Abstract
OBJECTIVES
To verify the ability to identify the layered structures of ureteral wall and to image a segment of ureter in three dimensions with a high speed endoscopic optical coherence tomography (EOCT).
METHODS
We imaged a porcine ureter ex vivo using a spectral domain EOCT with an specially designed circumferential scanning fiber catheter. The images were correlated with the histology to identify corresponding structures. Three-dimensional images and en face images at different depths from the luminal surface were reconstructed from the multiple cross-sectional images to visualize the layered structure of a segment of the ureter from different perspectives.
RESULTS
EOCT images can clearly reveal all layers of the ureteral wall as shown in the histological images. Especially, with the specially designed fiber catheter, the light beam was well centered during the rotation and pull back, which allowed constant acquisition of high fidelity images and unambiguous identification of the smooth muscle layers in all images. With high speed EOCT, a segment of ureter (20 mm) can be imaged in less than 90 seconds at a high resolution.
CONCLUSIONS
With its ability to visualize all layers of the ureteral wall, EOCT imaging offers the potential to stage urothelial cancers that have infiltrated the muscular wall (stage T2). This information will be complimentary to the diagnostic information obtained through ureteroscopic biopsy and CT urogram.
Keywords: Optical coherence tomography, Ureteral Cancer, Three dimensions, Endoscopic
Staging and grading of urothelial carcinoma in ureters have been problematic because of its narrow caliber, which makes biopsy difficult and unreliable1,2. Many efforts have been made to assess cancer stage and grade preoperatively, including the use of sophisticated biopsy devices and endoluminal ultrasound3,4. However, an aggressive biopsy technique involves a risk of ureteral perforation and has a low success rate; ultrasound does not have sufficient resolution to stage early ureteral cancer. In light of these difficulties, we have developed an endoscopic optical coherence tomography (EOCT) and have evaluated the feasibility of using the EOCT to image the layer structures of a segment of ureteral wall in three dimensions.
Optical coherence tomography (OCT) is analogous to ultrasound imaging, but enables much higher resolution by measuring the light reflection from different layers with an interferometer. As an emerging imaging technique, OCT has been widely accepted as a powerful diagnostic tool in ophthalmology5, and recently approved by FDA for intravascular imaging6, Because OCT can be implemented using fiber optics, endoscopic OCT (EOCT) has been explored as an affiliated microscopic imaging modality with white-light endoscopy to image various internal organs7, such as esophagus, cervix and lung. EOCT can acquire in vivo histology-resembling images, which suggests its potential of providing an imaging guidance of taking biopsy, assessing tumor margin and following treatment outcome.
EOCT with a forward-scanning fiber catheter has been used with cystoscopy to detect bladder cancer8. Staging of bladder cancer with EOCT is feasible by evaluating tumor-induced degradation of the layered structure of bladder wall9. Ureteral cancer is an uncommon cancer but more likely to be muscle invasive compared with bladder cancer due to the difficulty in accessing ureteral tumors and staging early ureteral cancer with currently available techniques10. Previously, ex vivo imaging of the porcine ureters with EOCT with a circumferential scanning fiber catheter produced some encouraging results11,12. However, due to the limitation of the imaging speed and the design of the fiber catheter, diagnostic-quality OCT images were not consistently acquired. In particular, the lack of the clear visualization of the smooth muscle layer would limit the staging ability of OCT. The purpose of this study was to demonstrate acquisition of high quality images of a segment of a porcine ureter in a short period of time with a high-speed EOCT equipped with a specially designed fiber catheter. The cross-sectional images were correlated with histological images, and a longitudinal image and en face images at different radical depths were reconstructed from the 3D volumetric image set.
MATERIAL AND METHODS
Porcine Ureter Preparation
A porcine ureter with a kidney from a normal female Yorkshire swine was obtained from the Research and Skills Labs at the University Hospitals of Cleveland. They were then transported to the imaging lab in cold saline immediately after animal sacrifice. Most soft tissue, such as fat and blood vessels, were separated from the ureter and discarded. To prevent dehydration, the ureter and kidney were immersed in cold saline during imaging.
Endoscopic OCT
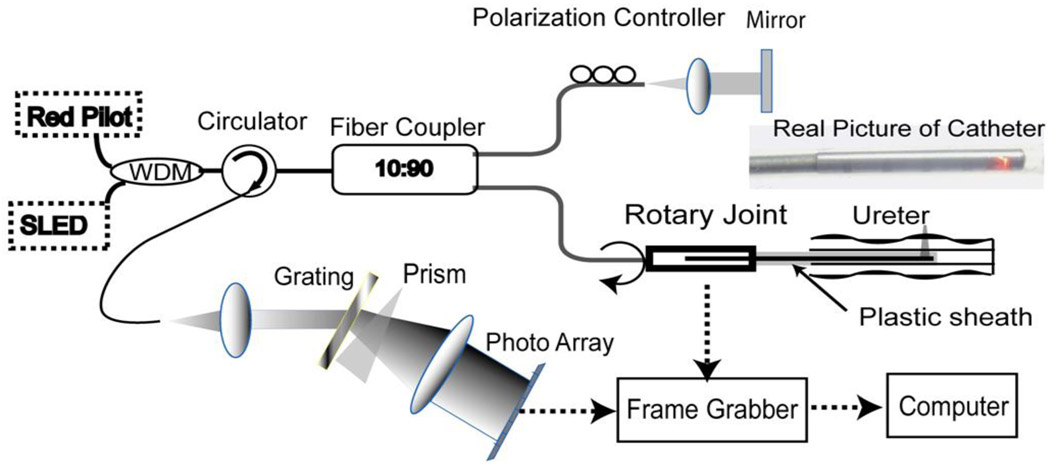
Figure 1 illustrates the schematic of the EOCT system and the OCT fiber catheter used in this study. A broadband light source centered at 1.3 µm with a full-width-at-half-maximum bandwidth of 70 nm was coupled into a fiber based spectral-domain OCT (SD-OCT) system. A red laser was also connected to the system through a wavelength-division multiplexer to visualize the imaging location. In contrast to previously-used time-domain OCT(TD-OCT)11,12, SD-OCT can achieve much higher imaging speed13, which is critical for in vivo imaging.
Figure 1.
Schematic of endoscopic OCT with a circumferential scanning fiber catheter shown as the inset picture. The prism used in the set up is to linearize the wavelength in wave-number space. WDM: wavelength-division multiplexer;
An optical fiber catheter build in-house was used to image a segment of the ureter through helical scanning. A photograph of the fiber catheter is shown as the inset in Fig.1. The light beam was guided by a single mode fiber and then focused on the ureter through a micro-optical lens group. The fiber was aligned in a flexible torsion cable, which conveyed the toque from the rotary joint to rotate the light beam. All these parts were housed in a plastic sheath, as a support, with an outer diameter of 2.3 mm. The focus of the light beam was around 0.5 mm from the outer surface of the sheath. The system enabled an axial resolution of ~12 µm and a lateral resolution of ~8 µm. The assembled catheter is flexible and can be readily inserted into the porcine ureter.
Imaging Acquisition and Processing
The fiber catheter was connected to the fiber rotary joint shown in Fig.1 and then inserted into the ureter. Two-dimensional, cross-sectional images of the porcine ureter were continually acquired during the rotation with automatic constant-speed pullback to form a helical scanning. Each cross-sectional image contained 4000 lines and was acquired at 10 frames-per-second (fps). A 20 mm ureter can be imaged within 90 seconds. A three-dimensional volume rendering and enface images at different depths from the lumenal surface were reconstructed using Amira software (Visage Imaging, German) from the 3D volumetric image set. The red pilot light was used to visualize the imaging locations. After the completion of imaging, the imaged segments were cut for standard histological processing. Three segments of the ureter starting from 1 cm, 3cm and 5 cm from the uretero-pelvic junction were imaged. Three histological slides were made from the middle of each segment for comparison with the EOCT images.
RESULTS
We acquired images from three segments of the ureter at different locations. Each segment was 20 mm long and 900–1000 cross-sectional OCT images were recorded during the helical scanning. All images showed similar quality. The images acquired from the segment starting from 1 cm away from the ureter-pelevic junction are shown in Figure 2. A representative cross-sectional image from the middle of the segment is shown in Fig.2 (a). A histological section of tissue from the same location is shown in Fig.2 (b). Because of 2.3 mm outer diameter of the OCT catheter, the porcine ureter was fully expanded in the OCT images, while the caliber is diminished in the histological section, partially due to the absence of the EOCT catheter and partially because of shrinkage due to formalin fixation and histological preparation. The reflection from the inner surface of the sheath and the internal reflection from the microlens group can be seen as two bright circles in Fig.2 (a).
Figure 2.
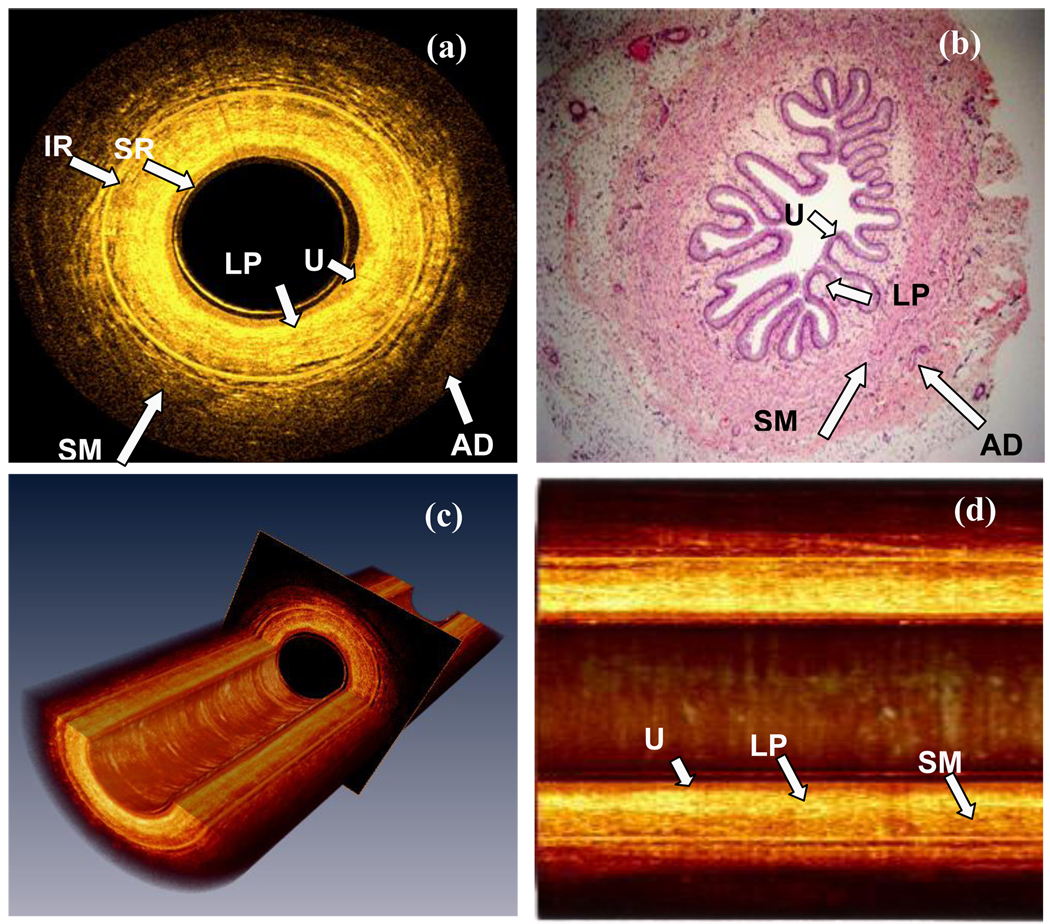
OCT ex vivo images of the porcine ureter. (a) A cross-sectional image of the porcine ureter. The diameter of the lumen in the image is not displayed at true size, but has been intentionally narrowed from 2.3 mm to 1.5 mm for better visualization of the tissue structures. (b) A histological image of the ureter at a location nearby (a). (c) Three dimensional renderings of a 20 mm long ureter segment consisting of 900 cross-sectional images. (Movie available on line) (d) The longitudinal view of a 5 mm long ureter. U: Urothelium; LP: Lamina propria; SM: Smooth muscle; AD: adventitia or adipose tissue; SR: Catheter surface reflection; IR: Internal reflection artifact. (Scale bar: 1mm) (Movie during the pull back shown in Video Clip 1)
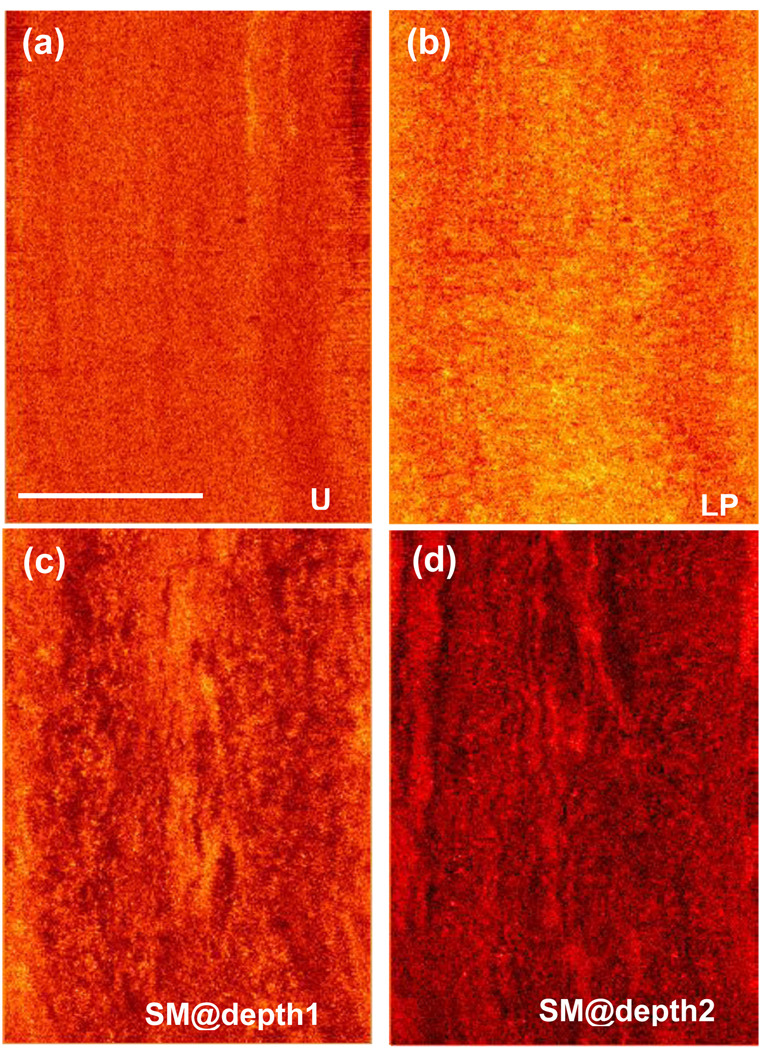
Except for unavoidable tissue deformation under different imaging conditions, comparison between the OCT images and the corresponding H&E-stained tissue sections shows excellent correlation. The three layers of the ureter: urothelium, lamina propria and smooth muscle, are readily differentiated in the OCT image as they are shown in the histological section. In the OCT image, urothelium is a thin layer with low signal, which indicates the strength of light back scattering, and lamina propria shows strong back scattering. Smooth muscle has two sub-layers: the inner layer is denser and has stronger back scattering than that of the outer layer, which shows more loosely arranged muscle fibers. These characteristics are also observed in the histological image. Through 3D reconstruction, the volumetric and longitudinal images of the ureter are displayed as Fig.2 (c) and (d). The movie (available on line) shows the real-time images during the pull back. The three layers are also easily identified along the ureter, which confirms that the EOCT can consistently acquire high quality images during the pull back. The 3D imaging made feasible by high-speed OCT also enables en face image reconstruction in the plane of the lumen surface. Figure 3 demonstrates the enface images at different depths (movie available on line). As shown in the cross-sectional image, urothelium [Fig.3 (a)] is a uniform layer with weak scattering. Histologically, urothelium consists of several layers of cells, but the individual cells are not resolved in the OCT images. Of the three principal layers of the ureteral wall, lamina propria [Fig.3 (b)] shows the strongest scattering, and its texture is uniform. Figure 3(c) shows the inner layer of the ureteral muscle, in which the densely packed muscle fiber can be observed, while the outer muscle layer as shown in [Fig.3(d)] is loose but organized. Some tissues outside the smooth muscle layer can be observed around the lumen as shown in Fig. 2 (a). They belong to adventitia or adipose tissue, but were near the imaging range limit of OCT.
Figure 3.
The enface images at different radical depths reconstructed from 10 mm long ureter. (Scale bar: 3.6 mm) U:urothelium; LP: lamina propria; SM: smooth muscle (Movie of the enface view of the ureteral wall at different depths shown in Video Clip 2)
COMMENT
The advance of ureteroscopy has been a milestone in managing ureteral cancer because it not only allows cancer diagnosis through biopsy, but also permits endoscopic resection and laser fulguration14–18 as an alternative to more radical surgical treatment for low-grade and low-stage upper urinary tract cancer. Previously, such treatment is largely limited to a group of highly selected patients who have solitary kidney, bilateral disease, and/or are high surgical risk for radical nephroureterectomy19. One of major causes for this limitation is the inadequate grading and staging capability for upper tract urothelial carcinoma, which leads to significant uncertainty associated with endoscopic treatment. Ureteroscopic biopsy and CT scan are current the mainstays of grading and staging of the ureteral cancer1. While ureteroscopic biopsy can potentially evaluate the grade of the tumors, currently the biopsy process is often inefficient, and in most instances unable to provide staging information due to the limited depth of tissue sampling. Not commonly, the tissue samples obtained ureterscopically are insufficient even for a cancer diagnosis, and urine cytology has to be relied upon as the substitute. In addition, ureteral biopsy also carries the risks of persistent bleeding and ureteral perforation, which may lead to tumor spillage. Similarly, CT scan with urogram phase has been shown to have limited value in differentiating organ-confined tumors from those that have extended beyond the confines of the ureter1. Endoluminal ultrasound (EUS) was evaluated as a staging tool, but its utility is very limited because the resolution is insufficient to delineate accurately the fine morphological features of the ureteral wall20. The OCT modality presented in this study provides a very promising tool for grading and staging of upper tract urothelial carcinoma without the need of ureteral biopsy, and therefore may completely change the current surgical practice and allow more individualized treatment.
EOCT enables cross-sectional imaging of biological tissue at micrometer resolution with imaging depth up to 1–2 mm. In previous studies, a commercially available TD-OCT was employed for ex vivo imaging of porcine ureters11,12. Urothelium and lamina proporia were differentiated in most of the OCT images, but not in the EUS images. However, the smooth muscle layer was identified in less than 40% of the OCT images. Identifying the muscle layer is critical in distinguishing stage of T1 from stage T2 tumors, because different treatments may be initiated based upon the apparent stage of the tumor. The TD-OCT used in previous studies was originally designed for intravascular imaging. The fiber catheter was very thin (~500 µm), which limited lateral resolution to about 40µm. Furthermore, the ureter was not uniformly imaged during the rotation because the fiber catheter was not always centered in the ureteral lumen, which resulted in part of the tissue being out of the light focus during imaging. In our study, we imaged the ureter with a specially designed fiber catheter, which has 2.3 mm (7F) outer diameter. The ureter was minimally distended during imaging by the plastic sheath. In this way, the light beam can always be centered during the rotation and the contrast between different layers was also enhanced by the gentle tissue stretch. In our images, all layers of the ureteral wall were reliably differentiated, even the two sub-layers of the smooth muscle. These results imply that EOCT may offer the capability of staging noninvasive cancers (stage Ta or Tis), those that invade only the lamina propria (stage T1), and those that invade into the ureteral muscle (stage > T2).
Objective interpretation of OCT images is the premise for accurate diagnosis. Although OCT images are similar to the histological images, the contrast of OCT images is from the backscattering induced by refractive index variation of the tissue, which is different from the histological images. The specific diagnostic criteria for OCT images should be established by using the histological diagnosis as the gold standard. Computer-aided diagnosis based on the quantification of OCT images may also have the potential to standardize the diagnosis and allows high throughout image evaluation.
Imaging speed is another essential factor for clinical diagnosis. For EOCT, high imaging speed enables dense sampling at a high lateral resolution during rotation, and thus provides high-quality images. Our system was based on SD-OCT, which has significantly higher imaging speed than TD-OCT. We were able to acquire a three-dimensional image set of a 20 mm segment of ureter in 90 seconds, instead of point-sampling images11,12. Now, it is feasible for physicians to surveying the entire ureter in a short period of time, which may be valuable for detecting endoscopically unapparent lesions such as urothelial carcinoma in situ. Three-dimensional images also allow the physicians to view the spatial relation of morphological features, which can not be appreciated in 2D cross-sectional images.
EOCT with a forward scanning fiber catheter has demonstrated its utility in clinical diagnosis of bladder cancer 9,21,22. With a circumferential scanning fiber catheter as shown here, EOCT may prove to be helpful both in staging ureteral cancer as well as in identifying subtle lesions such as urothelial carcinoma in situ, functions that would be tremendously important to the practice of urology. The results reported here are preliminary, but appear to justify further investigation of the potential roles of EOCT in the management of human ureteral cancer. The resolution of the system is insufficient to resolve the cellular details, which are crucial for grading of cancer. Improvement of both lateral and axial resolution should be the next technical goal. Further reducing the outer diameter of the fiber catheter to less than 2 mm without a compromise of lateral resolution is possible by optimizing the optical design.
CONCLUSION
With technical advances, we demonstrated ex vivo volume imaging of a porcine ureter with a circumferential scanning endoscopic spectral-domain OCT. The high imaging speed and specialized design of the fiber catheter permitted reliabe imaging of all layers of the ureteral wall, including the smooth muscle layer. More accurate assessment of the nature of cancerous abnormalities with human ureteral tissue and integrating forward scanning and circumferential scanning functions into a single device for imaging both ureter and bladder should be the next milestones works and will further illuminate the potential benefits of EOCT imaging in urological practice.
Supplementary Material
Real time recorded images of 20 mm porcine ureter during the pull back of the fiber catheter to demonstrate the layered structures of the ureteral wall
Enface view of the porcine ureter at different radical depths from the lumenal surface to show the texture features of the urothelium layer, lamina propria and muscularis propria
Acknowledgments
The authors acknowledge the contributions of Dr. Steve Schomisch in support of the swine experiments. The project described was support by the National Institute of Health grant numbers RO1 CA114276 and RO1 HL 083048. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Browne RFJ, Meehan CP, Colville J, Power R, Torreggiani WC. Transitional Cell Carcinoma of the Upper Urinary Tract: Spectrum of Imaging Findings1. Radiographics. 2005;25:1609–1627. doi: 10.1148/rg.256045517. [DOI] [PubMed] [Google Scholar]
- 2.van der Poel HG, Antonini N, van Tinteren H, Horenblas S. Upper Urinary Tract Cancer: Location is Correlated with Prognosis. European Urology. 2005;48:438–444. doi: 10.1016/j.eururo.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 3.El-Hakim A, Weiss GH, Lee BR, Smith AD. Correlation of ureteroscopic appearance with histologic grade of upper tract transitional cell carcinoma. Urology. 2004;63:647–650. doi: 10.1016/j.urology.2003.10.076. [DOI] [PubMed] [Google Scholar]
- 4.Lee DI, Bagley DH, Liu J-B. Experience with Endoluminal Ultrasonography in the Urinary Tract. Journal of Endourology. 2001;15:67–74. doi: 10.1089/08927790150500980. [DOI] [PubMed] [Google Scholar]
- 5.Sakata LM, DeLeon-Ortega J, Sakata V, Girkin CA. Optical coherence tomography of the retina and optic nerve – a review. Clinical & Experimental Ophthalmology. 2009;37:90–99. doi: 10.1111/j.1442-9071.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 6.Bezerra HG, Costa MA, Guagliumi G, Rollins AM, Simon DI. Intracoronary Optical Coherence Tomography: A Comprehensive Review: Clinical and Research Applications. J Am Coll Cardiol Intv. 2009;2:1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zara JM, Lingley-Papadopoulos CA. Endoscopic OCT Approaches Toward Cancer Diagnosis. Selected Topics in Quantum Electronics. IEEE Journal of. 2008;14:70–81. [Google Scholar]
- 8.Hugang R, Wayne CW, Rahuldev B, Jingxuan L, Zhijia Y, Christopher SDL, Frank D, David S, Howard LA, Jason K, et al. Diagnosis of Bladder Cancer With Microelectromechanical Systems-based Cystoscopic Optical Coherence Tomography. Urology. 2009 doi: 10.1016/j.urology.2009.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lerner SP, Goh AC, Tresser NJ, Shen SS. Optical Coherence Tomography as an Adjunct to White Light Cystoscopy for Intravesical Real-Time Imaging and Staging of Bladder Cancer. Urology. 2008;72:133–137. doi: 10.1016/j.urology.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Zigeuner R, Pummer K. Urothelial Carcinoma of the Upper Urinary Tract: Surgical Approach and Prognostic Factors. European Urology. 2008;53:720–731. doi: 10.1016/j.eururo.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Mueller-Lisse U, Meissner O, Babaryka G, Bauer M, Eibel R, Stief C, Reiser M, Mueller-Lisse U. Catheter-based intraluminal optical coherence tomography (OCT) of the ureter: ex-vivo correlation with histology in porcine specimens. European Radiology. 2006;16:2259–2264. doi: 10.1007/s00330-006-0191-8. [DOI] [PubMed] [Google Scholar]
- 12.Mueller-Lisse UL, Meissner OA, Bauer M, Weber C, Babaryka G, Stief CG, Reiser MF, Mueller-Lisse UG. Catheter-based Intraluminal Optical Coherence Tomography Versus Endoluminal Ultrasonography of Porcine Ureter Ex Vivo. Urology. 2009;73:1388–1391. doi: 10.1016/j.urology.2008.11.053. [DOI] [PubMed] [Google Scholar]
- 13.Yun S, Tearney G, Bouma B, Park B, de Boer J. High-speed spectral-domain optical coherence tomography at 1.3 ?m wavelength. Opt. Express. 2003;11:3598–3604. doi: 10.1364/oe.11.003598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwaszko MR, AE K. Conservative management of upper tract transitional cell carcinoma. Indian J Urol. 2008;24:159–163. doi: 10.4103/0970-1591.40608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koji S, Satoshi E, Jun M, Yoriaki K. Role of ureteroscopic biopsy in the management of upper urinary tract malignancy. International Journal of Urology. 2003;10:627–630. doi: 10.1046/j.1442-2042.2003.00721.x. [DOI] [PubMed] [Google Scholar]
- 16.Raman JD, Scherr DS. Management of patients with upper urinary tract transitional cell carcinoma. Nat Clin Pract Urol. 2007;4:432–443. doi: 10.1038/ncpuro0875. [DOI] [PubMed] [Google Scholar]
- 17.Bagley DH. Ureteroscopic Treatment of Upper Tract Neoplasms. Advanced Endourology. 2006:267–279. [Google Scholar]
- 18.Daniel JP, Anthony GT, Karin D, Peter A, Francis XK., Jr The modern management of upper urinary tract urothelial cancer: tumour diagnosis, grading and staging. BJU International. 2007;99:973–977. doi: 10.1111/j.1464-410X.2007.06801.x. [DOI] [PubMed] [Google Scholar]
- 19.Moore K, Khastgir J, Ghei M. Endoscopic Management of Upper Tract Urothelial Carcinoma. Advances in Urology. 2009;2009:1–6. doi: 10.1155/2009/620604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondabolu S, Khan SA, Whyard J, Diblasio C, Ayyala M, Pentyala S. The role of endoluminal ultrasonography in urology: current perspectives. International braz j urol. 2004;30:96–101. doi: 10.1590/s1677-55382004000200002. [DOI] [PubMed] [Google Scholar]
- 21.Hugang R, Wayne CW, Rahuldev B, Jingxuan L, Zhijia Y, Christopher SDL, Frank D, David S, Howard LA, Jason K, et al. Diagnosis of Bladder Cancer With Microelectromechanical Systems-based Cystoscopic Optical Coherence Tomography. Urology. 2009:1351–1357. doi: 10.1016/j.urology.2009.04.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joerg S, Mesut R, Tobias K, Matthias W, Julian M, Martin S, Michael M. Fluorescence Cystoscopy with High-Resolution Optical Coherence Tomography Imaging as an Adjunct Reduces False-Positive Findings in the Diagnosis of Urothelial Carcinoma of the Bladder. European Urology. 2009;56:914–919. doi: 10.1016/j.eururo.2009.07.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Real time recorded images of 20 mm porcine ureter during the pull back of the fiber catheter to demonstrate the layered structures of the ureteral wall
Enface view of the porcine ureter at different radical depths from the lumenal surface to show the texture features of the urothelium layer, lamina propria and muscularis propria