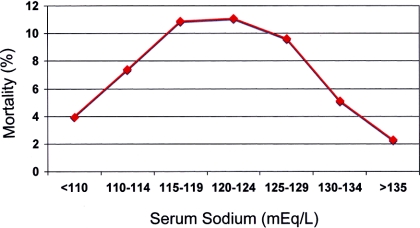
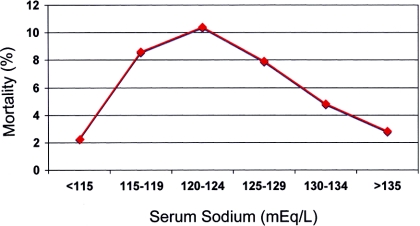
The overall mortality rate was 6.1% among all 45,693 hospitalized patients with hyponatremia (<135 mEq/L) compared with 2.3% among 164,146 patients with sNa >135 mEq/L, and mortality tended to increase as the sNa fell from 134 to 120 mEq/L (Figure 1). As the sNa fell below 120 mEq/L, the trend reversed, so that mortality among the 193 patients with sNa <115 mEq/L (6.8%) was considerably lower than the mortality among the 1844 patients in the 120 to 124 mEq/L bracket (11.2%; Figure 1). Results were similar for the 3-year data set corrected for the effect of hyperglycemia (Figure 2).
Figure 1.
Relationship between sNa uncorrected for the effect of hyperglycemia and mortality in patients admitted between 1996 and 2007.
Figure 2.
Relationship between sNa corrected for the effect of hyperglycemia and mortality in patients admitted between 2004 and 2007: 130 to 134 (n = 11,742), 125 to 129 (n = 2400), 120 to 124 (n = 474), 115 to 119 (n = 139), 110 to 114 (n = 39), and <110 mEq/L (n = 5).
Analysis of Fatal Cases with sNa ≤120 mEq/L (n = 53)
Thirty-nine (73%) of the patients who died had sNa <125 mEq/L at admission to the hospital; 14 (27%) were normonatremic or mildly hyponatremic at admission but their sNa fell below 120 mEq/L during hospitalization. Mean sNa at the time of death was 133 ± 11 mEq/L, and time from the lowest recorded sNa to death ranged from 1 to 163 days (median time, 8 days). About two thirds of the patients (66%) died despite return of their sNa to normal or near normal range (≥128 mEq/L) before death and, in >50% of cases (27 of 53 deaths), death occurred more than a week after the lowest sNa had been recorded.
The course of patients who died with uncorrected hyponatremia is of particular interest because some authors have attributed mortality in such cases to inadequate management. Data for all patients whose sNa at death was <8 mEq/L higher than their lowest recorded sNa are shown in Tables 2 (which includes patients whose sNa was <120 mEq/L at admission) and 3 (which includes patients with hospital acquired or hospital aggravated hyponatremia).
Table 2.
Fatal cases with admission-uncorrected hyponatremia
| Age/Gender | Admission SNa (mEq/L) | SNa at Death (mEq/L) | Day of Death | Clinical Condtion | Comfort Care? | Charlson Score |
|---|---|---|---|---|---|---|
| 79/F | 109 | 109 | 1 | Metastatic carcinoma, acute renal failure, hyperkalemia (8 mEq/L); died in 15 minutes | Yes | 5 |
| 68/F | 110 | ? | 4 | Metastatic carcinoma, acute renal failure (BUN 142 mg/dl), hyperkalemia (7.5 mEq/L) | Yes | 8 |
| 61/F | 112 | 117 | 4 | Metastatic disease with unknown primary, hepatic failure | Yes | 9 |
| 49/M | 118 | 121 | 12 | Hepatorenal syndrome, refractory shock. Corrected within 24 hours to 131 mEq/L; recurrent preterminal hyponatremia | Yes | 5 |
| 54/F | 118 | 118 | 1 | Cardiac arrest after azithromycin for pneumonia/pleural effusion | No | 4 |
F, female; M, male.
Table 3.
Fatal cases with uncorrected hospital-acquired or hospital-aggravated hyponatremia
| Age/Gender | Admission SNa (mEqL) | Lowest SNa (mEq/L) | SNa at Death (mEq/L) | Day of Death | Clinical Course | Comfort Care? | Charlson Score |
|---|---|---|---|---|---|---|---|
| 66/M | 145 | 114 | 114 | 27 | Metastatic carcinoma, cardiac arrest, respiratory failure, anoxic brain damage, acute kidney injury, refractory shock. First SNa <120 mEq/L 4 days before death | Yes | 11 |
| 67/M | 139 | 115 | 115 | 163 | Sepsis with several months in hospital with irreversible multiorgan failure; first sNa <120 mEq/L on day 155, 8 days before death | Yes | 5 |
| 63/M | 138 | 117 | 117 | 16 | Metastatic carcinoma, acute kidney injury, acute respiratory failure. First SNa <120 mEq/L 1 day before death | Yes | 11 |
| 87/M | 136 | 118 | 123 | 24 | ESRD. Comfort care on admission. | Yes | 4 |
| 65/M | 132 | 118 | 118 | 11 | End-stage liver disease, sepsis, multiorgan failure. First sNa <120 mEq/L on day of death | Yes | 5 |
| 65/M | 131 | 119 | 119 | 11 | Cardiogenic shock. First sNa <120 mEq/L 1 day before death | Yes | 4 |
| 55/M | 124 | 119 | 122 | 12 | End-stage heart disease, acute kidney injury | No | 7 |
| 54/F | 124 | 119 | 122 | 4 | End-stage lung disease | Yes | 3 |
| 83/M | 120 | 118 | 123 | 7 | End stage heart disease | No | 8 |
| 63/M | 121 | 119 | 122 | 2 | Advanced lung cancer | No | 7 |
M, male; F, female.
All patients who died had significant acute progressive underlying illnesses. Seventy percent had two or more of these severe illnesses; 51% were septic and 60% had acute kidney injury. Their Charlson comorbidity scores averaged 5.5. After a thorough review of patient records, we found only three deaths (5.6%) where recognized complications of hyponatremia seemed to play a causal role. Neurologic symptoms attributable to hyponatremia were uncommon, with only 4% of fatal cases presenting with hyponatremic seizures. The only patient to die of brain herniation became hyponatremic in the hospital (sNa = 118 mEq/L) because of administration of a thiazide to control hypertension after carotid endarterectomy; reperfusion cerebral edema and a large postoperative cerebral infarct contributed to the fatal outcome. With this exception, no other fatal case exhibited a clinical course suggestive of brain herniation and no neurology consultations or brain imaging suggested this diagnosis. No patient exhibited a biphasic neurologic course typical of osmotic demyelination (12,13,16,17). However, clinical features suggestive of central pontine myelinolysis (without confirmation with magnetic resonance images) were identified by the consulting neurologist in a patient with HIV and pneumococcal sepsis who was initially awake and talking after presenting with a seizure associated with sNa of 113 mEq/L but then suffered a cardiac arrest from an iatrogenic tension pneumothorax and never awoke; in addition to the anoxic insult from the arrest, his sNa had been corrected by 15 mEq/L in 24 hours and 21 mEq/L in 48 hours. No other neurology consultations or brain images suggested a diagnosis of osmotic demyelination. Finally, an elderly woman died of complications of an intertrochanteric hip fracture after she fell at home; her sNa on arrival in the hospital was 112 mEq/L.
Analysis of Survivors with an sNa <110 mEq/L (n = 32)
All of the survivors had become severely hyponatremic outside the hospital: 26 of them (81%) with a sNa <110mEq/L on admission, and 6 (19%) with an initial sNa of 111 to 112 mEq/L, falling below 110 mEq/L shortly after admission. Medications (thiazides and selective serotonin reuptake inhibitors) were the most common cause of hyponatremia (72% of cases), whereas the severe comorbidities that were so common in the fatal cases were seldom seen. Only 3% of this group had acute kidney injury, no patient had sepsis, and their Charlson comobidity scores averaged 1.8. Hyponatremic seizures occurred in 9% of these patients. Correction of hyponatremia averaged 9.3 ± 3.8 mEq/L in the first 24 hours and 16.3 ± 4.9 mEq/L in 48 hours; 69% were corrected by ≤10 mEq/L in 24 hours, 75% by ≤18 mEq/L in 48 hours, and 97% by <25 mEq/L in 48 hours. In 75% of survivors, the sNa was still <120 mEq/L, 24 hours after the lowest sNa was recorded, and no patient achieved an sNa >126 mEq/L in the first 24 hours.
Discussion
This study examined mortality associated with hyponatremia in a large cohort of patients identified over 12 years. In previously published smaller studies, mortality has ranged from 6.7 to 51% among hospitalized patients with sNa <120 mEq/L (1,7,8,17). The reason for the marked variability in these single-center series is not known, but we suspect that it may reflect differences in case mix in different populations. In our series, the mortality rate for patients with sNa <120 mEq/L (corrected for the effect of hyperglycemia) was 7.1%. A multiyear series from two hospitals in Boston found a mortality of 6.7% for patients with sNa <120 mEq/L (1), similar to our results.
As was first reported by Baran and Hutchison (18), our findings suggest that the sNa itself is seldom the cause of death but rather is a marker for the severity of underlying disease. Several lines of evidence from our analysis support this conclusion: (1) once sNa falls below 120 mEq/L, mortality rate does not seem to increase as the severity of hyponatremia worsens; in fact the trend is in the opposite direction; (2) deaths in patients with sNa <120 mEq/L were associated with serious comorbidities and were mostly attributable to conditions other than hyponatremia; (3) neurologic symptoms attributable to hyponatremia were uncommon among fatal cases, and deaths attributable to neurologic complications were rare.
Some previously reported case series have emphasized that mortality rates increase as the severity of hyponatremia worsens (3,4,9). One such analysis estimated that mortality associated with hyponatremia approximately doubles reaching 15% as sNa falls from 125 to 110 mEq/L, but the calculated regression (based on a restricted cubic spline) was driven by >20,000 cases with sNa 118 to 137 mEq/L compared with only 113 cases with sNa below 118 mEq/L. Although the brackets in this study differ from ours, the actual (rather than the projected) mortality rate of 6.1% in 113 patients with sNa <118 mEq/L is consistent with our findings showing that the trend toward increasing mortality does not continue as the sNa falls below 120 mEq/L. A paradoxical fall in mortality rate as the sNa falls can be found in other studies as well (1,19).
Our chart reviews of the patients who survived despite sNa <110 mEq/L suggest a possible explanation for the paradoxical fall in mortality as the severity of hyponatremia increases. These patients were typically admitted to the hospital because of severe hyponatremia, which was mostly medication-induced, and not because they were severely ill. By contrast, more moderate hyponatremia is common among patients who are admitted because of severe life-threatening illness. Larger multicenter prospective studies or registries will be needed to confirm this hypothesis.
A retrospective study of 168 patients with severe hyponatremia (<115 mEq/L) concluded that a slow rate of correction in severe hyponatremia is associated with higher mortality and speculated that aggressive therapy may improve outcome (10). Similarly, another study of patients with sNa <125 mEq/L found that mortality was highest among patients who were not treated for hyponatremia (11); noting that the recommended correction rates for hyponatremia have steadily declined over the years to approximately 8 mEq/L per day (20), the authors questioned whether “this decreasing trend has overstepped its goals and if part of the poor outcome could have been prevented by more aggressive therapy” (11). However, attributing excess mortality to inadequate therapy can be misleading. As shown in Tables 2 and 3, the patients with “uncorrected hyponatremia” tend to fall into two groups: those who died of severe underlying disease soon after admission and those whose hyponatremia developed near the end of a long hospital course, as an untreated preterminal event occurring while comfort care measures were being entertained. These patients with uncorrected or partially corrected hyponatremia had severe sepsis with multiorgan failure, end-stage heart disease or advanced malignancy, refractory to treatment; patients dying of irreversible illness are often not treated for hyponatremia (or anything else). Similarly, it is difficult to attribute survival in patients with sNa <110 mEq/L to aggressive therapy. In the past, it was suggested that correction of hyponatremia of this severity to sNa of 128 mEq/L by >0.7 mEq/L per hour was essential to avoid a fatal outcome (21); in our series, not a single survivor was corrected that rapidly.
It is well accepted that acute hyponatremia itself can be lethal if it is not treated promptly and that overcorrection of hyponatremia can cause potentially fatal neurologic sequelae (22,23). Over the 12-year span of this study, we found only one patient with sNa <120 mEq/L who died of cerebral edema, and this patient had coexistent intracranial pathology. Only one patient was thought to have central pontine myelinolysis, and, given the patient's severe comorbidities, it is doubtful that overcorrection of hyponatremia was responsible for his death.
Hyponatremic deaths from cerebral edema and osmotic demyelination are dramatic clinical events unlikely to be missed even in a retrospective chart review. Because our analysis of fatal cases was limited to patients with sNa <120 mEq/L, we could have missed deaths from cerebral edema among patients with less severe, but more acute, hyponatremia. However, series reporting a high incidence of hyponatremic deaths from cerebral edema are comprised of case referrals to experts in the field and do not reflect the true incidence of this complication (24); no studies to date have identified deaths from cerebral edema in a defined cohort of hospitalized patients with hyponatremia.
In addition to the well-documented and easily recognized neurologic complications of hyponatremia and its treatment, morbidity and even mortality could be related to more subtle effects of the electrolyte disturbance on other organ systems. For example, recent evidence has shown that hyponatremia is associated with osteoporosis, falls, and fractures (25–27); one of our patients died of complications from a hip fracture and her fall was likely caused in part by hyponatremia that developed before hospitalization. Hyponatremia contributing to hepatic encephalopathy or impairing cardiac function could also be clinically relevant, and more studies are needed to explore these possibilities.
Our data should not be taken to mean that hyponatremia should not be treated. However, they call into question the idea that large increases in sNa are indicated to prevent deaths from hyponatremia. A 4- to 6-mEq/L increase in the sNa, best achieved with 3% saline, seems to be sufficient for the most severely affected patients with acute hyponatremia (23). On the other hand, there is abundant evidence that overcorrection of severe chronic hyponatremia (>10 mEq/L in 24 hours and/or >18 mEq/L in 48 hours) can cause osmotic demyelination syndrome, an often devastating complication (12,13,17,23).
Potential morbidity and mortality from hyponatremia provide a rationale to try to maintain normonatremia in all patients, inside and outside hospital settings. However, there is as yet no evidence that the high mortality rates associated with hyponatremia can be altered by rapidly normalizing the sNa. Risk of death does not seem to be a good justification for exceeding current guidelines for safe rates of correction in patients with serum sodium concentrations <120 mEq/L.
Our retrospective study cannot exclude a causal relationship between hyponatremia and excess mortality. Prospective studies of both inpatient and ambulatory populations will be needed to better define this relationship and to establish what role therapy can play in reducing mortality. On the other hand, our detailed chart reviews and independent review of mortality events by multiple study investigators provide a more comprehensive understanding than previous studies based solely on International Statistical Classification of Diseases and Related Health Problems codes; they support our study hypothesis that the nature of the underlying illness rather than the severity of hyponatremia best explains mortality associated with hyponatremia.
Disclosures
None.
Acknowledgement
We remember our colleague and coauthor, Joseph D. Cappuccio, MD, whose untimely death preceded the submission of this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Hyponametria and Mortality: How Innocent is the Bystander?” on pages 951–953.
References
- 1. Waikar SS, Mount DB, Curhan GC: Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med 122: 857–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Asadollahi K, Beeching N, Gill G: Hyponatraemia as a risk factor for hospital mortality. QJM 99: 877–880, 2006 [DOI] [PubMed] [Google Scholar]
- 3. Gill G, Huda B, Boyd A, Skagen K, Wile D, Watson I, van Heyningen C: Characteristics and mortality of severe hyponatraemia: A hospital-based study. Clin Endocrinol (Oxf) 65: 246–249, 2006 [DOI] [PubMed] [Google Scholar]
- 4. Whelan B, Bennett K, O'Riordan D, Silke B: Serum sodium as a risk factor for in-hospital mortality in acute unselected general medical patients. QJM 102: 175–182, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE: Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 170: 294–302, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Erasmus RT, Matsha TE: The frequency, aetiology and outcome of severe hyponatraemia in adult hospitalised patients. Cent Afr J Med 44: 154–158, 1998 [PubMed] [Google Scholar]
- 7. Crook MA, Velauthar U, Moran L, Griffiths W: Review of investigation and management of severe hyponatraemia in a hospital population. Ann Clin Biochem 36: 158–162, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Saeed BO, Beaumont D, Handley GH, Weaver JU: Severe hyponatraemia: Investigation and management in a district general hospital. J Clin Pathol 55: 893–896, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Natkunam A, Shek CC, Swaminathan R: Hyponatremia in a hospital population. J Med 22: 83–96, 1991 [PubMed] [Google Scholar]
- 10. Nzerue CM, Baffoe-Bonnie H, You W, Falana B, Dai S: Predictors of outcome in hospitalized patients with severe hyponatremia. J Natl Med Assoc 95: 335–343, 2003 [PMC free article] [PubMed] [Google Scholar]
- 11. Hoorn EJ, Lindemans J, Zietse R: Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant, 21: 70–76, 2006 [DOI] [PubMed] [Google Scholar]
- 12. Sterns RH: Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med 107: 656–664, 1987 [DOI] [PubMed] [Google Scholar]
- 13. Sterns RH, Cappuccio JD, Silver SM, Cohen EP: Neurologic sequelae after treatment of severe hyponatremia: A multicenter perspective. J Am Soc Nephrol 4: 1522–1530, 1994 [DOI] [PubMed] [Google Scholar]
- 14. Birim O, Maat AP, Kappetein AP, van Meerbeeck JP, Damhuis RA, Bogers AJ: Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 23: 30–34, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA: New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol 57: 1288–1294, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Ayus JC, Krothapalli RK, Arieff AI: Treatment of symptomatic hyponatremia and its relation to brain damage. A prospective study. N Engl J Med 317: 1190–1195, 1987 [DOI] [PubMed] [Google Scholar]
- 17. Vu T, Wong RF, Hamblin PS, Zajac JF, Grossmann MF: Patients presenting with severe hypotonic hyponatremia: Etiological factors, assessment, and outcomes. Hosp Pract (Minneap) 37: 128–136, 2009 [DOI] [PubMed] [Google Scholar]
- 18. Baran D, Hutchinson TA: The outcome of hyponatremia in a general hospital population. Clin Nephrol 22: 72–76, 1984 [PubMed] [Google Scholar]
- 19. Clayton JA, Le Jeune IR, Hall IP: Severe hyponatraemia in medical in-patients: Aetiology, assessment and outcome. QJM 99: 505–511, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Martin PJ, Young CA: Central pontine myelinolysis: Clinical and MRI correlates. Postgrad Med J 71: 430–432, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ayus JC, Krothapalli RK, Arieff AI: Changing concepts in treatment of severe symptomatic hyponatremia. Rapid correction and possible relation to central pontine myelinolysis. Am J Med 78: 897–902, 1985 [DOI] [PubMed] [Google Scholar]
- 22. Arieff AI, Llach F, Massry SG: Neurological manifestations and morbidity of hyponatremia: Correlation with brain water and electrolytes. Medicine (Baltimore) 55: 121–129, 1976 [DOI] [PubMed] [Google Scholar]
- 23. Sterns RH, Nigwekar SU, Hix JK: The treatment of hyponatremia. Semin Nephrol 29: 282–299, 2009 [DOI] [PubMed] [Google Scholar]
- 24. Ayus JC, Arieff AI: Chronic hyponatremic encephalopathy in postmenopausal women: Association of therapies with morbidity and mortality. JAMA 281: 2299–2304, 1999 [DOI] [PubMed] [Google Scholar]
- 25. Verbalis JG, Barsony J, Sugimura Y, Tian Y, Adams DJ, Carter EA, Resnick HE: Hyponatremia-induced osteoporosis. J Bone Miner Res 25: 554–563, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gankam Kengne F, Andres C, Sattar L, Melot C, Decaux G: Mild hyponatremia and risk of fracture in the ambulatory elderly. QJM 101: 583–588, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Kinsella S, Moran S, Sullivan MO, Molloy MG, Eustace JA: Hyponatremia independent of osteoporosis is associated with fracture occurrence. Clin J Am Soc Nephrol 5: 275–280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]