Abstract
Purpose
To describe the prevalence and signs of early and late age-related macular degeneration (AMD) in an old cohort.
Design
Population based cohort study
Participants
We included 5,272 persons 66 years and older, randomly sampled from the Reykjavik area.
Methods
Fundus images were taken through dilated pupils using a 45°digital camera and were graded for drusen size, type, area, increased retinal pigment, retinal pigment epithelial depigmentation, neovascular lesions and geographic atrophy using the modified Wisconsin Age-Related Maculopathy Grading System.
Main outcome measure
Age-related macular degenerationin an old cohort.
Results
Mean age of participants was 76 years. The prevalence of early AMD was 12.4% (95% confidence interval [CI] 11.0–13.9) for those 66–74 year old and 36% (95% CI 30.9–41.1) for those 85 years and older. The prevalence of exudative AMD was 3.3% (95% CI 2.8–3.8) and for pure geographic atrophy 2.4% (95% CI 2.0–2.8). The highest prevalence for late AMD was among those 85 years and older 11.4% (95% CI 8.2–14.5) for exudative AMD and 7.6% (95% CI 4.8–10.4) for pure geographic atrophy.
Conclusion
Persons 85 years and older have 10-fold higher prevalence of late AMD than those 70–74 years old. The high prevalence of late AMD in the oldest age-group and expected increase of old people in the western world in coming years call for improved preventive measures and novel treatments.
Keywords: Age-related macular degeneration, exudative AMD, geographic atrophy
Introduction
Age-related macular degeneration (AMD) is the leading cause of blindness in the world.1,2,3,4 Data from population-based epidemiological studies have shown considerable variation in the prevalence of neovascular AMD and geographic atrophy among different racial/ethnic groups.5,6,7,8 We have in previous studies6,9,10,11, presented results suggesting that the geographic atrophy may be more common in Iceland than in other white populations. These estimates were based on rather small numbers in the oldest age-groups. Because treatment approaches and risk of visual impairment differ, precise prevalence estimates based on larger numbers of persons over 80 years of age are lacking and needed to understand the burden of different types of advanced AMD in the very old.12 In the western world, this age-group is expected greatly to increase in numbers in coming years. The purpose of this article is to report prevalence of early and late signs of AMD in this large and relatively old cohort, and to compare the prevalence with age specific estimates from other populations.
Material and Methods
The Age, Gene/Environment Susceptibility (AGES) Reykjavik Study is a population based study aimed to investigate genetic and environmental factors contributing to health, disability and disease in older people, including systemic disease as well as eye disease. The study design and assessment of the cohort have been described elsewhere.13, 14 In 2002, when the AGES Reykjavik Study began, 11,549 previously examined cohort members, from the Icelandic Heart Association's Reykjavik cohort (1967–1996), were still alive according to the Icelandic Census Database, and 5,764 individuals randomly chosen from the survivors, were examined for the AGES – Reykjavik Study during 2002–2006.15 The comprehensive AGES protocol required each participant to complete 3 visits to the Icelandic Heart Association (IHA) Research Center, within a window of 3–6 months.13 The ocular component was included as part of the third visit where 5,330 persons participated. As a part of the assessments at the IHA Research Center a questionnaire was administered, a clinical examination performed and images were acquired from the retina and brain.14 Transport to the IHA clinic was provided for those who asked for it, including persons in nursing homes, care units for the elderly and hospitals. In these latter institutions 251 persons (4.7%) were bedridden and unwilling or unable to visit the clinic, gave an interview, did however not have eye examination. The AGES – Reykjavik Study was approved by the Icelandic National Bioethics Committee (VSN: 00–063), which acts as the Institutional Review Board for the Icelandic Heart Association, and by the Institutional Review Board for the US National Institute of Ageing, National Institute of Health.
Fundus Photography
Fundus photography was performed using a standardized protocol that has been described in detail elsewhere.16,17,18 In brief, after pharmacologic dilation of the pupils, photography was performed in each eye using a 45° 6.3-megapixel digital nonmydriatic camera (Canon, Lake Success, NY). Two photographic fields were taken of each eye, the first centered on the optic disc and the second centered on the fovea. Software was used for image acquisition and archiving (Eye QSL, Digital Healthcare Inc., Cambridge, United Kingdom).
Fundus Image Grading
Retinal images were evaluated by the University of Wisconsin Ocular Epidemiology Reading Center for assessment of AMD in a semiquantitative fashion by a grader masked to any information about the participant using EyeQ Lite (an image-processing database for storage, retrieval, and manipulation of digital images), using a standard AMD grading protocol18, including the modified Wisconsin age-related maculopathy grading system used in the Multi Ethnic Study of Atherosclerosis.5 Among the AMD features evaluated were drusen size, type, and area; increased retinal pigment; retinal pigment epithelial depigmentation; pure geographic atrophy; and signs of exudative macular degeneration (subretinal hemorrhage, subretinal fibrous scar, retinal pigment epithelial detachment, and/or serous detachment of the sensory retina or laser or photodynamic treatment for neovascular AMD). Each image was graded twice (preliminary and detail grades) online using a modification of the Wisconsin Age-Related Maculopathy Grading scheme.17 Every digitized image was graded using the full complement of image enhancement tools (e.g., magnification, contrast enhancement, lightening, red-free) according to preset protocols. However, final scoring of an AMD lesion required its appearance on the nonenhanced image.
If the preliminary and detail gradings agreed (73.2%), the grading was considered final. If there was disagreement between gradings for a lesion, the image was sent to a third (edit) grader for reevaluation for that lesion without knowing what the specific disagreement was (25.9%). The preliminary, detail, and edit gradings were compared again for agreement. If the third grading agreed with either the preliminary or the detail grading, that grading was considered final. If a disagreement was present among all 3 graders, the image was evaluated by the Reading Center co-director (RK), whose grading was used (0.9%). Nine different graders performed the gradings.
Definitions of Variables
Soft distinct drusen were defined by size (usually between ≥63 and 300μm in diameter) and appearance (sharp margins and a round nodular appearance with a uniform density [color] from center to periphery). Soft indistinct drusen were the same size as the soft distinct, but have indistinct margins and a softer, less solid appearance. Increased retinal pigment appears as a deposition of granules or clumps of gray or black pigment in or beneath the retina. Retinal pigment epithelium (RPE) depigmentation is characterized by faint grayish–yellow or pinkish–yellow areas of varying density and configuration without sharply defined borders. Early AMD was defined by either the presence of any soft drusen (distinct or indistinct) and pigmentary abnormalities (either increased retinal pigment or RPE depigmentation) or the presence of a large soft drusen ≥125 μm in diameter with a large drusen area (>500 μm-diameter circle) or large (≥125 μm in diameter) soft instinct drusen in the absence of signs of late AMD. Late AMD was defined by the presence of any of the following: geographic atrophy or exudative AMD including at least one of the following: pigment epithelial detachment, subretinal hemorrhage or visible subretinal new vessel, or subretinal fibrous scar or laser treatment scar for AMD. When two eyes of a participant were discrepant for the severity of a lesion, the grade assigned for the participant was that of the more severely involved eye. For example, in assigning the prevalence of soft drusen, if soft drusen were present in one eye but not in the other eye, the participant was considered to have soft drusen. When drusen or signs of AMD could not be graded in an eye, the participant was assigned a score equivalent to that in the other eye.
Quality Control
Intergrader and intragrader agreements were assessed using the kappa statistic on a random subset of images of 25 eyes. There were excellent interobserver and intraobserver agreements among the 9 graders on the 3-level AMD classification (kappa statistic _0.88 to 1.0 and 0.82 to 1.0, respectively) and for exudative AMD (kappa statistic 0.88 to 1.0 for both). There were substantial interobserver and intraobserver agreements on presence of large drusen size (kappa statistic 0.76 to 1.0 and 0.71 to 1.0, respectively), soft distinct drusen (kappa statistic 0.75 to 1.0 and 0.61to 1.0, respectively), increased retinal pigment (kappa statistic 0.63 to 0.92 and 0.50 to0.85, respectively), and geographic atrophy (kappa statistic 0.53 to1.0 and 0.52 to1.0). There were moderate interobserver and intraobserver agreements on the presence of RPE depigmentation (kappa statistic 0.47 to 0.87 and 0.38 to 0.87, respectively).
Statistical analysis
For statistical analysis we used SPSS (version 13.0, 2004, SPSS Inc. Chicago, Illinois, USA). The prevalence of age-related maculopathy was calculated in 5-year age groups with 95% confidence intervals, using descriptive statistics. Chi-square and logistic regression analysis were used to compare differences in odds ratio and control for possible interactions and confounding variables.
Results
Of the 5,330 persons invited for eye examination 5,272 had readable photographs for Age-related macular degeneration (AMD) in at least one eye (98.9%).
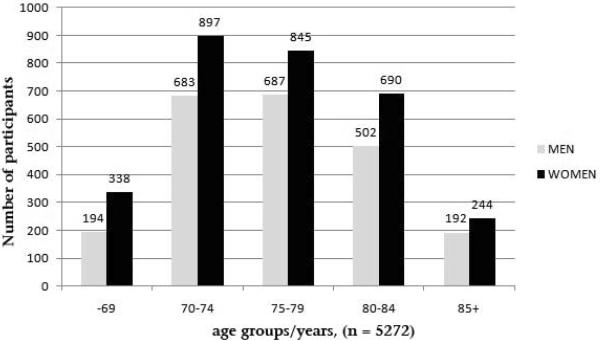
Figure 1 shows the number of participants with readable photographs from at least one eye, by age-groups and gender. The mean age was 76 years (SD +/− 6, range 66–91 years). Women represented 58% of the cohort. There were 3,160 participants, 75 years or older and 1,628 persons, 80 years and older who had readable photographs. Figure 2 displays for comparison the percentage, by sex and birth cohort, of the surviving members of the IHA's Reykjavik cohort in 2002, the AGES participants in 2004 (the median year of the study), and residents of comparable age, living in the greater Reykjavik area in 2004.
Figure 1.
Number of persons with readable photographs in at least one eye by age-groups and gender. Age, Gene/Environment Susceptibility Study - Reykjavik
Figure 2.
The age distribution, by sex and birth cohort, of members of the original Reykjavik Study cohort alive in 2002, the Age, Gene/Environment Study (AGES) participants, and of all residents of comparable age living in the greater Reykjavik area in 2004.
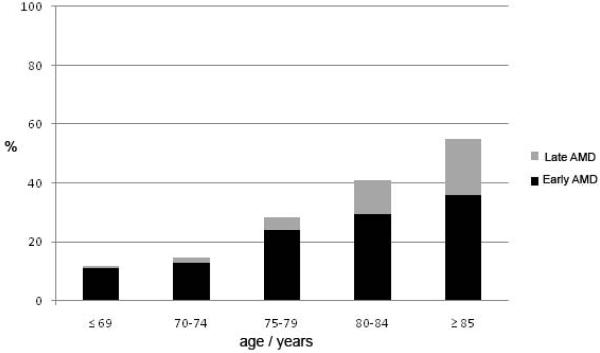
The frequency of various AMD lesions was highest in the oldest age group (Tables 1–3, Figure 3). Table 1 presents the age specific prevalence of various AMD lesions which do not differ significantly by gender, while there was a significant gender difference for phenotypes reported in tables 2 and 3. Drusen larger than 125μm were found in 18.1% of persons 70–74 year old and in 52.7% of those 85 years and older (table 1) a statistically significant increase with age (Odds Ratio (OR)=1.12; 95% confidence interval [CI] 1.10–1.13, p<.0001). Soft distinct drusen were found in 24.3% of those 70–74 years old and 60.5% of those 85 years and older. Overall age was significantly related to the risk of having soft indistinct drusen (OR=1.12; 95% CI 1.10–1.13, p<.0001) (table 2) and having soft drusen area larger than 500ȝm in worse eye (OR = 1.14; 95% CI 1.12–1.15, p<.0001)(table 2). After controlling for age, females had a significantly higher prevalence than males of soft indistinct drusen (OR=1.18; 95% CI 1.01–1.37, p=0.032) and soft drusen area larger than 500μm (OR=1.22; 95% CI 1.05–1.43, p= 0.012) (table 2). There was no significant gender difference for pigmentary abnormalities, nor for right and left eyes.
Table 1.
Prevalence of various phenotypes of age-related macular degeneration by age and worse eye, sexes combined. (95% confidence intervals)
| Age/years | Drusen ≥ 125 μm | Soft distinct drusen | Increased pigment | Retinal pigment epithelial depigmenation | Pigmentary abnormalities | Early age-related macular degeneration |
|---|---|---|---|---|---|---|
| 66–69 | 15.1% (11.9–18.2) | 17.5% (14.1–20.8) | 9.5% (7.0–12.1) | 3.8% (2.1–5.4) | 9.5% (7.0–12.1) | 10.9 (8.1–13.6) |
| 70–74 | 18.1% (16.2–20.1) | 24.3% (22.1–26.5) | 10.1% (8.5–11.6) | 4.8% (3.7–5.9) | 10.2% (8.7–11.7) | 13.0 (11.2–14.7 |
| 75–79 | 32.5% (30.1–35.0) | 41.7% (39.2–44.3) | 20.7% (18.6–22.8) | 10.8% (9.1–12.4) | 20.7% (18.6–22.8) | 23.9 (21.6–26.2) |
| 80–84 | 44.4% (41.4–47.3) | 53.9% (51.0–56.9) | 29.7% (27.0–32.4) | 17.9% (15.6–20.2) | 29.9% (27.1–32.6) | 29.5 (26.7–32.3) |
| ≥85 | 52.7% (47.7–57.7) | 60.5% (55.6–65.4) | 45.9% (40.9–50.8) | 28.6% (24.0–33.1) | 46.1% (41.1–51.1) | 36.0 (30.9–41.1) |
| Total | 30.7% (29.3–31.9) | 38.1% (36.8–39.5) | 20.3% (19.2–21.4) | 11.2% (10.3–12.1) | 20.4% (19.3–21.5) | 21.3 (20.1–22.5) |
| (p-values for age | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001) |
Table 3.
Prevalence (%) of Exudative Age-related Macular Degeneration and pure Geographic Atrophy by worse eyes and sex in 5 year age-groups (95% confidence intervals)
| Exudative AMD* | Pure Geographic Atrophy | |||||
|---|---|---|---|---|---|---|
| Age | Males | Females | Sexes combined | Males | Females | Sexes combined |
| ≤69 | 0.5% (0.0–1.6) | 0.6% (0.0–1.5) | 0.6% (0.0–1.3) | 0.0% (n.a.) | 0.3% (0.0–0.9) | 0.2% (0.0–0.6) |
| 70–74 | 0.6% (0.0–1.2) | 1.3% (0.5–2.1) | 1.0% (0.5–1.5) | 0.2% (0.0–0.5) | 0.7% (0.1–1.3) | 0.5% (0.1–0.8) |
| 75–79 | 1.5% (0.6–2.5) | 3.1% (1.9–4.39 | 2.4% (1.6–3.2) | 1.9% (0.8–2.9) | 1.9% (0.9–2.8) | 1.9% (1.2–2.6) |
| 80–84 | 6.3% (4.0–8.5) | 6.0% (4.1–7.8) | 6.1% (4.7–7.5) | 4.4% (2.4–6.3) | 5.8% (4.0–7.7) | 5.2% (3.9–6.6) |
| ≥85 | 11.4% (6.7–16.2) | 11.3% (7.0–15.6) | 11.4% (8.2–14.5) | 9.0% (4.5–13.6) | 6.4% (2.9–9.9) | 7.6% (4.8–10.4) |
| Total | 3.0% (2.3–3.7) | 3.5% (2.9–4.2) | 3.3% (2.8–3.8) | 2.2% (1.6–2.9) | 2.5% (1.9–3.1) | 2.4% (2.0–2.8) |
| (p-values for age) | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 |
= Age-related macular degeneration
Figure 3.
Prevalence (%) of early (black column) and late age-related macular degeneration (AMD) (gray column) by age groups (n=5272).
Table 2.
Prevalence (%) of soft indistinct drusen and soft drusen area larger than 500μ in worse eyes by sex and 5 year age-groups (95% confidence intervals)
| Soft indistinct drusen | Soft drusen larger than 500μ | |||||
|---|---|---|---|---|---|---|
| Age/years | Males | Females | Sexes combined | Males | Females | Sexes combined |
| ≤69 | 9.3% (5.1–13.6) | 7.5% (4.6–10.3) | 8.1% (5.7–10.5) | 6.0% (2.5–9.4) | 5.0% (2.6–7.4) | 5.4% (3.4–7.3) |
| 70–74 | 8.9% (6.7–11.1) | 9.8% (7.8–11.8) | 9.4% (7.9–10.9) | 6.4% (4.5–8.3) | 8.4% (6.5–10.3) | 7.5% (6.2–8.9) |
| 75–79 | 17.5% (14.6–20.4) | 20.6% (17.8–23.5) | 19.2% (17.2–21.3) | 16.6% (13.7–19.4) | 19.6% (16.8–22.4) | 18.2% (16.2–20.2) |
| 80–84 | 24.1% (20.2–28.0) | 31.8% (28.1–35.4) | 28.5% (25.9–31.2) | 24.1% (20.2–28.0) | 31.3% (27.7–35.0) | 28.3% (25.6–31.0) |
| ≥85 | 39.8% (32.4–47.2) | 35.1% (28.6–41.6) | 37.2% (32.3–42.0) | 37.9% (30.6–45.2) | 34.4% (28.0–40.9) | 36.0% (31.2–40.8) |
| Total | 17.4% (15.8–19.0) | 19.5% (18.0–20.9) | 18.6% (17.5–19.7) | 15.9% (14.4–17.5) | 18.3% (16.9–19.7) | 17.3% (16.2–18.3) |
| (p-values for age) | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 | < .0001 |
Table 3 shows a steep increase in prevalence of exudative AMD by age, sex and worse eye. In the age-group 70–74 year old, 1% is affected by exudative AMD, increasing to 11.4% among those 85 years and older. There is no significant gender difference.
Table 3 also includes pure geographic atrophy by age, sex and worse eyes excluding persons with exudative macular degeneration in the contralateral eye or concurrently in the same eye, both being included in the exudative AMD group only. Here there is an increase from 0.5% for those 70–74 years old to 7.6% for those 85 years and older. If however all persons with GA in either eye are included irrespective of also having exudative AMD the respective prevalence for GA is 1.2% for those 70–74 year old and 11.3% for those 85 years and older.
Unilateral exudative AMD was observed in 118 persons (163 eyes), 65 in the right eye, 53 in the left eye and 45 had bilateral AMD. GA was observed in 188 persons, 105 people had unilateral GA and 83 had GA in both eyes. Of these, 142 persons had pure GA, (89 unilateral and 53 bilateral). The prevalence of exudative AMD and pure GA is presented in table 3.0.2% of the participants and 1% of those 85 years and older had received laser and/or photodynamic therapy in at least one eye prior to their AGES Study visit.
Examining the relationships between pigmentary abnormalities and different drusen types and sizes we find that this relationship is strong and varies little between subtypes. An eye with increased pigment is significantly more likely to have drusen larger than 125μm (OR = 10.91; 95% CI 9.09–13.10, p<.0001), soft distinct drusen (OR = 9.40; 95% CI 7.78–11.35, p<.0001), or soft indistinct drusen (OR = 14.57; 95% CI 11.97–17.74, p<.0001). Eyes with RPE depigmentation are significantly more likely to have drusen larger than 125μm (OR = 11.10; 95% CI 8.81–13.99, p<.0001), to have soft distinct drusen (OR = 7.77; 95% CI 6.14–9.84, p<.0001), or soft indistinct drusen (OR = 15.76; 95% CI 12.74–19.50, p<.0001). All these calculations were done controlling for the effects of age and sex. We do not find similar significant associations between pigmentary abnormalities and drusen area large than 500μm.
Discussion
In this population based study of 5,272 old Caucasian adults, we found a steep age-related increase in the prevalence of late stages of AMD, including 277 persons with late AMD in at least one eye. The large size of our study ensured narrow 95% confidence intervals around the prevalence rates by 5-year age-groups. Our emphasis is on the oldest age-groups where population based estimates of prevalence of AMD are most limited.12 Comparisons of estimates of AMD prevalence with other studies are limited because of differences among studies age-group cut points and methods used to detect and define AMD.
Our data for late AMD for those ≥ 70 years seem higher than those reported in most population-based studies.7, 19, 20, 21 To our knowledge the only study outside of Iceland, found to have higher age specific prevalence of late AMD than the present study is a study of Greenland Inuits. A limitation of the Inuit study is the small numbers in the oldest age-groups.22 Pooled data from the United States of America (USA) finds GA to be about one third less prevalent than exudative AMD in the age-group examined23, other European, USA and Australian studies found exudative AMD twice as common as pure geographic atrophy,19,20, 24–26 and an Asian Indian study found exudative AMD to be 83% of all late AMD.27 We speculate that Icelanders may be more likely to develop geographic atrophy than Indians, because they are less pigmented and possibly more vulnerable to age-related retinal pigment epithelial loss than Indians. The Reykjavik Eye Study (RES) in 1996 had main emphasis on early AMD and therefore more than two – thirds of the participants were 50–69 years old.6 Older participants were few in particular those 80 years and older. The RES found the prevalence of late AMD for those 70–79 year old to be 4.8% with wide confidence margins while the prevalence of the present study for this age-group is 2.9% (95% CI 2.2 – 3.5). In RES there were too few participants 80 years and older for statistical analysis. Considering late AMD, the proportion of geographic atrophy compared to exudative AMD is however higher in the oldest age-group in the present study affecting comparisons with other studies. In the age-group 75–84 years, exudative AMD outnumbers pure GA by nearly times 1.3. We use the term pure GA for the endstage of atrophy of RPE with no evidence ever of exudative or neovascular process. Exudative process like e.g., serous detachment may however settle down into GA with no residual of exudative AMD and in cross sectional study be misclassified. In our experience, this is however uncommon in this population. We do not use the term pure exudative since this category may include cases with (primary) GA in one eye or even in both eyes, if occurred before seen in this study, as well as exudative AMD. This so called mixed type of late AMD where GA precedes the exudative AMD was found in about 15% of persons with late AMD in one of our previous studies.28 In the present study 2.4% of persons have pure GA and additionally 1.4% have GA where exudative AMD is also found in the same or in the contra lateral eye totalling 3.8%. Prevalence of exudative AMD is 3.3% including those with both pure exudative AMD and some additionally concurrently with primary GA (mixed type). This suggests that the prevalence may be similar for both the late forms of AMD in this aged population. Less than 0.5% of the participants had received photodynamic and/or laser therapy. Examining the exudative AMD subphenotypes it seems that at least 2/3rds of those with exudative AMD are likely to have received anti-vascular endothelial growth factor (anti-VEGF) treatment had it been available at the time.
Prevalence of drusen ≥125μm by worse eye, sexes combined for persons 70–74 year old and 75–79 year old is 18.1% and 32.5%, respectively in the present study is about third higher than the rate reported in the European Eye (EUREYE) Study24 and the pooled USA Study23. Our study also seems to have higher prevalence for drusen ≥125 μm and soft indistinct drusen than the white participants in the Multi-Ethnic Study of Atherosclerosis (MESA Study).5 Our prevalence of drusen in this age range may, however, be more similar to the Proyecto Vision and Eye Research (VER) Study.25 Although women and men of comparable age appear to develop early or late AMD at about the same rate we found that women were significantly more likely than men to develop soft indistinct drusen and soft drusen area >500 μm although for the latter lesions, the numbers are rather low and cautious interpretation is called for. Significant gender differences were not found for the other drusen phenotypes. Increased pigmentation (17.5%), decreased pigmentation (8.5%) and any pigmentary abnormalities (17.5%) for persons 70–79 years seem about twice as high as those in Hispanics in the Proyecto VER25, the Los Angeles Latino Eye Study7 and the Rotterdam Study.26 The estimates in the present study are more similar to the findings of the Beaver Dam Eye Study20 and the Blue Mountains Eye Study19. After correcting for age and gender eyes with increased pigment have 10.9 fold increased risk of having drusen ≥125 μm, 9.4 fold increased risk of having distinct drusen and 14.6 fold increased risk for having indistinct drusen and the same numbers for decreased pigmentation are 11.1, 7.8 and 15.8, respectively. The prevalence of early age-related macular degeneration for those 70–79 years old in our study is 18.3% (95% CI 16.8–19.7) as compared to 8.4% in the Blue Mountains Eye Study19, 12.8% for the Los Angeles Latino Study7, 29.6% for the Beaver Dam Eye Study20 and for those 80 years and older the prevalence across the studies are 31.2% (95% CI 28.7–33.6), 14.7%, 27.1%, 39.7%, respectively. When comparing across different studies, it is important to keep mind differences in study sampling of the oldest old, in the acquisition of retinal images and classification of graded lesions. Some of the older studies used stereoscopic 30° films while more recent studies, including ours use digital imaging, which may contribute, in part, to differences of the estimated prevalence for some of the phenotypes.
Anti-VEGF treatment currently available for exudative AMD may leave dry scars similar in appearance to a non-exudative phenotype of AMD and this may make identification of phenotypes more specifically of exudative AMD even more difficult to discern in future population based studies of AMD.
In summary: persons 85 years and older have a 10-fold higher prevalence of late AMD than those aged 70–74 years. GA is found in approximately half of all those affected with late AMD, a somewhat higher proportion than in other white populations of comparable age. Given the frequency of the advanced form of AMD observed in this elderly cohort of Icelanders, together with an increased life expectancy witnessed around the globe one may anticipate that the global burden AMD will increase in coming years. An improved understanding of the factors predictive of the development of specific AMD lesions, will take on increasing importance when developing new therapeutic strategies and novel treatment options.
Acknowledgments
Financial support: This research was supported by the NIH (Intramural Research Program of the National Institute on Ageing and the National Eye Institute, Z01-EY00401 NIH contract number N01-AG-1-2100), the Icelandic Heart Association, the Icelandic Parliament and the University of Iceland Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial interest: None
References
- 1).Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in East Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 2).Gunnlaugsdottir E, Arnarsson A, Jonasson F. Prevalence and causes of visual impairment and blindness in Icelanders aged 50 years and older: the Reykjavik Eye Study. Acta Ophthalmol. 2008;86:778–85. doi: 10.1111/j.1755-3768.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 3).Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103:357–64. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 4).Klaver CC, Wolfs RC, Vingerling JR, et al. Age-specific prevalence and causes of blindness and visual impairment in an older population: the Rotterdam Study. Arch Ophthalmol. 1998;116:653–8. doi: 10.1001/archopht.116.5.653. [DOI] [PubMed] [Google Scholar]
- 5).Klein R, Klein BE, Knudtson MD, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the Multi-Ethnic Study of Atherosclerosis. Ophthalmology. 2006;113:373–80. doi: 10.1016/j.ophtha.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6).Jonasson F, Arnarsson A, Sasaki H, et al. The prevalence of age-related maculopathy in Iceland: Reykjavik Eye Study. Arch Ophthalmol. 2003;121:379–85. doi: 10.1001/archopht.121.3.379. [DOI] [PubMed] [Google Scholar]
- 7).Varma R, Fraser-Bell S, Tan S, et al. Los Angeles Latino Eye Study Group Prevalence of age-related macular degeneration in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1288–97. doi: 10.1016/j.ophtha.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 8).Bressler SB, Munoz B, Solomon SD, West SK, Salisbury Eye Evaluation (SEE) Study Team Racial differences in the prevalence of age-related macular degeneration: the Salisbury Eye Evaluation (SEE) Project. Arch Ophthalmol. 2008;126:241–5. doi: 10.1001/archophthalmol.2007.53. [DOI] [PubMed] [Google Scholar]
- 9).Jonasson F, Thordarson K. Prevalence of ocular disease and blindness in a rural area in the eastern region of Iceland during 1980 through 1984. Acta Ophthalmol Suppl. 1987;182:40–3. doi: 10.1111/j.1755-3768.1987.tb02587.x. [DOI] [PubMed] [Google Scholar]
- 10).Jonasson F, Arnarsson A, Peto T, et al. 5-year incidence of age-related maculopathy in the Reykjavik Eye Study. Ophthalmology. 2005;112:132–8. doi: 10.1016/j.ophtha.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 11).Arnarsson A, Sverrisson Th, Stefansson E, et al. Risk factors for five-year incident age-related macular degeneration: the Reykjavik Eye Study. Am J Ophthalmol. 2006;142:419–28. doi: 10.1016/j.ajo.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 12).Kawasaki R, Yasuda M, Song SJ, et al. The prevelance of age-related macular degeneration in Asians: a systemic review and meta-analysis. Ophthalmology. 2010;117:921–7. doi: 10.1016/j.ophtha.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 13).Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Qiu C, Cotch MF, Sigurdsson S, et al. Retinal and cerebral microvascular signs and diabetes: the Age Gene/Environment Susceptibility-Reykjavik Study. Diabetes. 2008;57:1645–50. doi: 10.2337/db07-1455. [DOI] [PubMed] [Google Scholar]
- 15).Qiu C, Cotch MF, Sigurdsson S, et al. Microvascular lesions in the brain and retina: the Age, Gene/Environment Susceptibility-Reykjavik Study. Ann Neurol. 2009;65:569–76. doi: 10.1002/ana.21614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Klein R, Davis MD, Magli YL, et al. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98:1128–34. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 17).Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–95. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 18).Klein R, Meuer SM, Moss SE, et al. Detection of age-related macular degeneration using a nonmydriatic digital camera and a standard film fundus camera. Arch Ophthalmol. 2004;122:1642–6. doi: 10.1001/archopht.122.11.1642. [DOI] [PubMed] [Google Scholar]
- 19).Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia: the Blue Mountains Eye Study. Ophthalmology. 1995;102:1450–60. doi: 10.1016/s0161-6420(95)30846-9. [DOI] [PubMed] [Google Scholar]
- 20).Klein R, Klein BE, Linton KL. Prevalence of age-related maculopathy: the Beaver Dam Eye Study. Ophthalmology. 1992;99:933–43. doi: 10.1016/s0161-6420(92)31871-8. [DOI] [PubMed] [Google Scholar]
- 21).Friedman DS, Katz J, Bressler NM, et al. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106:1049–55. doi: 10.1016/S0161-6420(99)90267-1. [DOI] [PubMed] [Google Scholar]
- 22).Andersen MV, Rosenberg T, la Cour M, et al. Prevalence of age-related maculopathy and age-related macular degeneration among the Inuit in Greenland: the Greenland Inuit Eye Study. Ophthalmology. 2008;115:700–7. doi: 10.1016/j.ophtha.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 23).Eye Diseases Prevalence Research Group Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–72. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 24).Augood CA, Vingerling JR, de Jong PT, et al. Prevalence of age-related maculopathy in older Europeans: the European Eye Study (EUREYE) Arch Ophthalmol. 2006;124:529–35. doi: 10.1001/archopht.124.4.529. [DOI] [PubMed] [Google Scholar]
- 25).Munoz B, Klein R, Rodriguez J, et al. Prevalence of age-related macular degeneration in a population-based sample of Hispanic people in Arizona: Proyecto VER. Arch Ophthalmol. 2005;123:1575–80. doi: 10.1001/archopht.123.11.1575. [DOI] [PubMed] [Google Scholar]
- 26).Vingerling JR, Dielemans I, Hofman A, et al. The prevalence of age-related maculopathy in the Rotterdam Study. Ophthalmology. 1995;102:205–10. doi: 10.1016/s0161-6420(95)31034-2. [DOI] [PubMed] [Google Scholar]
- 27).Krishnan T, Ravindran RD, Murthy GV, et al. Prevalence of early and late age-related macular degeneration in India: the INDEYE study. Invest Ophthalmol Vis Sci. 2010;51:701–7. doi: 10.1167/iovs.09-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Magnusson KP, Duan S, Sigurdsson H, et al. CFH Y402H confers similar risk of soft drusen and both forms of advanced AMD. [Accessed August 7, 2010];PLoS Med. 2006 3:e5. doi: 10.1371/journal.pmed.0030005. report online. Available at: http://www.plosmedicine.org/article/info%3Adoi%2F10.1371%2Fjournal.pmed.0030005. [DOI] [PMC free article] [PubMed]