Abstract
Extracorporeal photopheresis (ECP) is emerging as a therapy for graft-versus-host-disease (GVHD), but the full mechanism of action and the impact on immunity have not been fully established. After murine minor histocompatibility antigen-mismatched bone marrow transplant (alloBMT), co-infusion of ECP-treated splenocytes with T cell-replete bone marrow attenuated GVHD irrespective of the donor strain of the ECP-treated splenocytes, and was associated with increased numbers of regulatory T cells. Co-culture of myeloid dendritic cells (mDC) with ECP-treated splenocytes resulted in increased interleukin (IL)-10 production after sub-maximal stimulation with lipopolysaccharide. Furthermore, male mDCs exposed to ECP-treated splenocytes were less potent at inducing CD8+ HY-responses when used as a vaccine in vivo. The efficacy of ECP-treated splenocytes was enhanced when administered just prior to delayed donor lymphocyte infusion (DLI) following T cell depleted alloBMT, allowing for the administration of sufficient numbers of T cells to respond to mDC vaccination in the absence of a thymus. Finally, the therapeutic effect of ECP-treated splenocytes were lost in recipients of IL-10 deficient bone marrow. We demonstrate that ECP-treated splenocytes attenuate GVHD irrespective of the source of ECP-treated cells via a mechanism that likely involves modulation of DCs, and requires IL-10 produced by bone marrow-derived cells. Importantly, attenuation of GVHD by ECP-treated splenocytes permits DLI-dependent responses to DC vaccines following alloBMT.
Introduction
Graft-versus-host-disease (GVHD) contributes to the curative potential of allogeneic blood and marrow transplantation (alloBMT) but results in substantial morbidity, and remains the leading cause of non-relapse mortality(1). While matching the donor and recipient to major histocompatibility complex (MHC) antigens reduces the chance of developing GVHD, the recognition of host minor histocompatibility antigens (mHA) by donor T cells can still lead to clinically significant GVHD(2). Steroids are the first-line therapy for treating GVHD, but fail in 50% of patients(1). In addition, steroids are globally immunosuppressive, increasing the risk of relapse and infections. Thus, strategies to mitigate GVHD with preservation of immune function will be important in improving outcomes following alloBMT.
Extracorporeal photopheresis (ECP) is a clinically utilized procedure that involves exposure of peripheral blood leukocytes isolated by apheresis to a photosensitizer such as 8-methoxypsoralen (8-MOP) and ultraviolet A (UVA) radiation followed by re-infusion into the patient. Cells treated in this manner have been shown in vitro to sustain damage to the cell membrane, with formation of monoadducts and cross-linking of DNA(3, 4), as well as activation of caspases, leading to protracted apoptosis(5) and immune modulation following reinfusion. ECP has demonstrated efficacy in treating both acute and chronic GVHD, resulting in a decrease in, or cessation of, immunosuppressive medications(6–9). Although the literature has been conflicting, it appears that the likelihood of response is dependent upon the organ(s) involved, with skin being one of the most responsive sites(10). There has been one published prospective randomized study of ECP in chronic GVHD involving the skin in adults which demonstrated a modest but statistically significant reduction in steroid dose in the ECP treatment arm(11). A number of prospective trials assessing the efficacy of ECP as therapy for acute or chronic GVHD are planned or ongoing. While the definitive mechanism of ECP in immune modulation has not been elucidated, a number of immunologic alterations are induced by ECP including expansion of regulatory T cells(6–9) and modulation of cytokine production and maturation of dendritic cells (DCs)(12–14). Thus, insights into the complex immunologic effects induced by ECP will be critical in optimization as a therapeutic approach for GVHD.
Murine models have begun to shed light on the immunologic effect of ECP. In recipients of ECP-treated cells, T cell responses to haptens are inhibited in an antigen-specific manner(15, 16), and this tolerance can be transferred to naïve animals(9, 15) with preferential trafficking of ECP treated cells to the spleen(9, 17). However, despite the availability of rodent and primate animal models for examining the mechanism of ECP in treating autoimmune diseases and preventing cardiac allograft rejection over the last 20 years(17, 18), mechanistic studies in alloBMT models have been limited(19, 20). Initial observations revealed that GVHD mediated by both MHC-mismatched and mHA-mismatched bone marrow could be minimized by ECP with some evidence for maintained graft-versus-leukemia (GVL) effects(20). More recent studies attributed the mechanism of ECP to the induction of regulatory T cells (Tregs)(6, 9). Finally, in preclinical autoimmune and solid organ allograft models of ECP(8), as well as in models of ultraviolet B exposure(21), selective deletion or suppression of pathogenic T cells has been described(22). Thus, ECP may offer the advantage of working in an antigen-specific manner, avoiding some of the global immunosuppression associated with prolonged steroid use. There is emerging evidence that apoptotic cells can impact immune responses through modulation of DC function(12, 14, 23, 24). Given that ECP involves the infusion of cells undergoing apoptosis, we hypothesized that modulation of DCs is the central mechanism by which ECP diminishes alloreactivity and may result in preserved immune responses to non-alloantigens targeted by vaccination.
Materials and Methods
Mice
C57/BL6 (B6), B6 IL-10−/−, C3H.SW x B6 (F1), C3H.SW, and BALB.B mice (all H-2b) were purchased from Jackson Laboratories (Bar Harbor, ME). These mice are MHC antigen-matched and mHA-mismatched at multiple antigens(25). C3H.HeNCr (H-2k) mice were purchased from the National Cancer Institute Animal Production Program (Frederick, MD). Mice were age-matched and used between 6 and 12 weeks of age. Where indicated, thymectomized mice underwent vacuum suction removal of the thymus according to standard protocol. These animals were housed in pathogen-free conditions throughout the study. The Animal Care and Use Committee at the National Institutes of Health approved all protocols.
Bone Marrow Transplant and Donor Lymphocyte Infusion
Bone marrow was harvested, and where indicated, T cell depleted as previously described(26). Lethally irradiated (10 Gy) C3H.SW or F1 recipient mice were injected intravenously through the tail vein with 10 × 106 T cell-replete (enriched with 5 × 106 lymph node cells, TCR) or 4 × 106 T cell-depleted (TCD) B6 bone marrow cells in serum-free RPMI media (Invitrogen, Carlsbad, CA). Engraftment was confirmed by flow cytometric analysis of spleens of F1 recipients (CD45.1+/CD45.2+) transplanted with TCD B6 (CD45.1−/CD45.2+) bone marrow. Donor lymphocyte infusions (DLI), obtained from single-cell suspensions of pooled inguinal and axillary lymph nodes, were administered on days +14 and +28 post-BMT where indicated. Cells were washed, counted and the entire lymph node suspension was resuspended in serum-free RPMI media for intravenous injection through the tail vein. No deletions of cell subsets were performed. Recipients were weighed individually twice every 7 days, and the mean weight of each treatment group was calculated at each timepoint and compared to the day 0 weight. Survival and clinical monitoring of GVHD, including observation for skin changes (ruffling or hair loss), diarrhea and hunched posture, occurred daily, although in this MHC-matched, mHA-mismatched BMT model, GVHD was best measured by weight loss and diminished splenic B cells counts as shown previously(26). “Attenuation” of GVHD was defined as weight loss that was significantly less than in GVHD controls. Immune reconstitution by flow cytometry is detailed in the Supplemental Methods. Examination for moribund mice was performed by a veterinarian and veterinary technicians blinded to experimental design and groups, who assessed mice daily according to approved institutional protocols.
ECP Treatment
ECP treatment involved harvesting spleens from appropriate donors and resuspending at 10 × 106 cells/mL. Where noted, depletion of B cells and T cells was achieved with magnetic cell selection with anti-B220 or anti-CD3 microbeads according to manufacturer’s directions (Miltenyi Biotec, Auburn, CA). 8-methoxypsoralen (8-MOP) was added to the suspension at a concentration of 0.2 mcg/mL unless otherwise noted. The cells were incubated with 8-MOP for 30 minutes at 37° C and 5% CO2. Following incubation, the cells were exposed to UVA light (5 J/cm2) in a calibrated light box (Therakos, Raritan, NJ). Treated cells were resuspended in serum-free RPMI media and were administered intravenously to recipient mice either one day after TCR BMT (therapeutic ECP) or on the same day as a DLI after TCD BMT (prophylactic ECP).
Dendritic Cell Culture
Bone marrow was harvested and processed as previously described(26). Mature hematopoietic cells and their committed precursors were depleted by magnetic lineage cell depletion over an LS column according to manufacturer’s directions (Miltenyi Biotec). The negative fraction was then resuspended in complete mouse media (CMM)(26) and cultured at 37 degrees Celsius, 5% CO2 for eight days with IL-4 at 500U/mL and GM-CSF at 1000U/mL. This culture system yields greater than 90% myeloid DCs (CD11c+CD11b+). DCs were activated with 4μg/mL anti-CD40 on day 7, and collected within 24 hours of activation. For experiments incorporating DC vaccines, DCs were then resuspended in serum-free RPMI media, and injected intraperitoneally at 1 × 105 cells per recipient at the time of DLI.
ELISPOT Analysis
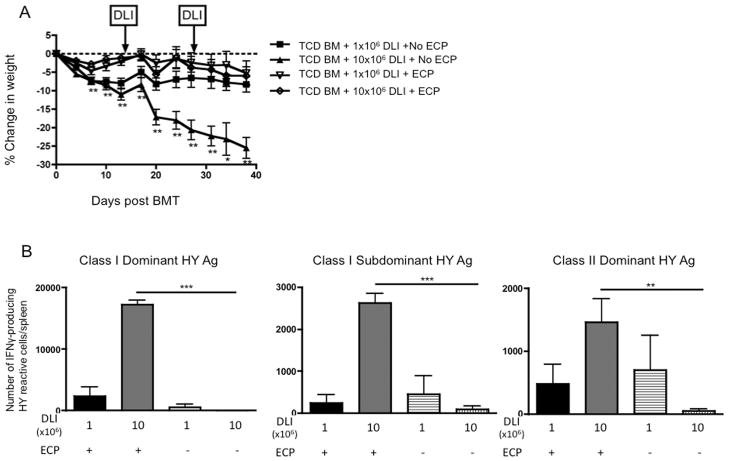
ELISPOT assays were performed on recipient splenocytes using IFN-γ mouse ELISPOT kits (R&D Systems, Minneapolis, MN), as previously described(26). Synthetic peptides representing the Class I dominant (UTY), Class I subdominant (SMCY) and Class II dominant (DBY) peptides from the male HY antigen complex were used to stimulate IFN-γ production. Two non-specific peptides (E7, class I and TC, class II) were used as control peptides to determine background, which was subtracted from the HY-peptide stimulated wells. Each sample was run in triplicate and the responder:stimulator ratio was 1:1. An automated ELISPOT plate reader was used to count the number of spots in each well (Cellular Technology Ltd., Shaker Heights, OH). The total number of HY-reactive T cells was calculated using the total splenocyte count and the number of spots/well as already described(27).
DC and ECP-treated cell co-culture assay
DCs were generated from female B6 bone marrow as described above and placed in a 24 well plate with CMM, IL-4 and GM-CSF. On days +2, 4, and 6, media and cytokines were exchanged. Additionally, titrated lipoloysaccharide (LPS) was added in serial dilutions on day +6. ECP and irradiated cells were added at 1 × 106 cells/well on day +6 such that a 1:1 ratio of dendritic cells to stimulatory cells was achieved. Each LPS concentration plus treatment was cultured in triplicate per experiment. Supernatants were analyzed on day +7 by IL-10, IL-12p70 and TNF-α ELISAs (R&D Systems) according to manufacturer instructions. Plates were read with a Versamax microplate and analyzed using SoftMAX Pro 5 reader (Molecular Devices, Sunnyvale, CA).
Statistical Analysis
Statistical tests were performed using GraphPad Prism version 4.0c for Macintosh (GraphPad Software, San Diego, CA). Significant differences when comparing two groups were determined by two-tailed Mann-Whitney test. Kruskal-Wallis with Dunn’s multiple comparison posttest was used to assess statistical differences between three or more groups. A p value less than 0.05 was considered significant.
Results
ECP leads to uniform apoptosis of treated splenocytes in an 8-MOP dose dependent fashion
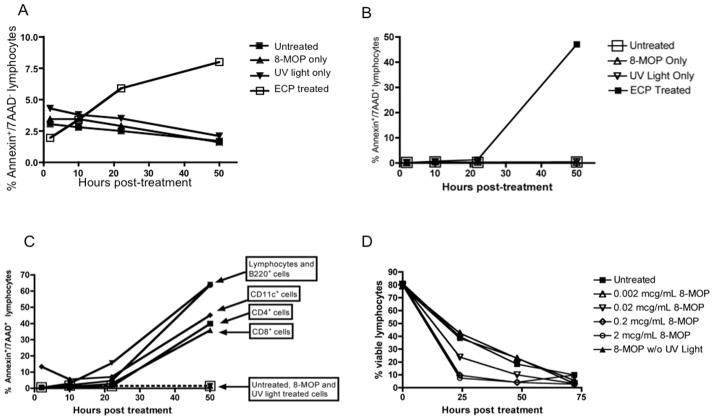
Although it has been well established that exposure of cells to 8-MOP and UVA light results in apoptosis, we first wanted to establish the timecourse of ECP-induced apoptosis of murine splenocytes in vitro and, given the heterogeneity of whole spleen, determine whether there are differences in the rate of apoptosis in splenocyte subsets. Following exposure of whole murine splenocytes to 8-MOP and UVA light, we observed an increase in Annexin V+ (Figure 1A) as early as 10 hours after treatment and in Annexin V+/7-AAD+ cells (Figure 1B) with ongoing cell death through 50 hours. The induction of apoptosis only occurred with exposure to both 8-MOP and UVA light, with no effect from either treatment alone. The kinetics of cell death did not vary much amongst cell subsets contained in the spleen (Figure 1C). Lastly there was a dose-dependent effect on apoptosis noted with increasing 8-MOP concentrations (Figure 1D). Based on these experiments, 0.2mcg/mL was the lowest concentration at which the rate of apoptosis plateaued, consistent with prior reports(6), and this dose was utilized for all subsequent experiments.
Figure 1. ECP-treated splenocytes induce apoptosis of lymphocytes in a time-dependent fashion, and is 8-MOP dose dependent.
A. Splenocytes were treated with 8-MOP, UV light or both (ECP) and apoptosis was determined by flow cytometric analysis of Annexin V over the course of 48 hours. B. Splenocytes were treated with 8-MOP, UV light or both (ECP) and apoptosis was determined by flow cytometric analysis of Annexin V and 7-AAD over the course of 48 hours. C. The rate of apoptosis was ascertained in individual splenic lymphocyte subsets and DCs by flow cytometric analysis of Annexin V and 7-AAD over 48 hours. D. Splenocytes were exposed to increasing doses of 8-MOP to determine the minimum concentration that caused maximal apoptosis.
Therapeutic ECP attenuates GVHD-associated weight loss in a strain-independent manner and expands regulatory T cells
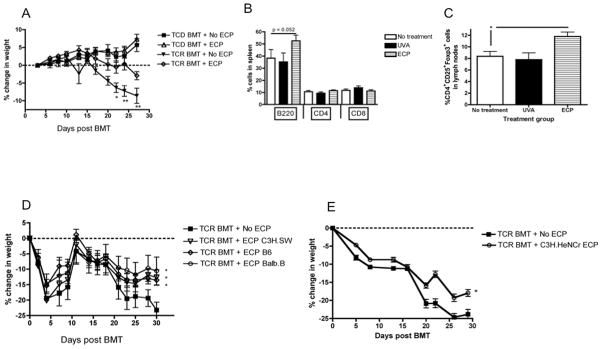
ECP-treated splenocytes were then applied as an early therapeutic intervention after T cell replete (TCR) mHA-mismatched alloBMT models which cause nonlethal GVHD(26). When injected intravenously 24 hours after infusion of TCR bone marrow, ECP-treated splenocytes modestly attenuated GVHD-associated weight loss (Figure 2A). Flow cytometric analysis demonstrated that recipients of ECP-treated splenocytes had a slight but statistically significant increase in splenic B220+ cells (Figure 2B), as well as in CD4+CD25+Foxp3+ Tregs in the lymph nodes (Figure 2C), as reported previously(6). In addition, donor strain, recipient strain and “third party” (strain different from the BMT B6 donor or C3H.SW recipient) MHC-matched ECP-treated splenocytes were equally effective at attenuating GVHD-associated weight loss (Figure 2D). The impact of ECP-treated splenocytes on GVHD did not require donor or recipient MHC class I or II antigen expression since MHC-mismatched ECP-treated splenocytes resulted in similar attenuation of weight loss (Figure 2E). Finally, ECP-treated splenocyte infusions depleted of T cells or B cells were equally effective at reducing GVHD-associated weight loss (Figure S1).
Figure 2. ECP-treated splenocytes attenuate GVHD-associated weight loss after TCR BMT, leading to an increase in B220+ cells and Tregs, and are effective independent of the source of ECP-treated cells.
A. C3H.SW recipients received either TCD or TCR B6 bone marrow on day +0, followed by untreated (No ECP) or ECP-treated B6 splenocytes on day +1, and followed for weight loss, 7–8 mice/group, * = p < 0.05, ** = p < 0.005. B. Flow cytometric analysis of splenic B220+ cells was performed after TCR BMT. C. Flow cytometric analysis of pooled axillary and inguinal lymph nodes for Tregs in mice treated with UVA-treated versus ECP-treated splenocytes after TCR BMT. * = p < 0.05. D. C3H.SW recipients received TCR B6 bone marrow on day +0, followed by either untreated splenocytes (No ECP), or ECP-treated splenocytes on day +1 from either the donor strain (ECP B6) * = p < 0.05, recipient strain (ECP C3H.SW) * = p < 0.05, MHC-matched, mHA-mismatched third party (ECP Balb.b), * = p < 0.05, and followed for weight loss, 7–8 mice/group. E. C3H.SW recipients received TCR B6 bone marrow on day +0, followed by untreated (No ECP) or ECP-treated MHC-mismatched (C3H.HeNCR ECP) splenocytes on day +1, and followed for weight loss, 5 mice/group, * = p < 0.05.
LPS-activated bone marrow-derived myeloid DCs exposed to ECP-treated splenocytes produce increased IL-10
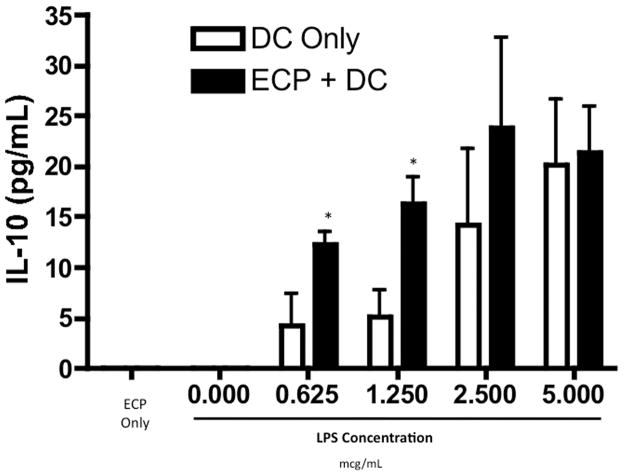
Apoptotic cells can regulate DC function(23, 24) and prior reports have suggested that DCs are required for the ECP effect in a non-alloBMT setting(9). Thus, we next tested whether ECP-treated splenocytes could modulate DCs in vitro. Myeloid DCs (mDCs) were generated from lineage-depleted bone marrow cells, and activated with lipopolysaccharide (LPS), a bacteria-derived toll-like receptor 4 agonist important in the pathophysiology of GVHD(28, 29). Co-culture of LPS-activated mDCs with ECP-treated splenocytes did not significantly change the expression of cell surface markers associated with mDC activation (Figure S2). There was also no change in TNF-α production (data not shown). However, at low concentrations of LPS, co-culture of mDCs with ECP-treated splenocytes resulted in a modest decrease in IL-12 production (data not shown) and a more pronounced increase in IL-10 production (Figure 3). Interestingly, this ECP effect was lost upon stimulation with higher concentrations of LPS.
Figure 3. ECP-treated splenocytes increase in vitro IL-10 production by DCs activated with LPS.
ELISA for IL-10 production in supernatants of cultures of B6 mDCs, co-cultured with or without ECP-treated B6 splenocytes, activated with increasing concentrations of LPS, * = p < 0.05. No IL-10 was detected at a LPS dose of 0.000 mcg/mL.
ECP-modulated DCs are less potent at inducing CD8+ immune responses in vivo
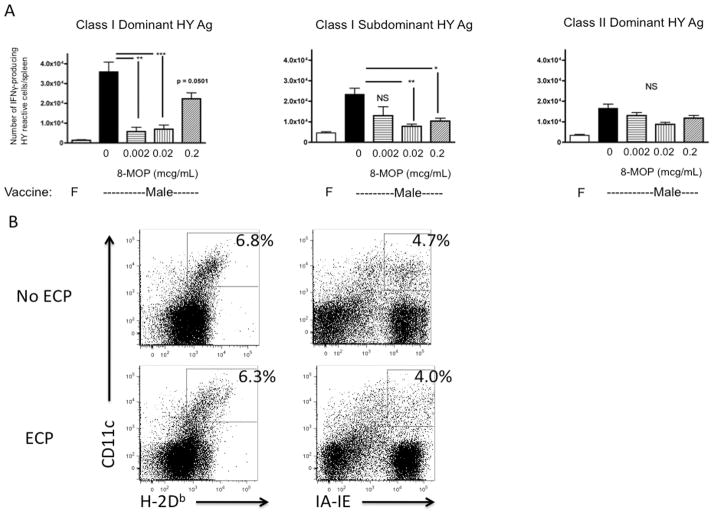
We next tested whether the changes in cytokine production in vitro resulted in diminished functional capacity of mDCs exposed to ECP-treated splenocytes to induce immune responses in vivo. Vaccination of female mice with male mDCs expressing the mHA complex, HY, results in expansion of CD8+ and CD4+ T cells recognizing dominant (UTY) and subdominant (SMCY) class I antigens and dominant class II (DBY) antigens enumerated by gamma interferon ELISPOT(27). Co-culture of male mDCs with female splenocytes exposed to UVA without 8-MOP (which do not undergo apoptosis, Figure 1) had no impact on vaccine responses (Figure 4A). However, exposure of male DCs to female ECP-treated splenocytes incubated with increasing concentrations of 8-MOP resulted in diminished CD8+ T cell responses to both dominant and subdominant class I antigens (Figure 4A). This was not due to diminished class I expression on mDCs exposed to ECP-treated splenocytes (Figure 4B). The CD4+ T cell responses to class II antigen were not affected by exposure of DCs to ECP-treated splenocytes.
Figure 4. ECP-treated splenocytes abrogate CD8+ T cell responses of nontransplanted female mice to male DC vaccination.
A. B6 mice were vaccinated with female or male B6 DCs that were exposed to ECP-treated splenocytes from varying doses of 8-MOP, and then ELISPOT was performed to quantitate IFN-γ production from UTY, SMCY and DBY-reactive T cells, 6–22 mice/group pooled from separate experiments, * = p < 0.05, ** = p < 0.01, *** = p < 0.001. F = female, NS = not significant. B. Activated B6 DCs were exposed to untreated (No ECP) or ECP-treated B6 splenocytes (ECP) and then examined by flow cytometric analysis for MHC class I and II expression.
ECP-treated splenocytes attenuate delayed DLI-induced GVHD while preserving responses to a DC vaccine
Although the effect of ECP-treated splenocytes was consistent and statistically significant when used early after TCR BMT (Figure 2), the attenuation of GVHD-associated weight loss was modest. Based on the increased IL-10 production by mDCs we observed in vitro, and that higher concentrations of LPS minimized the effect, we hypothesized that ECP might be more effective as a prophylactic treatment for GVHD mediated by donor lymphocyte infusions (DLI) given after inflammation associated with the preparative irradiation had diminished. To test this, a TCD BMT model was utilized with delayed infusion of T cells on days +14 and +28 to control the timing and degree of GVHD. Thymectomized BMT recipients were used to ensure that that all T cells were derived from the DLI, and to mimic the absence of thymic activity characteristic of the early post-alloBMT environment. ECP-treated splenocytes were prophylactically administered to BMT recipients as a separate injection just prior to the DLI. The use of prophylactic ECP in the setting of TCD BMT with DLI attenuated GVHD-associated weight loss from higher DLI doses of 10×106 cells (Figure 5A), and was substantially more effective than ECP used immediately after TCR BMT (Figure 2D).
Figure 5. ECP-treated splenocytes prevent GVHD-associated weight loss when used prior to alloreactive T cell infusion, and preserve responses to a male DC vaccine.
A. F1 mice were given TCD B6 bone marrow (BM) on day +0 followed by B6 DLI on days +14 and +28 to induce GVHD. ECP treatment was given as a separate injection just prior to DLI infusion as GVHD prophylaxis, and all groups were followed for weight loss, 8 mice/group, * = p < 0.05, ** = p < 0.01. B. ELISPOT was performed on day +42 to quantitate IFN-γ production from UTY, SMCY and DBY-reactive T cells, 6–8 mice/group, ** = p < 0.01, *** = p < 0.001.
We have previously validated responses to HY vaccination as a stringent measure of immune competence in thymectomized, T cell depleted hosts requiring a large T cell inocula(30). We next examined whether modulation of GVHD with prophylactic ECP preserved DLI-dependent DC vaccine responses in the absence of thymic T cell regeneration. Transplanted, thymectomized female mice received a male mDC vaccine as a separate intraperitoneal injection on the same day as DLI on days +14 and +28 post-BMT, and vaccine responses were measured by ELISPOT on day +42. At a DLI dose of 1×106 cells, the quantity of T cells caused a mild GVHD-associated weight loss (Figure 5A) but was insufficient to generate T cell responses to HY in the absence of thymic function (Figure 5B), as we have previously observed(26). Remarkably, at a DLI dose of 10×106 cells, which is sufficient to cause more severe GVHD-associated weight loss (Figure 5A), ECP-treated splenocytes permitted robust vaccine responses (Figure 5B), equivalent to thymus-bearing non-transplanted mice (Figure 4A).
Donor bone marrow-derived IL-10 contributes to the ECP effect on GVHD-associated weight loss
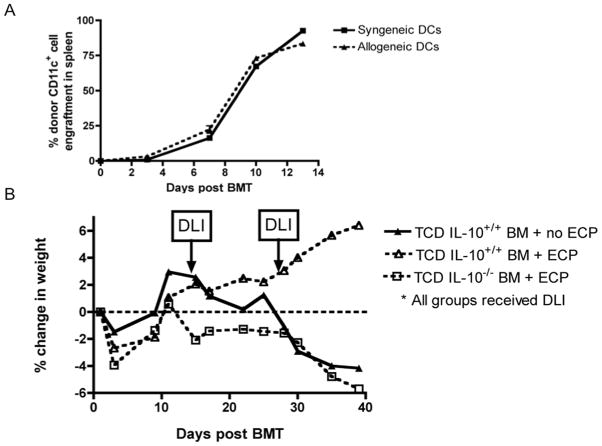
Since increased IL-10 production by mDCs was observed in the co-culture experiments, we next tested whether IL-10 was required for the therapeutic effect of ECP-treated splenocytes in vivo. AlloBMT was performed in thymectomized recipients (as in Figure 5A) using B6 IL-10-deficient or IL-10 producing wild-type marrow. Alloreactive DLI from wild-type mice was given on days +14 and +28 to induce GVHD. Importantly, by the time of DLI, CD11c+ DCs in spleen and lymph node are of donor origin (Figure 6A). Thus, the lack of IL-10 production was restricted to hematopoietic, non-T cells, including DCs. The efficacy of ECP-treated splenocytes in preventing GVHD induced by the delayed infusion of IL-10 producing T cells was completely lost in recipients of IL-10 deficient bone marrow, indicating that IL-10 produced by a bone marrow-derived, non-T cell is required for the attenuation of GVHD by ECP-treated splenocytes (Figure 6B).
Figure 6. Bone marrow derived IL-10 is necessary for ECP-treated splenocytes to attenuate GVHD-associated weight loss.
A. F1 recipients (CD45.1+/CD45.2+) transplanted with TCD B6 (CD45.2+) bone marrow on day +0, then sacrificed on day +3, +7, +10 and +13 for flow cytometric analysis of CD45.2+CD11c+ reconstitution in the spleen prior to DLI, 3 mice/timepoint. B. Thymectomized F1 recipients were transplanted with TCD B6 wild type or B6 IL-10−/− bone marrow on day +0 and then treated with 10 × 106 cells DLI on days +14 and +28. ECP was given as a separate injection to indicated groups just prior to DLI on days +14 and +28. All groups were followed for weight loss, 7 mice/group.
Discussion
While a variety of therapies are available to manage steroid-refractory GVHD (albeit with poor response rates), infection and relapse remain major complications, and strategies to prevent GVHD while preserving immunocompetence remain elusive. ECP represents one potential strategy to treat GVHD with demonstrated efficacy in the clinic, but the impact of ECP on immunity is quite complex and diverse in different systems. Although there have been a number of murine models that have assessed the immunomodulatory properties of ECP in non-alloBMT settings, as well as correlative clinical data, there is limited data directly addressing the mechanism of ECP in the complex millieu that characterizes the post alloBMT environment. We utilized a MHC-matched, mHA-mismatched alloBMT model to examine the impact of ECP-treated splenocytes on GVHD-associated weight loss using both TCR bone marrow models and TCD bone marrow with delayed DLI models.
Our data confirm prior reports that ECP can modulate GVHD in murine models and is associated with modest increases in B220+ cells and Tregs. The increase in B220+ cells may reflect attenuation of GVHD, as loss of B220+ cells correlating with GVHD has been previously demonstrated in this model(26), and other BMT models(31). We cannot rule out that B220 expansion contributes to the mechanism of ECP, but B220+ cells are not required in the ECP inocula to attenuate weight loss. The contribution of Tregs to the therapeutic effect of ECP has been demonstrated in other alloBMT models(6) and is suggested by clinical data(32). We show for the first time that the therapeutic effect of ECP is not dependent on the source of ECP-treated cells, since “third-party” ECP donor cells are equally effective, even when mismatched at MHC class I and class II. From a clinical perspective, the efficacy of “third-party” cells does indicate that infusion of “off the shelf” ECP-treated cells, not expressing mHA antigens against which immune modulation is desired, could be utilized. It is important to note that the severity of GVHD-associated weight loss was attenuated, but not prevented, implying ECP-treated recipients still have some degree of GVHD, which is consistent with clinical experience with ECP in treating established GVHD.
Previous reports in non-alloBMT models suggest that ECP preserves immune competence via antigen-specific immune suppression(9) but the impact of ECP on immune competence in alloBMT has not been established. Our data support that the infusion of ECP-treated splenocytes acts at least in part through DCs, consistent with the clinical observation of altered DC profiles in the peripheral blood of ECP treated patients and the impact of ECP on human APCs in vitro(12–14). Prior reports indicated that the efficacy of ECP is lost with depletion of DCs from the ECP-treated cells(9). Our results indicated that, while direct modulation of DCs in the ECP-treated inocula may be occurring in the first 48 hours, and that these DCs may directly contribute to the therapeutic effect of ECP, all cell populations, including DCs, undergo apoptosis at the same rate with near complete death by 48 hours. We also demonstrate that exposure to ECP-treated splenocytes can indirectly attenuate the capacity for DCs not treated with ECP to induce immune responses in vivo, further supporting that DCs contribute, at least in part, to the therapeutic effect of ECP. Indeed, DCs are critical for the induction of GVHD(33), and the ability for apoptotic cells to alter the functional capacity of APCs has been described previously(34).
Importantly, in our model, donor T cells exposed to ECP-treated splenocytes in the recipient at the time of infusion were less able to mediate GVHD, but responded to activated mDC vaccination expressing non-alloantigens, suggesting that ECP-treated splenocytes do not directly affect the function of non-alloreactive T cells. We cannot state conclusively that immune responses to tumor or infectious antigens, that may be dependent on mDCs resident in the recipient, are also preserved. The ability for ECP to preserve immune competence is supported by reports demonstrating that patients undergoing long-term ECP therapy, have no reported higher risk of developing infections and respond normally to both novel and recall antigens(35).
ECP-treated splenocytes co-cultered with mDCs modulated cytokine production resulting in increased IL-10. We confirmed the role for IL-10 in vivo using IL-10 deficient bone marrow allografts, which completely abrogates the therapeutic effect of ECP. Furthermore, since the T cells in the DLI and ECP-treated splenocytes were both capable of producing IL-10 in this model, the data support the in vitro studies demonstrating that IL-10 production by bone marrow-derived, non-T cells is required for ECP-treated splenocytes to diminish alloreactivity. Both in clinical chronic GVHD studies(36) and in tumor models(37), IL-10 can prevent DC differentiation, making them poor APCs. In addition, IL-10-producing DCs have been shown to expand Tregs in humans, potentially linking DC modulation by ECP to the observed expansion of Tregs in murine models and in humans(7). Collectively, we favor a model wherein infusion of ECP-treated splenocytes indirectly modulates T cell-mediated alloreactivity via an IL-10 producing DC not in the ECP-treated inocula, resulting in expansion of Tregs.
The inability for ECP-treated splenocytes to modulate DC cytokine production at higher concentrations of LPS, in addition to the modest impact of ECP-treated splenocytes administered immediately after alloBMT at the height of inflammatory cytokines, indicates that the ECP effect on DCs can be overcome by a strong activation stimulus and suggests that ECP may have enhanced efficacy if given prior to the onset of GVHD. Compared to TCR BMT (Figures 2A vs. 2C/D), there was markedly improved attenuation of DLI-induced GVHD-associated weight loss after TCD BMT when ECP was given before high doses of alloreactive T cells were infused (Figures 5A and 6B). Thus, the discrepancy of GVHD-associated weight loss, between TCR vs. TCD BMT models may reflect the fact that GVHD mediated by TCR BMT may be more severe or difficult to treat because of the additional inflammation from the preparative irradiation. Interestingly, ECP has been shown to be potentially efficacious when use prophylactically in pilot studies in humans(38).
Taken together, we conclude that the therapeutic effect mediated by the infusion of apoptotic cells induced by ECP involves modulation of mDCs with increases in B220+ cells and Tregs after alloBMT(6), but our data add several key findings. First, the source of ECP-treated cells does not have to be recipient-derived, as is practiced clinically, but can be from a third party source, including MHC-mismatched cells. ECP-treated splenocytes appear to skew mDCs toward increased IL-10 production in vitro, and IL-10 production by donor bone marrow-derived, non-T cells in vivo is critical for the therapeutic effect of ECP-treated splenocytes on GVHD-associated weight loss. In addition, mDCs exposed to ECP-treated splenocytes promote diminished CD8+ T cell responses. Thus, our data support that the therapeutic effect of ECP involves modulation of DC cytokine production in the recipient toward a more immunosuppressive phenotype consistent with the importance of DC in the induction of GVHD. Finally, ECP-treated splenocytes were more effective when used as a prophylactic modality for DLI-induced GVHD after TCD BMT, permitting CD4+ and CD8+ T cell responses to an activated DC vaccine. These observations may have implications as investigators apply immunotherapeutic strategies targeting tumor-associated or infection-associated antigens in the setting of alloBMT where the development of GVHD, and current therapies for GVHD, results in immune suppression.
Supplementary Material
F1 recipients received TCD B6 bone marrow on day +0. On days +14 and +28, recipients received either no DLI (no T cells), 10 × 106 DLI only (No ECP), or ECP with whole splenocytes, CD3-depleted splenocytes or B220-depleted splenocytes followed by 10 × 106 DLI, and followed for weight loss, 7 mice/group.
Bone marrow-derived DCs were co-cultured with untreated, irradiated, or ECP-treated splenocytes and examined for cell surface expression of costimulatory molecules by FACS analysis. All panels show histograms of the indicated costimulatory molecule compared to an isotype control.
Acknowledgments
Financial disclosure: This work was supported by the intramural research program at N.I.H. and grants from the Children’s Cancer Foundation and Lisa’s Heart Kids Cancer Research Fund (T.J.F.).
Footnotes
Disclaimer
The content of this publication does not necessarily reflect the views of policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Authorship
Contribution: C.M.C. and J.P.E.D. designed and performed research, collected, analyzed, and interpreted data, performed statistical analysis, and drafted the manuscript; S.M.L., S.H and N.M.N. performed research, collected, analyzed, and interpreted data, and performed statistical analysis; and T.J.F. designed and supervised research, analyzed and interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Messina C, Faraci M, de Fazio V, Dini G, Calo MP, Calore E. Prevention and treatment of acute GvHD. Bone Marrow Transplant. 2008;41:S65–S70. doi: 10.1038/bmt.2008.57. [DOI] [PubMed] [Google Scholar]
- 2.Simpson E, Scott D, James E, et al. Minor H antigens: genes and peptides. Transplant immunology. 2002;10:115–123. doi: 10.1016/s0966-3274(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 3.Guiotto A, Rodighiero P, Manzini P, et al. 6-Methylangelicins: a new series of potential photochemotherapeutic agents for the treatment of psoriasis. Journal of medicinal chemistry. 1984;27:959–967. doi: 10.1021/jm00374a005. [DOI] [PubMed] [Google Scholar]
- 4.Hearst JE. Psoralen Photochemistry and Nucleic Acid Structure. J Investig Dermatol. 1981;77:39–44. doi: 10.1111/1523-1747.ep12479229. [DOI] [PubMed] [Google Scholar]
- 5.Tambur AR, Ortegel JW, Morales A, Klingemann H, Gebel HM, Tharp MD. Extracorporeal photopheresis induces lymphocyte but not monocyte apoptosis. Transplantation Proceedings. 2000;32:747–748. doi: 10.1016/s0041-1345(00)00966-0. [DOI] [PubMed] [Google Scholar]
- 6.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biagi E, Di Biaso I, Leoni V, et al. Extracorporeal Photochemotherapy Is Accompanied by Increasing Levels of Circulating CD4+CD25+GITR+Foxp3+CD62L+ Functional Regulatory T-Cells in Patients With Graft-Versus-Host Disease. Transplantation. 2007;84:31–39. doi: 10.1097/01.tp.0000267785.52567.9c. [DOI] [PubMed] [Google Scholar]
- 8.George JF, Gooden CW, Guo WH, Kirklin JK. Role for CD4(+)CD25(+) T cells in inhibition of graft rejection by extracorporeal photopheresis. The Journal of heart and lung transplantation. 2008;27:616–622. doi: 10.1016/j.healun.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Maeda A, Schwarz A, Kernebeck K, et al. Intravenous Infusion of Syngeneic Apoptotic Cells by Photopheresis Induces Antigen-Specific Regulatory T Cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 10.Baird K, Wayne AS. Extracorporeal photo-apheresis for the treatment of steroid-resistant graft versus host disease. Transfusion and Apheresis Science. 2009;41:209–216. doi: 10.1016/j.transci.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flowers MED, Apperley JF, van Besien K, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008;112:2667–2674. doi: 10.1182/blood-2008-03-141481. [DOI] [PubMed] [Google Scholar]
- 12.Di Renzo M, Sbano P, De Aloe G, et al. Extracorporeal photopheresis affects co-stimulatory molecule expression and interleukin-10 production by dendritic cells in graft-versus-host disease patients. Clinical and experimental immunology. 2008;151:407–413. doi: 10.1111/j.1365-2249.2007.03577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Renzo M, Rubegni P, Pasqui AL, et al. Extracorporeal photopheresis affects interleukin (IL)-10 and IL-12 production by monocytes in patients with chronic graft-versus-host disease. British journal of dermatology. 2005;153:59–65. doi: 10.1111/j.1365-2133.2005.06482.x. [DOI] [PubMed] [Google Scholar]
- 14.Spisek R, Gasova Z, Bartunkova J. Maturation state of dendritic cells during the extracorporeal photopheresis and its relevance for the treatment of chronic graft-versus-host disease. Transfusion. 2006;46:55–65. doi: 10.1111/j.1537-2995.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 15.van Iperen H, Beijersbergen van Henegouwen G. An animal model and new photosensitizers for phbotopheresis. Photochemistry and Photobiology. 1993;58:571–574. doi: 10.1111/j.1751-1097.1993.tb04934.x. [DOI] [PubMed] [Google Scholar]
- 16.van Iperen HP, Beijersbergen van Henegouwen GM. An animal model for extracorporeal photochemotherapy based on contact hypersensitivity. Journal of photochemistry and photobiology B, Biology. 1992;15:361–366. doi: 10.1016/1011-1344(92)85142-h. [DOI] [PubMed] [Google Scholar]
- 17.Berger CL. Experimental murine and primate models for dissection of the immunosuppressive potential of photochemotherapy in autoimmune disease and transplantation. The Yale Journal of Biology and Medicine. 1989;62:611–620. [PMC free article] [PubMed] [Google Scholar]
- 18.Cavaletti G, Perseghin P, Dassi M, et al. Extracorporeal photochemotherapy reduces the incidence of relapses in experimental allergic encephalomyelitis in DA rats. Journal of Neurology. 2001;248:535–536. doi: 10.1007/s004150170169. [DOI] [PubMed] [Google Scholar]
- 19.Ulirich SB. Photoinactivation of T-Cell Function with Psoralen and UVA Radiation Suppresses the Induction of Experimental Murine Graft-Versus-Host Disease Across Major Histocompatibility Barriers. J Investig Dermatol. 1991;96:303–308. doi: 10.1111/1523-1747.ep12465134. [DOI] [PubMed] [Google Scholar]
- 20.Truitt RL, Johnson BD, Hanke C, Talib S, Hearst JE. Photochemical Treatment with S-59 Psoralen and Ultraviolet A Light to Control the Fate of Naive or Primed T Lymphocytes In Vivo After Allogeneic Bone Marrow Transplantation. J Immunol. 1999;163:5145–5156. [PubMed] [Google Scholar]
- 21.Schwarz A, Maeda A, Wild MK, et al. Ultraviolet Radiation-Induced Regulatory T Cells Not Only Inhibit the Induction but Can Suppress the Effector Phase of Contact Hypersensitivity. J Immunol. 2004;172:1036–1043. doi: 10.4049/jimmunol.172.2.1036. [DOI] [PubMed] [Google Scholar]
- 22.Cohen I. Regulation of Autoimmune Disease Physiological and Therapeutic. Immunological Reviews. 1986;94:5–21. doi: 10.1111/j.1600-065x.1986.tb01161.x. [DOI] [PubMed] [Google Scholar]
- 23.Fry T, Shand J, Milliron M, Tasian S, Mackall C. Antigen loading of DCs with irradiated apoptotic tumor cells induces improved anti-tumor immunity compared to other approaches. Cancer Immunology, Immunotherapy. 2009;58:1257–1264. doi: 10.1007/s00262-008-0638-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morelli A, Larregina A. Apoptotic cell-based therapies against transplant rejection: role of recipient’s dendritic cells. Apoptosis. 2010 Sep;15(9):1083–97. doi: 10.1007/s10495-010-0469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korngold R, Sprent J. Variable capacity of L3T4+ T cells to cause lethal graft-versus-host disease across minor histocompatibility barriers in mice. J Exp Med. 1987;165:1552–1564. doi: 10.1084/jem.165.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capitini CM, Herby S, Milliron M, Anver MR, Mackall CL, Fry TJ. Bone marrow deficient in gamma interferon signaling selectively reverses GVHD-associated immunosuppression and enhances a tumor-specific GVT effect. Blood. 2009 May 14;113(20):5002–9. doi: 10.1182/blood-2008-11-187385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. The Journal of Clinical Investigation. 2005;115:1177–1187. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penack O, Holler E, van den Brink MRM. Graft-versus-host disease: regulation by microbe-associated molecules and innate immune receptors. Blood. 2010;115:1865–1872. doi: 10.1182/blood-2009-09-242784. [DOI] [PubMed] [Google Scholar]
- 29.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. The Journal of experimental medicine. 1992;175:405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001;97:1525–1533. doi: 10.1182/blood.v97.6.1525. [DOI] [PubMed] [Google Scholar]
- 31.Puliaev R, Nguyen P, Finkelman FD, Via CS. Differential Requirement for IFN-{gamma} in CTL Maturation in Acute Murine Graft-versus-Host Disease. J Immunol. 2004;173:910–919. doi: 10.4049/jimmunol.173.2.910. [DOI] [PubMed] [Google Scholar]
- 32.Di Biaso I, Di Maio L, Bugarin C, et al. Regulatory T cells and extracorporeal photochemotherapy: correlation with clinical response and decreased frequency of proinflammatory T cells. Transplantation. 2009;87:1422–1425. doi: 10.1097/TP.0b013e3181a27a5d. [DOI] [PubMed] [Google Scholar]
- 33.Shlomchik WD, Couzens MS, Tang CB, et al. Prevention of Graft Versus Host Disease by Inactivation of Host Antigen-Presenting Cells. Science. 1999;285:412–415. doi: 10.1126/science.285.5426.412. [DOI] [PubMed] [Google Scholar]
- 34.Albert ML. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat Rev Immunol. 2004;4:223–231. doi: 10.1038/nri11308. [DOI] [PubMed] [Google Scholar]
- 35.Suchin KR, Cassin M, Washko R, et al. Extracorporeal photochemotherapy does not suppress T- or B-cell responses to novel or recall antigens. Journal of the American Academy of Dermatology. 1999;41:980–986. doi: 10.1016/s0190-9622(99)70257-4. [DOI] [PubMed] [Google Scholar]
- 36.Radek S, Zdena G, Jirina B. Maturation state of dendritic cells during the extracorporeal photopheresis and its relevance for the treatment of chronic graft-versus-host disease. Transfusion. 2006;46:55–65. doi: 10.1111/j.1537-2995.2005.00670.x. [DOI] [PubMed] [Google Scholar]
- 37.David MM, Xia Z. Interleukin-10: new perspectives on an old cytokine. Immunological Reviews. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaughnessy PJ, Bolwell BJ, van Besien K, et al. Extracorporeal photopheresis for the prevention of acute GVHD in patients undergoing standard myeloablative conditioning and allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2010;45:1068–1076. doi: 10.1038/bmt.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
F1 recipients received TCD B6 bone marrow on day +0. On days +14 and +28, recipients received either no DLI (no T cells), 10 × 106 DLI only (No ECP), or ECP with whole splenocytes, CD3-depleted splenocytes or B220-depleted splenocytes followed by 10 × 106 DLI, and followed for weight loss, 7 mice/group.
Bone marrow-derived DCs were co-cultured with untreated, irradiated, or ECP-treated splenocytes and examined for cell surface expression of costimulatory molecules by FACS analysis. All panels show histograms of the indicated costimulatory molecule compared to an isotype control.