Abstract
Summary
Background and objectives
Sleep-disordered breathing (SDB) and excessive daytime sleepiness (EDS) are highly prevalent among hemodialysis (HD) patients. It is unclear to what extent SDB is associated with advanced chronic kidney disease (CKD; stages 4 to 5). This paper describes and compares the prevalence, severity, and patterns of SDB and EDS among patients with advanced CKD, HD-dependent patients, and community individuals without known renal disease.
Design, setting, participants, & measurements
Eighty-nine CKD and 75 HD patients were compared with 224 participants from the Sleep-Strategies Concentrating on Risk Evaluation Sleep-SCORE study of sleep and cardiovascular risk. Participants had in-home unattended polysomnography for quantifying SDB. EDS was defined by a score ≥10 on the Epworth Sleepiness Scale.
Results
The sample had a median age 58.1 years, was predominantly male (57.4%) and white (62.5%), and had a median body mass index of 28.1 kg/m2. Controls and Sleep-SCORE Study CKD patients had significantly higher median total sleep time and sleep efficiency compared with HD patients. The adjusted odds of severe SDB were higher for CKD and HD groups compared with the controls. Nocturnal hypoxemia was significantly elevated in the HD group compared with the CKD group. There were similar proportions of participants with EDS between the controls (33%), the CKD patients (29.3%), and the HD patients (40.6%).
Conclusions
Severe SDB (predominantly obstructive) and EDS are common among advanced CKD and HD patients. EDS correlated modestly with severe SDB and its obstructive and mixed patterns in the HD group.
Introduction
Sleep-disordered breathing (SDB) is the most common cause of poor sleep in kidney disease patients, with manifestations ranging from obstructive apneas, in which upper airway obstruction leads to cessation or reduction of airflow despite persistent ventilatory efforts, to central apnea, in which airflow is absent because of cessation of ventilatory efforts or mixed (central and obstructive) apnea. Studies in the past have consistently shown a high prevalence of SDB (>50%) in patients on hemodialysis (HD) because of compromised upper airway stability (extracellular fluid volume overload) (1,2), ventilatory control instability (altered central and peripheral chemosensitivity), and reduced upper airway muscle tone (uremia) (3–7). Risk factors of SDB in the general population such as older age, male gender, obesity, smoking, increased neck circumference and diabetes are also prevalent in the CKD population (8).
SDB has been associated with increased cardiovascular risk (9–11) and may contribute to the morbidity and mortality of patients with advanced (stages 4 to 5) chronic kidney disease (CKD) or on HD (9). The pathophysiologic link between SDB and adverse outcomes in the HD population may be mediated through increased cardiac and peripheral sympathetic activity, vasoconstriction, cardiac arrhythmia, oxidative stress, and vascular inflammation promoting coronary calcification (12) and atherogenesis (13,14), respectively. Nocturnal hypoxemia due to SDB has been associated with left ventricular hypertrophy (15), hypertension (16), and cognitive dysfunction (17). Finally, daytime somnolence associated with SDB may lead to diminished quality of life and cognitive dysfunction (5,18,19). Hence, it is crucial to better understand the prevalence and risk factors for SDB and nocturnal hypoxemia in the CKD and HD patients.
Although CKD patients outnumber HD patients, most of the sleep disorder epidemiology in kidney disease patients has focused on the latter. We previously reported a fourfold greater prevalence of sleep apnea in older conventional HD patients compared with a control sample (20). Furthermore, current clinical SDB practice for CKD and HD patients is largely informed by data obtained from small studies (21–23), limited channel sleep monitors (18,23,24), or studies primarily comprised of symptomatic patients (5,22,25). The aim of this report is to describe and compare the prevalence and various patterns of SDB and the prevalence of excessive daytime sleepiness (EDS) among patients with advanced CKD, HD patients, and a control group without known CKD. We hypothesized that HD patients would demonstrate more severe SDB and prevalent EDS compared with advanced CKD patients and controls. CKD patients would have more severe SDB and EDS than the controls. Finally, we examined the extent to which other population characteristics may correlate with the severity of SDB and EDS.
Materials and Methods
Study Setting, Samples, and Design
Patients.
For this study, 164 CKD and HD patients were enrolled from outpatient nephrology clinics, local dialysis centers, and the Thomas E. Starzl Transplant Institute in Western Pennsylvania between March 2004 and December 2008. Patients were eligible to participate if they were >18 years of age and had advanced CKD (Modification of Diet in Renal Disease [MDRD]-derived estimated GFR [eGFR] ≤40 ml/min per 1.73 m2). Exclusion criteria have been previously described. Briefly, patients were excluded for use of continuous positive airway pressure and for active medical or psychiatric disease (e.g., unstable angina, alcohol abuse). Forty-six older HD patients included in this study sample were previously published in a comparison with patients from the Sleep Heart Health Study (20).
HD patients' preference determined the night of polysomnography (PSG) conduction relative to their HD day. Of the 67 HD patients with available data, 31 (41.3%) and 36 (48%) patients were studied the evening after and before their session, respectively. Of the 57 HD participants with available shift data, 40 were on morning (5:30 to 10:00 a.m.), 16 on afternoon (10:00 a.m. to 3:30 p.m.), and 1 on evening shift (3:30 to 5:30 p.m.).
Controls.
For this report, 224 controls were taken from the Sleep Strategies Concentrating on Risk Evaluation (SCORE) study (26,27), which recruited participants from the Heart-SCORE study, a single-center, prospective, community-based cohort study investigating the mechanisms accountable for population disparities in cardiovascular risk (28). Baseline enrollment began on June 16, 2003 and ended on October 11, 2006.
The Sleep-SCORE study recruited participants with high-, moderate-, or low-risk Framingham scores and approximately equal male/female and black/white ratios of participants. Exclusion criteria for the Sleep-SCORE study included pregnancy; use of continuous positive airway pressure for SDB treatment; regular use of sleep medications; nighttime work schedule; medication for diabetes; and prior diagnosis of stroke, myocardial infarction, or interventional cardiology procedures. The University of Pittsburgh Institutional Review Board approved both studies. All participants provided written informed consent.
Data Collection
Baseline data collection for all participants included a brief standardized health interview, questionnaire administration, assessment of antihypertensives (average number used at the time of the study) and antidepressants, systolic BP (SBP) and diastolic BP (DBP) before the PSG assessment, weight, height, neck circumference, and unattended home PSG.
In both studies, two measurements of SBP and DBP were performed within at least 2 to 3 minutes before each PSG using an automated cuff. The Sleep-SCORE project standardized these measurements additionally with a mercury cuff. If there was a 4- to 6-mmHg discrepancy between the two cuffs, a third measurement was performed. The within-6-month from the date of the study serum creatinine (Scr), eGFR, and serum glucose for all groups were also recorded.
Sleep Assessment—PSG
Unattended in-home PSG was performed using an ambulatory Compumedics Siesta monitor (Charlotte, NC) at habitual sleep times for both studies. The PSG montage (same for both studies) included bilateral central and occipital electroencephalogram channels (C3-P3, C4-P4 and CZ-PZ), bilateral electrooculogram, and bipolar submentalis electromyogram. Bipolar electrocardiogram and position sensors were used to monitor heart rate and body position, respectively. Participants were also monitored for respiratory parameters, nasal pressure, and for abdominal and thoracic effort using finger pulse oximetry (Nonin, Minneapolis, MN), nasal-oral thermocouple, and inductance plethysmography, respectively. The Sleep-SCORE participants were studied using two nights of PSG. This report uses the first night sleep study (26,27).
Scoring of Polysomnograms—Sleep Parameter Definitions
Sleep study data processing and scoring followed identical procedures (26,27). The same centrally trained PSG technologists scored sleep records for all study groups according to the Rechtschaffen and Kales guidelines using standard sleep stage scoring criteria for each 20-second epoch (29). All scorers were blinded to the renal function of the patients. Standard definitions were used to identify apneas and hypopneas; oximetry readings were used to quantify average and minimum oxyhemoglobin saturation levels. Apnea was defined as a complete or an almost complete (≤25% of baseline) airflow cessation; measured by the amplitude of the ≥10-second nasal pressure signal. Hypopnea was defined as a ≥10-second abnormal respiratory event with ≥30% airflow reduction (compared with baseline) and was associated with ≥4% oxyhemoglobin desaturation.
PSG outcome variables in the analysis included total sleep time (TST; sleep time excluding periods of wakefulness during the night), sleep efficiency (percentage of TST as a proportion of the total study duration), parameters of sleep architecture (percentage of TST spent in stage 1, stage 2, stages 3 to 4, and rapid eye movement sleep), apnea/hypopnea index (AHI; number of apneas and hypopneas/hour of sleep), arousal index (number of arousals/hour of sleep), type of sleep apnea (obstructive, central, mixed apnea index), and nocturnal hypoxemia (≥3% of TST with oxyhemoglobin saturation <90%) (30). Severe SDB was defined as having AHI ≥ 30.
Daytime Sleepiness Self-Report
Participants also completed the Epworth Sleepiness Scale (ESS) (31), an eight-item subjective measure of the likelihood of falling asleep in specific situations. Scores ≥ 10 reflected EDS.
Statistical Analyses
Nonparametric tests were used to examine the statistical significance of the differences between the study groups (Kruskal–Wallis test). For population characteristics and sleep parameters significantly different, the Mann–Whitney U test was performed for pairwise comparisons. The strength of the relationship between self-reported EDS and severe SDB, nocturnal hypoxemia, and various patterns of SDB was examined using the φ coefficient for the correlation between two dichotomous variables and the point biserial coefficient (rpb) for the correlation between dichotomous and continuous variables.
Univariate binary logistic regression was performed to quantify the degree of association of each covariate with severe SDB, nocturnal hypoxemia, and EDS. All multivariate logistic regression analyses were adjusted for age, gender, and body mass index (BMI) and were performed with SPSS, version 16 statistical software (SPSS, Inc., Chicago, IL).
Results
Study Population
The characteristics for all study participants are shown in Table 1. Patients in the CKD group were the youngest. Compared with the control group, the CKD group had higher proportions of men and whites, lower proportion of employed participants, lower BMI, and higher SBP and DBP. The HD group had higher SBP and used more antihypertensives than the controls. Finally, the HD patients had higher serum glucose levels compared with the CKD patients and the controls. The study groups did not differ significantly on the use of antidepressants.
Table 1.
Characteristics of patients and community controls
| Characteristic | CKD Stages 4 to 5 (n = 89) | HD (n = 75) | Sleep-SCORE Controls (n = 224) | P |
|---|---|---|---|---|
| Age (years) | 51 (42.5, 64) | 57.5 (46, 67.2) | 59.8 (54.5, 65.2) | <0.001 |
| Controls > CKD,a HD > CKDc | ||||
| Male | 60 (67.4%) | 49 (66.2%) | 113 (50.4%) | 0.006 |
| CKD > controls,b HD > controlsc | ||||
| White | 70 (78.7%) | 45 (60.8%) | 127 (56.7%) | 0.001 |
| CKD > controls,a CKD > HDb | ||||
| High school education | 82 (92.1%) | 63 (85.1%) | 221 (98.7%) | 0.001 |
| Controls > CKD,b controls > HDa | ||||
| Employed | 40 (44.9%) | 11 (14.9%) | 136 (60.7%) | <0.001 |
| Controls > CKD,b controls > HD,a CKD > HDa | ||||
| BMI (kg/m2) | 27.6 (25, 31.2) | 27.2 (23.5, 31.2) | 28.6 (25.8, 32.7) | 0.01 |
| Controls > CKD,b controls > HDb | ||||
| Neck circumference (cm) | 38 (36, 41.5) | 40 (37.2, 43.7) | 38.6 (36.7, 42.7) | 0.06 |
| SBP (mmHg) | 148.2 (132.1, 165.5) | 145.7 (126.6, 169.4) | 131 (122.5, 141.5) | <0.001 |
| CKD, HD > controlsa | ||||
| DBP (mmHg) | 83.7 (73.4, 90.9) | 78.5 (70.0, 88.9) | 79.7 (73.5, 86.5) | 0.040 |
| CKD > controls,b CKD > HDb | ||||
| Glucose (mg/dl) | 93.7 (85, 121) | 119 (96.5, 147.2) | 95 (86, 103) | <0.001 |
| HD > controls,a HD > CKDa | ||||
| Total number of antihypertensives | 3 (2, 4) | 2 (1, 3) | 0 (0, 1) | <0.001 |
| CKD, HD > controls,a CKD > HDb | ||||
| Antidepressants | 13 (14.6%) | 10 (13.7%) | 18 (8%) | 0.15 |
| Smoking | 0.7 | |||
| current | 13 (14.6%) | 8 (10.8%) | 19 (8.5%) | |
| former | 28 (31.5%) | 33 (44.6%) | 106 (47.3%) | |
| never | 48 (53.9%) | 33 (44.6%) | 99 (44.2%) |
Results are presented as medians and interquartile ranges or as percentages. Numbers in parentheses reflect the 25th and the 75th percentile of the variables.
P < 0.001;
P < 0.01;
P < 0.05 for the pairwise comparisons.
The controls and the CKD sample had a mean MDRD-derived (32) eGFR of 91.8 ± 19.2 ml/min per 1.73 m2 and 18.9 ± 7.6 ml/min per 1.73 m2, respectively, and a mean Scr of 0.96 ± 0.2 and 4.5 ± 2.7, respectively. The cause of CKD/ESRD is presented in Table 2. The HD patients had been on thrice weekly in-center HD for 21.5 months (25th to 75th percentiles, 9.0 to 49.7 months) and were receiving adequate dialysis dose (mean single-pool Kt/V, 1.6 ± 0.31 or mean urea reduction ratio of 72.6 ± 6.2).
Table 2.
Etiology of CKD and ESRD
| Cause | CKD (n = 89) | ESRD (n = 73) |
|---|---|---|
| Diabetic nephropathy, n | 29 (32.6%) | 24 (32%) |
| Hypertension, n | 15 (16.9%) | 14 (18.7%) |
| Glomerulonephritis, n | 16 (18%) | 11 (14.7%) |
| Liver- or small-bowel transplant related, n | 2 (2.2%) | 3 (4%) |
| Other causes, n | 21 (23.6%) | 16 (21.3%) |
| Unknown or uncertain causes, n | 6 (6.7%) | 5 (6.7%) |
Sleep Characteristics and Parameters of SDB
Parameters of sleep, SDB, and EDS and their unadjusted differences across study groups are shown in Table 3. Compared with the HD group, the CKD patients and controls had significantly greater TST and sleep efficiency. The HD group had significantly greater stage 1 and stage 3 to 4 sleep and significantly less stage 2 and rapid eye movement sleep, whereas CKD patients had significantly greater stage 3 to 4 sleep compared with the controls. Median AHI was higher in the HD group compared with the other groups, but it did not differ between the CKD and the control group. CKD and HD groups had more participants with severe SDB compared with the controls with no significant difference in the prevalence of severe SDB. Very few central and/or mixed apneas were recorded among any of the participants.
Table 3.
Polysomnographic parameters and EDS results for all study groups
| Parameter | CKD Stage 4 to 5 (n = 89) | HD (n = 75) | Sleep-SCORE Controls (n = 224) | P |
|---|---|---|---|---|
| TST (minutes) | 366.3 (298.3, 433.2) | 313.3 (216.2, 388.2) | 374 (314.3, 421.1) | <0.001 |
| CKD > HDa controls > HDa | ||||
| Sleep efficiency (%) | 77.8 (67.3, 85.1) | 69.8 (59.5, 78.9) | 79.3 (70.1, 86.3) | <0.001 |
| CKD > HDb controls > HDa | ||||
| Stage 1 (% TST) | 10.1 (6.2, 15.9) | 11.6 (6.8, 18.1) | 8.7 (5.5, 12.5) | 0.014 |
| Controls > HDb | ||||
| Stage 2 (% TST) | 61.2 (53.9, 69.8) | 57.5 (51, 67) | 63.5 (56.5, 68.4) | 0.004 |
| Controls > HDb | ||||
| Stage 3 and 4 (% TST) | 5.4 (1.3, 10.9) | 7.3 (1.3, 18.5) | 2.5 (0.3, 8) | <0.001 |
| CKD > controlsb HD > controlsa | ||||
| REM (% TST) | 20.3 (14.5, 26) | 17.9 (11.2, 22.3) | 21.8 (17.2, 26.2) | <0.001 |
| Controls > HDa | ||||
| AHI | 8.8 (3.2, 27.6) | 18.2 (6.7, 30.2) | 8.6 (4.3, 16.4) | <0.001 |
| HD > CKDc HD > controlsa | ||||
| AHI ≥ 30 | 20 (22.5%) | 19 (25.7%) | 25 (11.5%) | 0.002 |
| CKD > controlsb HD > controlsb | ||||
| Arousal index | 8.9 (5.4, 13.4) | 8.8 (3.9, 15.5) | 8.5 (5.4, 13.9) | 0.94 |
| Type of apnea | ||||
| obstructive apnea index | 6.2 (2, 17.3) | 11.3 (4.1, 23) | 6 (2.6, 13.2) | 0.006 |
| HD > controlsb HD > CKDc | ||||
| central apnea index | <0 (0, 0.3) | 0.2 (0, 0.7) | <0 (0, 0.2) | 0.001 |
| HD > controlsa HD > CKDc | ||||
| mixed apnea index | <0 (0, 0.2) | <0 (0, 0.3) | <0 (0, 0.2) | 0.34 |
| nocturnal hypoxemia ≥ 3% TST | 28 (37.8%) | 39 (54.9%) | 49 (27.1%) | <0.001 |
| HD > controlsa HD > CKDc | ||||
| Self-reported sleep | ||||
| ESS ≥ 10 | 24 (29.3%) | 28 (40.6%) | 74 (33.0%) | 0.33 |
Results are presented as medians and interquartile ranges or as percentages. Numbers in parentheses reflect the 25th and the 75th percentile of the variables. REM, rapid eye movement; sleep efficiency, percentage of TST as a proportion of the total study duration; AHI ≥ 30, percentage of subjects who had at least 30 apneas/hypopneas during TST; obstructive apnea index, number of obstructive apneas per hour of TST; central apnea index, number of central apneas per hour of TST; mixed apnea index, number of obstructive and central apneas per hour of TST; nocturnal hypoxemia ≥ 3% TST, percentage of subjects who had at least 3% of TST with oxyhemoglobin saturation <90%.
P < 0.001;
P < 0.01;
P < 0.05 for the pairwise comparisons.
Compared with HD patients, nocturnal hypoxemia was lower in the CKD patients and controls, but there was no difference between the latter. There were no significant differences in arousal index and self-reported EDS across all groups.
In ESRD patients, there was no significant difference in nocturnal hypoxemia (median: 4.6 versus 3.8; P = 0.2), AHI (median: 18.3 versus 17.8, P = 0.4), or total ESS score (median: 8.0 versus 9.0, P = 0.8) between dialysis and nondialysis evenings, respectively. Nor were there significant differences in nocturnal hypoxemia (median: 4.0 versus 5.8; P = 0.4), AHI (median: 14.3 versus 17.5, P = 0.7), or total ESS score (median: 9.0 versus 6.0, P = 0.09) between morning and afternoon/evening dialysis shifts, respectively.
Multivariate Analysis Results
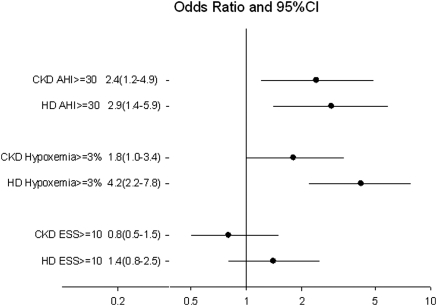
Figure 1 demonstrates the adjusted odds ratios (ORs) for severe SDB, nocturnal hypoxemia, and EDS in the CKD and HD patients compared with controls after adjustment for age, gender, and BMI. CKD and HD each had significantly higher odds for severe SDB (P = 0.01 and 0.003, respectively) and nocturnal hypoxemia (P = 0.05 and P < 0.001, respectively). However, compared with the controls, CKD and HD were not associated with increased risk of EDS. As a sensitivity analysis, we tested the effect of using an AHI > 15 to classify patients with moderate to severe SDB. HD (OR 4.14, 95% confidence interval [CI] 2.26 to 7.60) and advanced CKD (OR 2.19, 95% CI 1.22 to 3.92) had higher odds of moderate to severe SDB compared with controls under this definition of SBD.
Figure 1.
Association of treatment (CKD or HD) with polysomnographic parameters and EDS after adjustment for age, gender, and BMI. Error horizontal lines represent the 95% CIs and black dots represent the ORs. CKD and HD were significantly (P < 0.05) associated with an AH ≥ 30 and with nocturnal hypoxemia (oxyhemoglobin saturation [SaO2] < 90%) for ≥3% of the TST. Reference group is the control group.
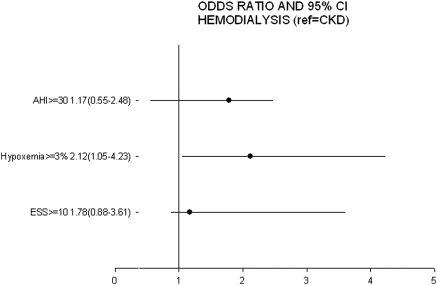
Figure 2 demonstrates the adjusted ORs for severe SDB, nocturnal hypoxemia, and EDS in the HD group compared with the CKD group after adjustment for age, gender, and BMI. Participants on HD had higher odds for nocturnal hypoxemia (OR 2.12, 95% CI 1.05 to 4.23, P = 0.04) compared with advanced CKD participants. However, compared with the CKD group, HD was not associated with increased risk of EDS.
Figure 2.
Association of HD treatment with polysomnographic parameters and EDS after adjustment for age, gender, and BMI. Error horizontal lines represent the 95% CIs and black dots represent the ORs. HD was significantly (P < 0.05) associated with nocturnal hypoxemia (SaO2 < 90%) for ≥3% of the TST. Reference group is the advanced CKD group.
After adjusting for age, gender, and BMI, men with advanced CKD had higher odds for severe SDB than women with advanced CKD. Men undergoing HD were less likely to report EDS than women undergoing HD. Among the controls, men were more likely to have severe SDB and EDS compared with women, whereas controls with a higher BMI were more likely to be hypoxemic.
Among the CKD population and after adjustment for age, gender, and BMI, Scr and eGFR were not significantly correlated with severe SDB, nocturnal hypoxemia, or EDS.
Correlation of Self-Reported EDS with Severe SDB, Nocturnal Hypoxemia, and Patterns of Sleep Apnea
The relationships between self-reported EDS and severe SDB, nocturnal hypoxemia, and SDB patterns for all participants and for each subgroup are shown in Table 4. A weak correlation between EDS and severe SDB, nocturnal hypoxemia, and obstructive and mixed apneas was observed for all participants, and between EDS and obstructive apneas for the controls, respectively. Finally, a weak to moderate correlation was observed between EDS and severe SDB and obstructive and mixed apneas for the HD group.
Table 4.
Correlation of self-reported EDS (ESS ≥ 10) with severe sleep apnea, nocturnal hypoxemia, and patterns of sleep apnea among the CKD patients, the HD patients, and the Sleep-SCORE controls
| CKD Stage 4 to 5 (n = 89) | HD (n = 75) | Sleep-SCORE Controls (n = 224) | |
|---|---|---|---|
| AHI ≥ 30 | ϕ = 0.13, P = 0.23 | ϕ = 0.32, P = 0.009b | ϕ = 0.09, P = 0.19 |
| Nocturnal hypoxemia ≥ 3% TST | ϕ = 0.16, P = 0.18 | ϕ = 0.08, P = 0.54 | ϕ = 0.13, P = 0.07 |
| Obstructive apnea index | rpb = 0.12, P = 0.29 | rpb = 0.27, P = 0.02a | rpb = 0.14, P = 0.03a |
| Central apnea index | rpb = 0.12, P = 0.27 | rpb = 0.18, P = 0.13 | rpb = 0.07, P = 0.28 |
| Mixed apnea index | rpb = 0.06, P = 0.56 | rpb = 0.25, P = 0.03a | rpb = 0.09, P = 0.18 |
Results are expressed as correlation coefficient (ϕ) or r point biserial (rpb) and two-tailed statistical significance (P). Nocturnal hypoxemia ≥ 3% TST, percentage ≥3% of total sleep time with oxyhemoglobin saturation <90%.
Significance at the a0.05 and b0.01 level.
Discussion
In this report we undertook home PSG in a relatively large community-based sample of advanced CKD patients, HD patients, and controls. We found that severe SDB is highly prevalent among advanced CKD patients and among patients undergoing conventional thrice-weekly HD, whereas obstructive sleep apneas were predominant in CKD and HD patients. Although subjectively reported EDS was commonly reported in all groups, EDS only modestly correlated with severe SDB and its obstructive and mixed patterns in the HD group. These findings highlight the challenges in the identification of CKD or HD patients at risk for SDB.
In this report, presence of advanced CKD was associated with a 2.4-fold higher risk of severe SDB after adjustment compared with the control group. Previous studies using PSG to examine SDB in advanced CKD were smaller and uncontrolled. A study of 35 patients with CKD (mean creatinine clearance: 27 ml/min per 1.73 m2) demonstrated that 50% of the patients had mild SDB and approximately one-third had moderate SDB (33). Furthermore, a large cross-sectional study of patients in the United States also demonstrated a weak association of eGFR <30 ml/min per 1.73 m2 with SDB diagnosis, with a nonsignificant 1.16-fold increase in risk for SDB that was further attenuated after adjustment for comorbidities (34). One could posit that the discrepancy between the latter and our study is due to underdiagnosis of SBD among CKD and HD patients in the community.
Furthermore, we found a higher risk for severe SDB and nocturnal hypoxemia in the HD group compared with the controls and in the HD group compared with the CKD group. In addition, the severity of SDB did not vary between dialysis and off-dialysis evenings or morning and afternoon/evening dialysis shifts, in concordance with previous reports (20,23,35). Observations that nocturnal HD (36) and nocturnal peritoneal dialysis (37,38) significantly improve sleep apnea suggest that factors originating from the deterioration of renal function, rather than from dialysis per se or from comorbidities, may partially explain the increased prevalence of SDB in all stages of CKD. In both patient groups, it is important to investigate the extent to which fluid retention may lead to airway edema, nocturnal hypoxemia, and the observed severity and obstructive patterns of SDB (39,40).
Male gender was strongly associated with increased likelihood of severe SDB in the control and CKD group, but it was not significantly associated with severe SDB in the HD population. This finding extends established knowledge from the general population; SDB occurs in 24% of young, middle-aged men and 9% of women and in 70% of older men and 56% of older women (41). Inherent differences in fat distribution, upper airway anatomy, neurochemical control mechanisms, arousal response, and sex hormones may underline these gender differences in the general population (41) and are possibly operational in patients with renal dysfunction.
In our sample we observed a weak, inconsistent correlation between severe SDB and nocturnal hypoxemia as well as between obstructive and mixed SDB patterns and EDS. A moderate correlation was also observed between EDS and severe SDB and between obstructive and mixed apneas for the HD group. The similarity in the prevalence of EDS, reflected by the ESS scores, is likely because the three groups were screened for SDB in the community and in renal clinics but not in a sleep clinic. Consequently, patients did not present because of sleep-related complaints. This observation can be viewed as a limitation of this report and raises the question about the diagnostic utility of the ESS in CKD or ESRD patients with significant SDB. Other screening questionnaires or portable sleep devices should be considered in the CKD and HD population to better assess for SDB.
The findings of this report should also be interpreted in light of several other limitations. First, the control group was drawn from a large community-based study of participants screened for cardiovascular disease that are not entirely representative of the general population or the population without advanced CKD (27). Second, differences in the exclusion criterion of actively treated sleep apnea and cardiovascular risk between the studies may limit comparability between patient and control groups. Finally, the cross-sectional design of our study does not allow conclusions regarding the causal direction of the link between SDB and advancing CKD. A longitudinal follow-up study is needed to better characterize the natural history of SDB in patients with progressive CKD.
In conclusion, our findings confirm that SDB is highly prevalent among those with advanced CKD as well as those undergoing thrice-weekly HD. Nephrologists should have a high index of suspicion for the diagnosis and treatment of SDB in symptomatic CKD and HD patients. Potential pathophysiological mechanisms and mediators for the higher prevalence of SDB in the CKD and HD population include increased pharyngeal cross-sectional area (42) and abdominal circumference (43), overhydration (38), metabolic acidosis (44), high levels of proinflammatory cytokines (45), C-reactive protein, and triglycerides (46). These mediators should be examined as possible markers or even mediators in future studies to develop targeted interventions for the prevention and therapy of SBD in patients with renal disease.
Disclosures
Mark H. Sanders is a scientific consultant to Philips-Respironics, which manufactures devices used to monitor sleep and diagnose and treat sleep-related breathing disorders. He is also a co-inventor of BiPAP and has a financial interest in this brand and related technologies by Philips-Respironics. In the past he received research support from Respironics and he was on their Speakers Bureau. In the past he was on an Advisory Panel for Sanofi and on an Advisory Panel for Cephalon. Dr. Buysse serves as a consultant for Actelion, Arena, Cephalon, Eli Lilly, GlaxoSmithKline, Merck, Neurocrine, Neurogen, Pfizer, Respironics, Sanofi-Aventis, Sepracor, Servier, Somnus Therapeutics, Stress Eraser, Takeda, and Transcept Pharmaceuticals, Inc. Dr. Unruh has served as a consultant for Merck and on the Medical Advisory Board of Baxter CRRT. Dr. Unruh also has received grant support from the Baxter Extramural Grant Program.
Acknowledgments
This study was supported by National Institute of Health (NIH) grants HL076379, HL076852, HL076858, and Clinical and Translational Science Award/N-Clinical and Translational Research Center #RR024153. This project was funded in part under a grant with the Pennsylvania Department of Health (contract ME-02–384). This work also was supported by an American Society of Nephrology-Hartford–Association of Specialty Professors Junior Development Grant in Geriatric Nephrology, the Paul Teschan Research Fund, and DK66006 (Unruh), and this publication was supported by funds received from the NIH/National Center for Research Resources/General Clinical Research Centers grant MO1-RR000056. We acknowledge Dr. Karen A. Matthews for sharing the data from the Sleep-SCORE study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Sleep Apnea in Individuals with Chronic Kidney Disease: A Wake-up Call,” on pages 954–956.
Supplemental information for this article is available online at www.cjasn.org.
References
- 1. Hanly P: Sleep apnea and daytime sleepiness in end-stage renal disease. Semin Dial 17: 109–114, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Beecroft JM, Pierratos A, Hanly PJ: Clinical presentation of obstructive sleep apnea in patients with end-stage renal disease. J Clin Sleep Med 5: 115–121, 2009 [PMC free article] [PubMed] [Google Scholar]
- 3. Kraus MA, Hamburger RJ: Sleep apnea in renal failure. Adv Perit Dial 13: 88–92, 1997 [PubMed] [Google Scholar]
- 4. Zoccali C, Mallamaci F, Tripepi G: Sleep apnea in renal patients. J Am Soc Nephrol 12: 2854–2859, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Kimmel PL, Miller G, Mendelson WB: Sleep apnea syndrome in chronic renal disease. Am J Med 86: 308–314, 1989 [DOI] [PubMed] [Google Scholar]
- 6. Mendelson WB, Wadhwa NK, Greenberg HE, Gujavarty K, Bergofsky E: Effects of hemodialysis on sleep apnea syndrome in end-stage renal disease. Clin Nephrol 33: 247–251, 1990 [PubMed] [Google Scholar]
- 7. Fletcher EC: Obstructive sleep apnea and the kidney. J Am Soc Nephrol 4: 1111–1121, 1993 [DOI] [PubMed] [Google Scholar]
- 8. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM: Predictors of sleep-disordered breathing in community-dwelling adults: The Sleep Heart Health Study. Arch Intern Med 162: 893–900, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Zoccali C, Mallamaci F, Tripepi G: Nocturnal hypoxemia predicts incident cardiovascular complications in dialysis patients. J Am Soc Nephrol 13: 729–733, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Zoccali C, Mallamaci F, Tripepi G: Nocturnal hypoxemia: A neglected cardiovascular risk factor in end-stage renal disease? Blood Purif 20: 120–123, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Molnar MZ, Lazar AS, Lindner A, Fornadi K, Czira ME, Dunai A, Zoller R, Szentkiralyi A, Rosivall L, Shapiro CM, Novak M, Mucsi I: Sleep apnea is associated with cardiovascular risk factors among kidney transplant patients. Clin J Am Soc Nephrol 5: 125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung HH, Han H, Lee JH: Sleep apnea, coronary artery disease, and antioxidant status in hemodialysis patients. Am J Kidney Dis 45: 875–882, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, Le Jemtel TH: Vascular inflammation in obesity and sleep apnea. Circulation 121: 1014–1021, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arnaud C, Dematteis M, Pepin JL, Baguet JP, Levy P: Obstructive sleep apnea, immuno-inflammation, and atherosclerosis. Semin Immunopathol 31: 113–125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zoccali C, Mallamaci F, Tripepi G, Benedetto FA: Autonomic neuropathy is linked to nocturnal hypoxaemia and to concentric hypertrophy and remodelling in dialysis patients. Nephrol Dial Transplant 16: 70–77, 2001 [DOI] [PubMed] [Google Scholar]
- 16. Zoccali C, Benedetto FA, Tripepi G, Cambareri F, Panuccio V, Candela V, Mallamaci F, Enia G, Labate C, Tassone F: Nocturnal hypoxemia, night-day arterial pressure changes and left ventricular geometry in dialysis patients. Kidney Int 53: 1078–1084, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Row BW: Intermittent hypoxia and cognitive function: Implications from chronic animal models. Adv Exp Med Biol 618: 51–67, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Kuhlmann U, Becker HF, Birkhahn M, Peter JH, von Wichert P, Schutterle S, Lange H: Sleep-apnea in patients with end-stage renal disease and objective results. Clin Nephrol 53: 460–466, 2000 [PubMed] [Google Scholar]
- 19. Shayamsunder AK, Patel SS, Jain V, Peterson RA, Kimmel PL: Sleepiness, sleeplessness, and pain in end-stage renal disease: Distressing symptoms for patients. Semin Dial 18: 109–118, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Unruh ML, Sanders MH, Redline S, Piraino BM, Umans JG, Hammond TC, Sharief I, Punjabi NM, Newman AB: Sleep apnea in patients on conventional thrice-weekly hemodialysis: Comparison with matched controls from the Sleep Heart Health Study. J Am Soc Nephrol 17: 3503–3509, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Wadhwa NK, Mendelson WB: A comparison of sleep-disordered respiration in ESRD patients receiving hemodialysis and peritoneal dialysis. Adv Perit Dial 8: 195–198, 1992 [PubMed] [Google Scholar]
- 22. Benz RL, Pressman MR, Hovick ET, Peterson DD: A preliminary study of the effects of correction of anemia with recombinant human erythropoietin therapy on sleep, sleep disorders, and daytime sleepiness in hemodialysis patients (The SLEEPO study). Am J Kidney Dis, 34: 1089–1095, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Venmans BJ, van Kralingen KW, Chandi DD, de Vries PM, ter Wee PM, Postmus PE: Sleep complaints and sleep disordered breathing in hemodialysis patients. Neth J Med 54: 207–212, 1999 [DOI] [PubMed] [Google Scholar]
- 24. Sanner BM, Tepel M, Esser M, Klewer J, Hoehmann-Riese B, Zidek W, Hellmich B: Sleep-related breathing disorders impair quality of life in haemodialysis recipients. Nephrol Dial Transplant 17: 1260–1265, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Millman RP, Kimmel PL, Shore ET, Wasserstein AG: Sleep apnea in hemodialysis patients: The lack of testosterone effect on its pathogenesis. Nephron 40: 407–410, 1985 [DOI] [PubMed] [Google Scholar]
- 26. Matthews KA, Kamarck TW, M, HH, Strollo PJ, Owens JF, Buysse DJ, Lee L, Reis SE: Blood pressure dipping and sleep disturbance in African-American and Caucasian men and women. Am J Hypertens 21: 826–831, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mezick EJ, Matthews KA, Hall M, Strollo PJ, Jr., Buysse DJ, Kamarck TW, Owens JF, Reis SE: Influence of race and socioeconomic status on sleep: Pittsburgh Sleep-SCORE project. Psychosom Med 70: 410–416, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aiyer AN, Kip KE, Marroquin OC, Mulukutla SR, Edmundowicz D, Reis SE: Racial differences in coronary artery calcification are not attributed to differences in lipoprotein particle sizes: The Heart Strategies Concentrating on Risk Evaluation (Heart SCORE) Study. Am Heart J 153: 328–334, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Rechtschaffen A, Kales A. Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects, NIH Publication 204, Bethesda, MD, U.S. National Institute of Neurological Diseases and Blindness, Neurological Information Network, 1968 [Google Scholar]
- 30. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O'Connor GT, Rapoport DM, Redline S, Resnick HE, Robbins JA, Shahar E, Unruh ML, Samet JM: Sleep-disordered breathing and mortality: A prospective cohort study. PLoS Med 6: e1000132, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Johns MW: A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep 14: 540–545, 1991 [DOI] [PubMed] [Google Scholar]
- 32. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 33. Markou N, Kanakaki M: Sleep-disordered breathing and reductions in renal function: Are they associated in the elderly? Sleep Med 9: 598–600, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Sim JJ, Rasgon SA, Kujubu DA, Kumar VA, Liu IL, Shi JM, Pham TT, Derose SF: Sleep apnea in early and advanced chronic kidney disease: Kaiser Permanente Southern California cohort. Chest 135: 710–716, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Hanly PJ, Gabor JY, Chan C, Pierratos A: Daytime sleepiness in patients with CRF: Impact of nocturnal hemodialysis. Am J Kidney Dis 41: 403–410, 2003 [DOI] [PubMed] [Google Scholar]
- 36. Hanly PJ, Pierratos A: Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med 344: 102–107, 2001 [DOI] [PubMed] [Google Scholar]
- 37. Tang SC, Lam B, Ku PP, Leung WS, Chu CM, Ho YW, Ip MS, Lai KN: Alleviation of sleep apnea in patients with chronic renal failure by nocturnal cycler-assisted peritoneal dialysis compared with conventional continuous ambulatory peritoneal dialysis. J Am Soc Nephrol 17: 2607–2616, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Tang SC, Lam B, Lai AS, Pang CB, Tso WK, Khong PL, Ip MS, Lai KN: Improvement in sleep apnea during nocturnal peritoneal dialysis is associated with reduced airway congestion and better uremic clearance. Clin J Am Soc Nephrol 4: 410–418, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shiota S, Ryan CM, Chiu KL, Ruttanaumpawan P, Haight J, Arzt M, Floras JS, Chan C, Bradley TD: Alterations in upper airway cross-sectional area in response to lower body positive pressure in healthy subjects. Thorax 62: 868–872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yumino D, Redolfi S, Ruttanaumpawan P, Su MC, Smith S, Newton GE, Mak S, Bradley TD: Nocturnal rostral fluid shift: A unifying concept for the pathogenesis of obstructive and central sleep apnea in men with heart failure. Circulation 121: 1598–1605, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Lin CM, Davidson TM, Ancoli-Israel S: Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 12: 481–496, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beecroft JM, Hoffstein V, Pierratos A, Chan CT, McFarlane P, Hanly PJ: Nocturnal haemodialysis increases pharyngeal size in patients with sleep apnoea and end-stage renal disease. Nephrol Dial Transplant 23: 673–679, 2008 [DOI] [PubMed] [Google Scholar]
- 43. Sakkas GK, Karatzaferi C, Liakopoulos V, Maridaki MD, Lavdas E, Giannaki CD, Gourgoulianis KI, Stefanidis I: Polysomnographic evidence of sleep apnoea disorders in lean and overweight haemodialysis patients. J Ren Care 33: 159–164, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Tada T, Kusano KF, Ogawa A, Iwasaki J, Sakuragi S, Kusano I, Takatsu S, Miyazaki M, Ohe T: The predictors of central and obstructive sleep apnoea in haemodialysis patients. Nephrol Dial Transplant 22: 1190–1197, 2007 [DOI] [PubMed] [Google Scholar]
- 45. Erten Y, Kokturk O, Yuksel A, Elbeg S, Ciftci TU, Pasaoglu H, Ozkan S, Bali M, Arinsoi T, Sindel S: Relationship between sleep complaints and proinflammatory cytokines in haemodialysis patients. Nephrology (Carlton) 10: 330–335, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Chiu YL, Chuang YF, Fang KC, Liu SK, Chen HY, Yang JY, Pai MF, Peng YS, Wu KD, Tsai TJ: Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant 24: 247–251, 2009 [DOI] [PubMed] [Google Scholar]