Abstract
Reactive astrocytes are associated with a vast array of central nervous system (CNS) pathologies. The activation of astrocytes is characterized by changes in their molecular and morphological features, and depending on the type of damage can also be accompanied by inflammatory responses, neuronal damage, and in severe cases, scar formation. Although reactive astrogliosis is the normal physiological response essential for containing damage, it can also have detrimental effects on neuronal survival and axon regeneration, particularly in neurodegenerative diseases. It is believed that progressive changes in astrocytes as they become reactive are finely regulated by complex intercellular and intracellular signaling mechanisms. However, these have yet to be sorted out. Much has been learned from gain-of-function approaches in vivo and culture paradigms, but in most cases, loss-of-function genetic studies, which are a critical complementary approach, have been lacking. Understanding which signaling pathways are required to control different aspects of astrogliosis will be necessary for designing therapeutic strategies to improve their beneficial effects and limit their detrimental ones in CNS pathologies. In this article, we review recent advances in the mechanisms underlying the regulation of aspects of astrogliosis, with the main focus on the signaling pathways that have been studied using loss-of-function genetic mouse models.
Keywords: Astrogliosis, Gliosis, Reactive gliosis, Genetics
The CNS is susceptible to many types of pathological insults such as traumatic injury, ischemia, neurotoxic chemicals, tumors, infections, and neurodegenerative diseases. In all these cases, the response to damage includes at a minimum the activation of astrocytes, characterized by changes in their morphology and molecular expression profile. In addition to these changes, the response to damage often includes increased proliferation of several cell types, infiltration of leukocytes, scar formation, and neuronal death [1–4]. In this review, we limit our discussion primarily to what molecular mechanisms regulate astrocyte activation.
Many cell types including neurons, microglia, oligodendrocytes, endothelial cells, and leukocytes are likely to interact with astrocytes and influence the duration and amount of reactive astrogliosis [1–3, 5]. Although many extracellular signals are known to be up-regulated in different CNS pathologies, the cells that produce or respond to these signals in vivo in each case and how these signals affect astrocytes in particular is poorly understood. An informative approach will undoubtedly be to use mouse models of CNS damage combined with cell type-specific deletions of genes encoding intercellular and intracellular signaling components postulated to regulate astrogliosis. In this article, we discuss examples of what has been learned to date from such approaches.
In response to damage, the activation of astrocytes can be characterized by several features. First, the most commonly used marker of activated astrocytes is their upregulation of intermediate filaments (glial fibrillary acidic protein (GFAP), vimentin, and to some extent Nestin) coincident with cellular hypertrophy. Second, activated astrocytes are also likely to increase their expression of cytokines, chemokines, and extracellular matrix components [3]. However, in vivo data demonstrating this has remained sparse ([6]), since other cell types are also likely to secrete similar factors in response to damage making the cell source of these extracellular factors difficult to pinpoint. Hence, it remains to be determined just which factors are secreted by reactive astrocytes under what pathological conditions. Third, lineage tracing experiments in adult astrocytes in vivo using lentivirus and genetic approaches have shown that in some cases, such as after a stab wound injury, reactive astrocytes appear to become proliferative [7]. Olig2-expressing NG2 progenitors also proliferate in response to injury and may in some cases generate reactive astrocytes. NG2 cells have been reported to generate astrocytes in the cortex after stab wound or cryoinjuries [8, 9]. However, this is still a matter of debate as no new astrocytes appear to be generated from NG2 progenitors in other models of neurodegeneration, spinal cord injury, or cortical stab wound injury [10–12]. The differences may arise from the distinct combinations of fate mapping approaches and injury models. Further studies in which the progeny of defined glial cell types are marked are still needed to distinguish what cells can generate astrocytes under what injury conditions.
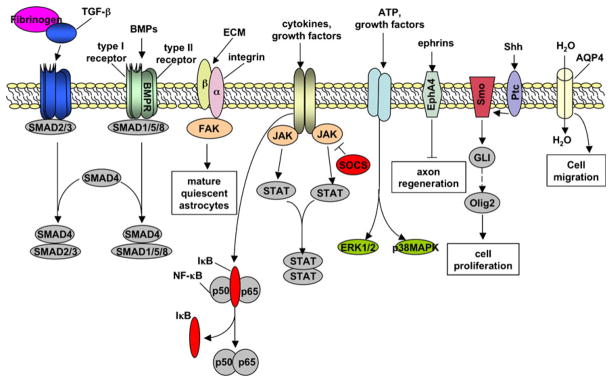
Aspects of reactive astrogliosis can exert both beneficial and harmful effects. For example, in transgenic mice in which reactive astrocytes are ablated or disabled by loss of GFAP and vimentin, traumatic injury leads to the lack of normal scar formation, prolonged and more widespread inflammation, and a failure to reconstruct the blood–brain barrier (BBB) and to maintain tissue integrity [13, 14]. However, ablation or impairment of reactive astrocytes also leads to increased nerve fiber growth in the immediate vicinity of the injury site [13]. Despite this example, the positive roles and negative consequences of astrogliosis in most CNS pathologies remain largely unclear. For example, whether the reactive astrogliosis that occurs in neurodegenerative diseases is beneficial or detrimental is unknown. Moreover, the genetic pathways, of which there are likely many (examples are illustrated in Fig. 1), that regulate the different aspects and extent of astrogliosis in vivo remain largely unaddressed. An understanding of these pathways will be necessary to target specific aspects of reactive astrogliosis to maximize its beneficial effects and minimize its unwanted effects for each type of pathology.
Fig. 1.
Molecules and signaling pathways involved in activating different aspects of reactive astrogliosis. See text for details
NF-κB and Inflammation
Nuclear transcription factor-κB (NF-κB), which can be activated by stimuli associated with damage and in turn can promote expression of pro-inflammatory cytokines, is thought to play a central role in most inflammatory responses. When the CNS is damaged, the resident cell types that activate NF-κB to mount a response have not been fully defined, but are likely to include astrocytes. Mice deficient for a major subunit of NF-κB, p50, exhibit a smaller infarct size after middle cerebral artery occlusion (a model of stroke), which presumably reflects less neuronal death [15]. However, in this study, activated NF-κB was only detected in neurons, not astrocytes, of control animals. Likewise, activated NF-κB was only detected in neurons and microglia, but not astrocytes, after spinal cord injury in rats [16]. On the other hand, in a number of brain pathologies, NF-κB levels are up-regulated in cell types that include astrocytes based on cell morphology [17–20]. Consistent with a role in astrocytes, mice in which expression of a constitutively active form of the inhibitor of NF-κB, IκBα is driven by the hGFAP promoter exhibit reduced lesion volumes, reduced glial scar formation, increased preservation of white matter, and a significant improvement in their ability to recover mobility after spinal cord injury [21]. Similarly, using the same transgenic mice, expression of pro-inflammatory genes, neuronal death, and overall disease severity were reduced following experimental autoimmune encephalomyelitis [22]. Importantly, however, the cell-type specificity of expression of the hGFAP-IκBα transgene in these mice was not examined for adult tissues in vivo, leaving open the possibility that NF-κB function was directly inhibited in cells other than astrocytes and that astrocytes were subsequently activated. Nevertheless, these studies together suggest that inhibition of NF-κB signaling results in protective effects after injury and is a potential target for pharmacological intervention in CNS pathologies.
MAPK Signaling
Reports describing whether the mitogen-activated protein kinase (MAPK) pathways are upregulated in astrocytes in vivo are mixed. For example, an increase in the level of phosphorylated extracellular signal-regulated kinase (ERK), a component of MAPK signaling, is detected specifically in astrocytes after traumatic spinal cord injury [23]. On the other hand, activation of p38 MAPK is detected solely in microglia, and not in astrocytes, in a mouse model of Alzheimer’s disease [24]. Moreover, pharmacological inhibition of p38 MAPK or ERK appeared protective in some models of ischemia or brain injury [25–28], whereas inhibition of p38 MAPK appeared to worsen brain damage after ischemia in another study [29]. Further experiments are clearly needed to delineate which components of MAPK signaling are activated under what pathological conditions and in which cell types.
In addition, the ligands and receptors that activate components of MAPK signaling in astrocytes remain to be identified and will likely require deleting the genes encoding candidate receptors specifically in these cells upon CNS damage. Such candidates include the P2 purinergic receptor P2X7, FGF receptors, and EGF receptors, all of which are upregulated in astrocytes upon CNS damage [30–36]. The functionally relevant downstream genes that are regulated by MAPK signaling in vivo also remain to be identified.
SHH and Olig2 in Astrocyte Activation and Proliferation
The factors that regulate cell proliferation in response to CNS damage are not fully known, but are likely to include the mitogen SHH and the basic helix–loop–helix transcription factor Olig2, which can be induced by SHH. Olig2 expression is upregulated in most GFAP-positive reactive astrocytes after injury, ischemia, and a model for multiple sclerosis [37–40]. Conditionally knocking out Olig2 in neurons or oligodendrocytes does not affect reactive astrogliosis after cortical injury whereas conditionally knocking this gene out in all cortical cells results in reduced activation and proliferation of reactive astrocytes after injury [37]. This strongly suggests that Olig2 is required specifically in astrocytes for their activation. SHH expression and activity (measured using a Gli1-luciferase reporter transgene) are induced after cortical injury and are required to promote the proliferation of Olig2-expressing cells [41]. Although likely, whether SHH-induced proliferation is dependent on Olig2 activity remains to be confirmed.
Endothelins in Astrocyte Activation and Proliferation
Endothelins are a family of potent vasoactive peptides. Following brain injury, expression of the endothelin ET-1 and endothelin receptor ET-B is strongly upregulated in reactive astrocytes [42, 43]. Moreover, infusion of exogenous ET-1 or ET receptor agonists causes astrocytes to become hypertrophic and proliferate [44], whereas receptor antagonists attenuate astrocyte activation and proliferation after stab wound injury [45]. In these studies, although the effect of endothelins is likely to be directly on astrocytes, indirect effects through other cell types cannot be ruled out. Targeting of endothelin receptors directly in astrocytes and other cell types, for example, will clarify the role of endothelins in astrogliosis.
Fibrinogen, TGFβ and Scar Formation
Reactive astrocytes build a glial scar to contain damage and protect surrounding tissue. However, the glial scar is likely to form a biochemical and mechanical impediment to axon regeneration. Given the therapeutic potentials of manipulating scar formation, understanding how this process is regulated is of great interest.
After vascular damage with disruption of the BBB, the soluble blood coagulation protein fibrinogen leaks into the CNS and is converted to insoluble fibrin by the action of thrombin. Depletion of fibrin pharmacologically or genetically results in decreased inflammation and delayed onset of demyelination in mouse models of multiple sclerosis, which leads to increased lifespan and delayed symptoms [46]. Both in vivo and in vitro studies show that fibrinogen can activate astrocytes, leading to deposition of scar components after CNS injury. In fibrinogen knockout mice or mice treated with the fibrinogen-depleting agent ancrod, GFAP expression and deposition of neurocan, an inhibitory chondroitin sulfate proteoglycan (CSPG) secreted by astrocytes, are significantly reduced after stab wound injury [47]. However, the effect of fibrinogen on astrocytes is likely to be indirect through the transforming growth factor beta (TGFβ) pathway. Fibrinogen can act as carrier of latent TGFβ to sites of injury. In both fibrinogen-deficient mutants and ancrod-treated mice, active TGFβ levels after injury are dramatically reduced. Moreover, injection of fibrinogen into the cortex is sufficient to induce astrogliosis, while inhibition of the TGFβ pathway abolishes the fibrinogen-induced effects on glia scar formation [47].
Multiple Roles for BMPs
Interestingly, the bone morphogenetic proteins (BMPs), members of the TGFβ superfamily of ligands, are also involved in astrocyte reactivity. Both BMP ligands and receptors are increased following CNS injury in neurons, astrocytes, oligodendrocytes, and microglia [48–51]. Using a GFAP-Cre transgene to conditionally delete the type 1 BMP receptor gene, Bmpr1a, specifically in astrocytes has no obvious effect under normal conditions. However, after spinal cord injury, Bmpr1a mutant astrocytes exhibit less hypertrophy and lower levels of GFAP than control astrocytes, leading to increased infiltration of inflammatory cells and greater loss of tissue [52]. Interestingly, mice that are null for a different type 1 receptor gene, Bmpr1b, exhibit an opposite phenotype after spinal cord injury including higher levels of GFAP, a more compact inflammation area, and smaller lesion volume [52]. These data suggest that BMPR1A and 1B signaling exert opposite effects on astrocytes following injury. However, because BMPR1B is a null allele that is lost in all cell types, the effects observed on astrogliosis in the Bmpr1b mutant may not be direct. Alternatively, the function of the two receptors within astrocytes may differ. In any case, the difference in phenotypes underscores the complexicity of astrogliosis regulation.
STAT3 and Astrocyte Migration
The Signal transducer and activator of transcription 3 (STAT3) is a member of the Jak-STAT signaling family. It may transduce signals for several cytokines and growth factors implicated in the injury response. The activation of STAT3 by phosphorylation increases markedly in astrocytes, microglia, endothelial cells, and neurons shortly after CNS insults [53–58]. GFAP-Cre and Nestin-Cre were used to conditionally delete Stat3 in astrocytes from developmental stages onward [58, 59]. Although conditional deletion of Stat3 specifically in adult astrocytes are still needed to exclude potential developmental and indirect effects, the results obtained from these studies nevertheless suggest a key role for this gene in the healing process after spinal cord injury. In the mutants, astrocyte hypertrophy and scar formation fail after injury and there was an increased spread of inflammation and lesion volume with impaired motor recovery [58, 59]. Consistent with these findings, the conditional deletion of Socs3 in astrocytes, which negatively feedsback on STAT3, exhibits the opposite phenotypes [59]. Interestingly, STAT3 appears to function by promoting the migration of reactive astrocytes to the injury site [58, 59].
Aquaporin-4 and Astrocyte Migration
Aquaporins (AQPs) are water channels that regulate water homeostasis in physiological and pathological conditions. Analysis of mutant mice lacking AQP4 indicates that this protein facilitates water flux into and out of the brain parenchyma and may be involved in brain edema in multiple pathological conditions [60–65]. In mutant mice lacking AQP4, glia scar formation after cortical stab injury is significantly impaired, perhaps due to a failure of astrocytes to migrate, as suggested by the behavior of AQP4-deficient astrocytes in culture [66, 67].
Integrins and Astrocyte Maturation
Integrins act as receptors for extracellular matrix proteins and as such are likely to play multiple roles in response to CNS damage. These roles have yet to be clearly outlined, although β1-integrin is likely to be required specifically in astrocytes for their normal maturation in the uninjured brain. Conditional deletion of the β1-integrin gene in astrocytes and neurons during embryogenesis leads to many features of astrogliosis in postnatal and adult cortex and spinal cord including astrocyte hypertrophy, upregulation of GFAP, and microglia activation [68]. Deletion of this gene specifically in neurons, however, has no phenotype, suggesting that β1-intergrin is required specifically in astrocytes. A conditional knockout of Fak in embryos, a gene encoding a kinase that can be activated by integrins, also exhibits numerous GFAP-positive astrocytes in postnatal cortex [69]. However, it remains unclear to what extent the observed astrogliosis in the adult brain is due to developmental defects or due to abnormal astrocytes activation in the absence of obvious injury. In addition, whether the response of astrocytes to exogenously induced injury would be exacerbated in these mutants is not known. In any case, it is reasonable to postulate that the down-regulation of integrin after injury may be required for astrocyte activation.
Eph Signaling in Axon Regeneration
Although factors such as myelin-associated glycoprotein, Nogo-A, oligodendrocyte myelin glycoprotein, CSPGs, and KSPGs have previously been identified as potential inhibitors of axon regeneration after injury, experiments aimed at perturbing these factors in vivo to promote axon regrowth have met with limited success [70–79]. The Eph receptor tyrosine kinase family and its ligands, the ephrins, regulate axon guidance through contact repulsion during CNS development. Normally, continued low level expression of Ephs and ephrins occurs in the adult CNS. After injury or in CNS diseases, many Ephs and ephrins are upregulated in different cell types including astrocytes, neurons, and oligodendrocytes [80]. In vitro evidence has suggested that the increased Eph/ephrin signaling in CNS pathological conditions is inhibitory to axon regeneration of spinal cord, cortical neurons, and retinal ganglion neurons [81–84]. Analysis of EphA4−/− mutant mice has clearly demonstrated the inhibitory effects of Eph/ephrin signaling on axon regeneration following spinal cord hemisection [85]. In EphA4−/− mice, large numbers of newly formed axons grow through the lesion site. Functionally, several parameters of movement recovery are greatly improved in EphA4−/− mice compared with the controls presumably due to improved axon regeneration. In addition to inhibiting axon regrowth, upregulation of Ephs and ephrins in astrocytes in CNS pathologies appears to play a role in mediating astrogliosis and scar formation. In EphA4−/− mice, astrogliosis, assessed by GFAP expression, and scar formation are greatly reduced [85]. Together, these data indicate that Eph/ephrins are produced during astrogliosis and are inhibitory molecules for axon regeneration.
Proteoglycans
A hallmark of reactive astrocytes is their secretion of proteoglycans, extracellular matrix molecules comprised of a protein core and variable glycosaminoglycan side chains. One major class of proteoglycans, CSPGs, are upregulated and secreted rapidly after a wide range of CNS injuries and can persist for months [86–88]. Although it is likely that these proteoglycans can interact with various signaling pathways [89], these have yet to be identified in vivo in the context of CNS damage. One exception is EGFR signaling, which can mediate inhibition of axon regeneration by myelin and CSPGs in a Ca2+-dependent manner [90]. Consistent with this finding, blocking EGFR function by an inhibitor can promote axonal regrowth and functional recovery following optic nerve and spinal cord injuries [90, 91]. Despite this example, further in vivo studies are clearly required to define the effects of CSPGs on reactive astrocytosis.
Conclusion and Prospects
We currently only have a superficial grasp of the mechanisms regulating the initiation and extent of astrocyte activation under different pathological conditions. These mechanisms likely involve interactions with several cell types including infiltrating leukocytes, endothelial cells, microglia, and neurons. Our understanding of how the inflammatory response to CNS damage is eventually resolved, including the de-activation of astrocytes, is even more rudimentary. As with most biological processes, the state of astrocytes even under non-pathological conditions may be regulated by a balance between pro- and anti-reactive signals.
Nevertheless, with the advent of new genetic tools and experimental paradigms our knowledge of how astrocytes and other cells respond to CNS damage is growing quickly. Of particular interest would be to develop a therapeutic ability to modulate the negative effects of certain aspects of inflammation to maximize tissue viability and in some cases promote neuronal and axonal regeneration.
References
- 1.Norton WT, Aquino DA, Hozumi I, Chiu FC, Brosnan CF. Quantitative aspects of reactive gliosis: a review. Neurochem Res. 1992;17:877–885. doi: 10.1007/BF00993263. [DOI] [PubMed] [Google Scholar]
- 2.Ridet JL, Malhotra SK, Privat A, Gage FH. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/s0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 3.Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009;32:638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babcock AA, Kuziel WA, Rivest S, Owens T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J Neurosci. 2003;23:7922–7930. doi: 10.1523/JNEUROSCI.23-21-07922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tatsumi K, Takebayashi H, Manabe T, Tanaka KF, Makinodan M, Yamauchi T, Makinodan E, Matsuyoshi H, Okuda H, Ikenaka K, Wanaka A. Genetic fate mapping of Olig2 progenitors in the injured adult cerebral cortex reveals preferential differentiation into astrocytes. J Neurosci Res. 2008;86:3494–3502. doi: 10.1002/jnr.21862. [DOI] [PubMed] [Google Scholar]
- 9.Burns KA, Murphy B, Danzer SC, Kuan CY. Developmental and post-injury cortical gliogenesis: a genetic fate-mapping study with Nestin-CreER mice. Glia. 2009;57:1115–1129. doi: 10.1002/glia.20835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW, Meletis K, Frisen J. Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell. 2010;7:470–482. doi: 10.1016/j.stem.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Dimou L, Simon C, Kirchhoff F, Takebayashi H, Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang SH, Fukaya M, Yang JK, Rothstein JD, Bergles DE. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron. 2010;68:668–681. doi: 10.1016/j.neuron.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- 14.Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 2004;24:2143–2155. doi: 10.1523/JNEUROSCI.3547-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider A, Martin-Villalba A, Weih F, Vogel J, Wirth T, Schwaninger M. NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat Med. 1999;5:554–559. doi: 10.1038/8432. [DOI] [PubMed] [Google Scholar]
- 16.Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez-Otano I, McMillian MK, Chen J, Bing G, Hong JS, Pennypacker KR. Induction of NF-kB-like transcription factors in brain areas susceptible to kainate toxicity. Glia. 1996;16:306–315. doi: 10.1002/(SICI)1098-1136(199604)16:4<306::AID-GLIA3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 18.Terai K, Matsuo A, McGeer EG, McGeer PL. Enhancement of immunoreactivity for NF-kappa B in human cerebral infarctions. Brain Res. 1996;739:343–349. doi: 10.1016/s0006-8993(96)01073-6. [DOI] [PubMed] [Google Scholar]
- 19.Terai K, Matsuo A, McGeer PL. Enhancement of immunoreactivity for NF-kappa B in the hippocampal formation and cerebral cortex of Alzheimer’s disease. Brain Res. 1996;735:159–168. doi: 10.1016/0006-8993(96)00310-1. [DOI] [PubMed] [Google Scholar]
- 20.Gabriel C, Justicia C, Camins A, Planas AM. Activation of nuclear factor-kappaB in the rat brain after transient focal ischemia. Brain Res Mol Brain Res. 1999;65:61–69. doi: 10.1016/s0169-328x(98)00330-1. [DOI] [PubMed] [Google Scholar]
- 21.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR. Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med. 2005;202:145–156. doi: 10.1084/jem.20041918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brambilla R, Hurtado A, Persaud T, Esham K, Pearse DD, Oudega M, Bethea JR. Transgenic inhibition of astroglial NF-kappa B leads to increased axonal sparing and sprouting following spinal cord injury. J Neurochem. 2009;110:765–778. doi: 10.1111/j.1471-4159.2009.06190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito M, Natsume A, Takeuchi H, Shimato S, Ohno M, Wakabayashi T, Yoshida J. Type I interferon inhibits astrocytic gliosis and promotes functional recovery after spinal cord injury by deactivation of the MEK/ERK pathway. J Neurotrauma. 2009;26:41–53. doi: 10.1089/neu.2008.0646. [DOI] [PubMed] [Google Scholar]
- 24.Koistinaho M, Kettunen MI, Goldsteins G, Keinanen R, Salminen A, Ort M, Bures J, Liu D, Kauppinen RA, Higgins LS, Koistinaho J. Beta-amyloid precursor protein transgenic mice that harbor diffuse A beta deposits but do not form plaques show increased ischemic vulnerability: role of inflammation. Proc Natl Acad Sci USA. 2002;99:1610–1615. doi: 10.1073/pnas.032670899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barone FC, Irving EA, Ray AM, Lee JC, Kassis S, Kumar S, Badger AM, White RF, McVey MJ, Legos JJ, Erhardt JA, Nelson AH, Ohlstein EH, Hunter AJ, Ward K, Smith BR, Adams JL, Parsons AA. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J Pharmacol Exp Ther. 2001;296:312–321. [PubMed] [Google Scholar]
- 26.Namura S, Iihara K, Takami S, Nagata I, Kikuchi H, Matsushita K, Moskowitz MA, Bonventre JV, Alessandrini A. Intravenous administration of MEK inhibitor U0126 affords brain protection against forebrain ischemia and focal cerebral ischemia. Proc Natl Acad Sci USA. 2001;98:11569–11574. doi: 10.1073/pnas.181213498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Wang H, Xu L, Rozanski DJ, Sugawara T, Chan PH, Trzaskos JM, Feuerstein GZ. Significant neuroprotection against ischemic brain injury by inhibition of the MEK1 protein kinase in mice: exploration of potential mechanism associated with apoptosis. J Pharmacol Exp Ther. 2003;304:172–178. doi: 10.1124/jpet.102.040246. [DOI] [PubMed] [Google Scholar]
- 28.Mori T, Wang X, Aoki T, Lo EH. Downregulation of matrix metalloproteinase-9 and attenuation of edema via inhibition of ERK mitogen activated protein kinase in traumatic brain injury. J Neurotrauma. 2002;19:1411–1419. doi: 10.1089/089771502320914642. [DOI] [PubMed] [Google Scholar]
- 29.Lennmyr F, Ericsson A, Gerwins P, Ahlstrom H, Terent A. Increased brain injury and vascular leakage after pretreatment with p38-inhibitor SB203580 in transient ischemia. Acta Neurol Scand. 2003;108:339–345. doi: 10.1034/j.1600-0404.2003.00129.x. [DOI] [PubMed] [Google Scholar]
- 30.Logan A, Frautschy SA, Gonzalez AM, Baird A. A time course for the focal elevation of synthesis of basic fibroblast growth factor and one of its high-affinity receptors (flg) following a localized cortical brain injury. J Neurosci. 1992;12:3828–3837. doi: 10.1523/JNEUROSCI.12-10-03828.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly JF, Kumari VG. Alterations in fibroblast growth factor receptor expression following brain injury. Exp Neurol. 1996;140:139–150. doi: 10.1006/exnr.1996.0124. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 33.Planas AM, Justicia C, Soriano MA, Ferrer I. Epidermal growth factor receptor in proliferating reactive glia following transient focal ischemia in the rat brain. Glia. 1998;23:120–129. doi: 10.1002/(sici)1098-1136(199806)23:2<120::aid-glia3>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 34.Ferrer I, Alcantara S, Ballabriga J, Olive M, Blanco R, Rivera R, Carmona M, Berruezo M, Pitarch S, Planas AM. Transforming growth factor-alpha (TGF-alpha) and epidermal growth factor-receptor (EGF-R) immunoreactivity in normal and pathologic brain. Prog Neurobiol. 1996;49:99–123. doi: 10.1016/0301-0082(96)00009-3. [DOI] [PubMed] [Google Scholar]
- 35.Birecree E, Whetsell WO, Jr, Stoscheck C, King LE, Jr, Nanney LB. Immunoreactive epidermal growth factor receptors in neuritic plaques from patients with Alzheimer’s disease. J Neuropathol Exp Neurol. 1988;47:549–560. doi: 10.1097/00005072-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 2006;26:7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Miles DK, Hoang T, Shi J, Hurlock E, Kernie SG, Lu QR. The basic helix-loop-helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J Neurosci. 2008;28:10983–10989. doi: 10.1523/JNEUROSCI.3545-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci USA. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cassiani-Ingoni R, Coksaygan T, Xue H, Reichert-Scrivner SA, Wiendl H, Rao MS, Magnus T. Cytoplasmic translocation of Olig2 in adult glial progenitors marks the generation of reactive astrocytes following autoimmune inflammation. Exp Neurol. 2006;201:349–358. doi: 10.1016/j.expneurol.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 40.Magnus T, Coksaygan T, Korn T, Xue H, Arumugam TV, Mughal MR, Eckley DM, Tang SC, Detolla L, Rao MS, Cassiani-Ingoni R, Mattson MP. Evidence that nucleocytoplasmic Olig2 translocation mediates brain-injury-induced differentiation of glial precursors to astrocytes. J Neurosci Res. 2007;85:2126–2137. doi: 10.1002/jnr.21368. [DOI] [PubMed] [Google Scholar]
- 41.Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29:10299–10308. doi: 10.1523/JNEUROSCI.2500-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP, Schmidt JA, Ghilardi JR, Maggio JE, Mantyh PW. Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol. 2003;180:1–13. doi: 10.1016/s0014-4886(02)00023-7. [DOI] [PubMed] [Google Scholar]
- 43.Li JJ, Wu LH, Cao Q, Yuan Y, Yang L, Guo ZY, Kaur C, Sivakumar V, Ling EA, Wu CY. Endothelins-1/3 and endothelin-A/B receptors expressing glial cells with special reference to activated microglia in experimentally induced cerebral ischemia in the adult rats. Neuroscience. 2010;167:665–677. doi: 10.1016/j.neuroscience.2010.02.062. [DOI] [PubMed] [Google Scholar]
- 44.Koyama Y, Takemura M, Fujiki K, Ishikawa N, Shigenaga Y, Baba A. BQ788, an endothelin ET(B) receptor antagonist, attenuates stab wound injury-induced reactive astrocytes in rat brain. Glia. 1999;26:268–271. doi: 10.1002/(sici)1098-1136(199905)26:3<268::aid-glia8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 45.Gadea A, Schinelli S, Gallo V. Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci. 2008;28:2394–2408. doi: 10.1523/JNEUROSCI.5652-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Akassoglou K, Adams RA, Bauer J, Mercado P, Tseveleki V, Lassmann H, Probert L, Strickland S. Fibrin depletion decreases inflammation and delays the onset of demyelination in a tumor necrosis factor transgenic mouse model for multiple sclerosis. Proc Natl Acad Sci USA. 2004;101:6698–6703. doi: 10.1073/pnas.0303859101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK, Degen JL, Margolis RU, Akassoglou K. Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci. 2010;30:5843–5854. doi: 10.1523/JNEUROSCI.0137-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J, Leong SY, Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur J Neurosci. 2005;22:1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- 49.Setoguchi T, Nakashima K, Takizawa T, Yanagisawa M, Ochiai W, Okabe M, Yone K, Komiya S, Taga T. Treatment of spinal cord injury by transplantation of fetal neural precursor cells engineered to express BMP inhibitor. Exp Neurol. 2004;189:33–44. doi: 10.1016/j.expneurol.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Setoguchi T, Yone K, Matsuoka E, Takenouchi H, Nakashima K, Sakou T, Komiya S, Izumo S. Traumatic injury-induced BMP7 expression in the adult rat spinal cord. Brain Res. 2001;921:219–225. doi: 10.1016/s0006-8993(01)03123-7. [DOI] [PubMed] [Google Scholar]
- 51.Matsuura I, Taniguchi J, Hata K, Saeki N, Yamashita T. BMP inhibition enhances axonal growth and functional recovery after spinal cord injury. J Neurochem. 2008;105:1471–1479. doi: 10.1111/j.1471-4159.2008.05251.x. [DOI] [PubMed] [Google Scholar]
- 52.Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, Stupp SI, Kessler JA. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci. 2010;30:1839–1855. doi: 10.1523/JNEUROSCI.4459-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi JS, Kim SY, Cha JH, Choi YS, Sung KW, Oh ST, Kim ON, Chung JW, Chun MH, Lee SB, Lee MY. Upregulation of gp130 and STAT3 activation in the rat hippocampus following transient forebrain ischemia. Glia. 2003;41:237–246. doi: 10.1002/glia.10186. [DOI] [PubMed] [Google Scholar]
- 54.Planas AM, Soriano MA, Berruezo M, Justicia C, Estrada A, Pitarch S, Ferrer I. Induction of Stat3, a signal transducer and transcription factor, in reactive microglia following transient focal cerebral ischaemia. Eur J Neurosci. 1996;8:2612–2618. doi: 10.1111/j.1460-9568.1996.tb01556.x. [DOI] [PubMed] [Google Scholar]
- 55.Satriotomo I, Bowen KK, Vemuganti R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J Neurochem. 2006;98:1353–1368. doi: 10.1111/j.1471-4159.2006.04051.x. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol. 2001;170:63–71. doi: 10.1006/exnr.2001.7701. [DOI] [PubMed] [Google Scholar]
- 57.Justicia C, Gabriel C, Planas AM. Activation of the JAK/STAT pathway following transient focal cerebral ischemia: signaling through Jak1 and Stat3 in astrocytes. Glia. 2000;30:253–270. doi: 10.1002/(sici)1098-1136(200005)30:3<253::aid-glia5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 58.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H. Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med. 2006;12:829–834. doi: 10.1038/nm1425. [DOI] [PubMed] [Google Scholar]
- 60.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–1293. doi: 10.1096/fj.04-1723fje. [DOI] [PubMed] [Google Scholar]
- 61.Papadopoulos MC, Verkman AS. Aquaporin-4 gene disruption in mice reduces brain swelling and mortality in pneumococcal meningitis. J Biol Chem. 2005;280:13906–13912. doi: 10.1074/jbc.M413627200. [DOI] [PubMed] [Google Scholar]
- 62.Verkman AS, Binder DK, Bloch O, Auguste K, Papadopoulos MC. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim Biophys Acta. 2006;1758:1085–1093. doi: 10.1016/j.bbamem.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 63.Saadoun S, Papadopoulos MC, Krishna S. Water transport becomes uncoupled from K+ siphoning in brain contusion, bacterial meningitis, to brain tumours: immunohistochemical case review. J Clin Pathol. 2003;56:972–975. doi: 10.1136/jcp.56.12.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saadoun S, Papadopoulos MC, Davies DC, Krishna S, Bell BA. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry. 2002;72:262–265. doi: 10.1136/jnnp.72.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 66.Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–5698. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- 67.Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Robel S, Mori T, Zoubaa S, Schlegel J, Sirko S, Faissner A, Goebbels S, Dimou L, Gotz M. Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia. 2009;57:1630–1647. doi: 10.1002/glia.20876. [DOI] [PubMed] [Google Scholar]
- 69.Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- 71.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 72.Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 73.Lee JK, Zheng B. Axon regeneration after spinal cord injury: insight from genetically modified mouse models. Restor Neurol Neurosci. 2008;26:175–182. [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng B, Ho C, Li S, Keirstead H, Steward O, Tessier-Lavigne M. Lack of enhanced spinal regeneration in Nogo-deficient mice. Neuron. 2003;38:213–224. doi: 10.1016/s0896-6273(03)00225-3. [DOI] [PubMed] [Google Scholar]
- 75.Zheng B, Atwal J, Ho C, Case L, He XL, Garcia KC, Steward O, Tessier-Lavigne M. Genetic deletion of the Nogo receptor does not reduce neurite inhibition in vitro or promote corticospinal tract regeneration in vivo. Proc Natl Acad Sci USA. 2005;102:1205–1210. doi: 10.1073/pnas.0409026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simonen M, Pedersen V, Weinmann O, Schnell L, Buss A, Ledermann B, Christ F, Sansig G, van der Putten H, Schwab ME. Systemic deletion of the myelin-associated outgrowth inhibitor Nogo-A improves regenerative and plastic responses after spinal cord injury. Neuron. 2003;38:201–211. doi: 10.1016/s0896-6273(03)00226-5. [DOI] [PubMed] [Google Scholar]
- 77.Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. doi: 10.1016/0896-6273(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 78.Kim JE, Liu BP, Park JH, Strittmatter SM. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 79.Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldshmit Y, McLenachan S, Turnley A. Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev. 2006;52:327–345. doi: 10.1016/j.brainresrev.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 81.Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA. 2005;102:10694–10699. doi: 10.1073/pnas.0504021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kullander K, Croll SD, Zimmer M, Pan L, McClain J, Hughes V, Zabski S, DeChiara TM, Klein R, Yancopoulos GD, Gale NW. Ephrin-B3 is the midline barrier that prevents corticospinal tract axons from recrossing, allowing for unilateral motor control. Genes Dev. 2001;15:877–888. doi: 10.1101/gad.868901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wahl S, Barth H, Ciossek T, Aktories K, Mueller BK. Ephrin-A5 induces collapse of growth cones by activating Rho and Rho kinase. J Cell Biol. 2000;149:263–270. doi: 10.1083/jcb.149.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yue Y, Su J, Cerretti DP, Fox GM, Jing S, Zhou R. Selective inhibition of spinal cord neurite outgrowth and cell survival by the Eph family ligand ephrin-A5. J Neurosci. 1999;19:10026–10035. doi: 10.1523/JNEUROSCI.19-22-10026.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM. Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci. 2004;24:10064–10073. doi: 10.1523/JNEUROSCI.2981-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tang X, Davies JE, Davies SJ. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 2003;71:427–444. doi: 10.1002/jnr.10523. [DOI] [PubMed] [Google Scholar]
- 88.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- 89.Schaefer L, Schaefer RM. Proteoglycans: from structural compounds to signaling molecules. Cell Tissue Res. 2010;339:237–246. doi: 10.1007/s00441-009-0821-y. [DOI] [PubMed] [Google Scholar]
- 90.Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 91.Erschbamer M, Pernold K, Olson L. Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J Neurosci. 2007;27:6428–6435. doi: 10.1523/JNEUROSCI.1037-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]