Abstract
Objective
To assess the cross-sectional relation of planus foot morphology to ipsilateral knee pain and compartment-specific knee cartilage damage in older adults.
Methods
In the Framingham Studies, we adapted the Staheli Arch Index (SAI) to quantify standing foot morphology from pedobarographic recordings. We inquired about knee pain and read 1.5 Tesla MRIs using whole-organ magnetic resonance imaging scoring. Logistic regression compared the odds of knee pain among the most planus feet to the odds among all other feet, and estimated odds within categories of increasing SAI. Similar methods estimated the odds of cartilage damage in each knee compartment. Generalized estimating equations adjusted for age, sex, BMI, and non-independent observations.
Results
Among 1903 participants (mean age 65± 9 years; 56% female), 22% of knees were painful most days. Cartilage damage was identified in 45% of medial TF, 27% of lateral TF, 58% of medial PF, and 42% of lateral PF compartments. Compared with other feet, the most planus feet had 1.3 (95% CI: 1.1, 1.6) times the odds of knee pain (p=0.009), and 1.4 (95% CI: 1.1, 1.8) times the odds of medial TF cartilage damage (p=0.002). Odds of pain (ptrend=0.05) and medial TF cartilage damage (ptrend=0.001) increased linearly across categories of increasing SAI. There was no association between foot morphology and cartilage damage in other knee compartments.
Conclusion
Planus foot morphology is associated with frequent knee pain and medial TF cartilage damage in older adults.
Nearly a quarter of men and women over 55 years of age report knee pain on most days (1). At least half of these older adults have radiographic knee osteoarthritis (OA) (2), and many more exhibit signs of cartilage damage that are visible on MRI. Despite the high prevalence of knee OA, its etiology remains poorly understood.
Evidence suggests that many of the characteristic features of knee OA are related to mechanical loading (3). Excessive loading of the knee can result from factors that increase compressive and/or shear stress on the tibiofemoral (TF) or patellofemoral (PF) compartments. Much of the research has focused on the consequences of local knee malalignment (4–7). However, the foot plays an even more immediate role in absorbing the mechanical stresses of ground contact and sculpting the pattern of postural alignment and joint motion at the knee and throughout the lower extremity (8). Despite its central role in lower extremity biomechanics, little is known about the consequences of abnormal foot morphology (planus or cavus) for the risk of knee tissue damage or frequent knee symptoms.
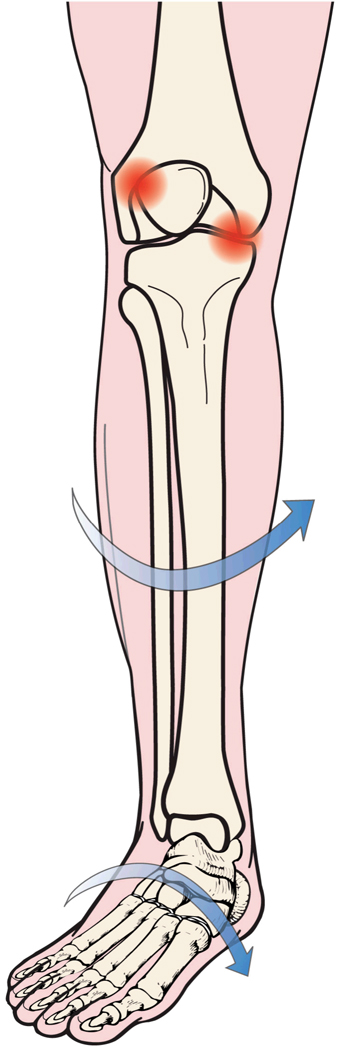
Planus foot morphology (“flat-footedness”) has been posited to contribute to both TF (9) and PF pathology (10, 11), and preliminary findings suggest that cases of older adults with medial TF OA may differ from age-matched controls in several common clinical indicators of flat-footedness in standing (12). During most weight bearing activities, the posture and motion of the foot and knee are coupled within a closed kinematic chain. Closed chain coupling may link excessively planus foot morphology to excessive internal rotation of the lower limb (13, 14). The consequences of this rotation are unknown, but it may have effects on mechanical stress across the knee, possibly resulting in increased rotational stress on the load bearing tissues of the TF compartments and increased contact between the articulating surfaces of the lateral patella and the lateral trochlea femoris (Figure 1). While the details of this biomechanical model remain speculative and three-dimensional knee kinematics can be difficult to infer from static morphologic measures alone, a growing body of evidence supports the basic premise that foot and knee mechanics are interdependent (14, 15). Their interdependence may contribute causally to some knee pathologies, including OA.
Figure 1.
Hypothesized relationship between standing planus foot morphology and increased postural stress on tibiofemoral and patellofemoral tissues.
Despite evidence suggesting a biomechanical link between excessively planus foot morphology and potential for mechanical stress on TF and PF compartments (13, 16), we are not aware of any studies that have investigated the relationship of planus foot morphology to the occurrence of frequent knee pain or cartilage damage in older adults. The implications of a detected relation of foot morphology to knee pain and cartilage damage are great, since it would then be conceivable that altering foot posture via appropriate shoes, arch supports, or foot orthoses might serve to reduce the risk of symptomatic OA in targeted knee compartments.
The purpose of this study was to assess the cross-sectional relationship of standing foot morphology to the prevalence of frequent knee pain and compartment-specific knee cartilage damage in a population-based sample of older adults. We hypothesized that planus foot morphology would be associated with knee pain and medial tibiofemoral and lateral patellofemoral cartilage damage.
Methods
Study Sample
The Framingham Foot and OA Studies consist of a large population-based sample of older adult male and female residents of Framingham, Massachusetts. Participants originate from two groups. The first is the Framingham Heart Study (FHS) Offspring cohort which consists of sons and daughters (and their spouses) of participants in the original FHS (17). In 1992–1993, as part of a study of the inheritance of osteoarthritis, we recruited members (and their spouses) of the Framingham Offspring cohort whose parents had been studied during osteoarthritis investigations in the original FHS. These offspring were evaluated again for osteoarthritis in 2002–2005 when the Framingham Foot Study was introduced into the examination.
A second group of participants was newly selected using random digit dialing and census tracts of the Framingham community. To increase awareness of the studies, community leaders, local television stations, and senior centers were informed and flyers were hung in public places within the town of Framingham. To be included, subjects had to be at least 50 years of age and ambulatory. Subjects with rheumatoid arthritis or other forms of inflammatory arthritis were identified and excluded using a validated screening questionnaire (18).
Foot Morphology
Plantar pressure recordings of foot morphology were obtained during relaxed bipedal standing using a TekScan MatScan pedobarographic device with MatScan 5.0 software (Tekscan, Inc. Boston, MA). Each subject was instructed to stand quietly on the pressure-sensing device with their body weight distributed equally over the two feet and their toes pointed ahead. A high resolution (1.4 sensors/cm2) digital recording was made of both footprints. From these recordings, standing foot morphology was quantified with the assistance of Matlab technical computing software (MathWorks, Inc. Natick, MA.) to calculate the Staheli Arch Index (SAI) (19). The SAI was originally developed using the chalk footprints of younger adults and children (19). Our use of the SAI was adapted in order to quantify foot morphology from the digital footprints of older adults. We label this as the Staheli Arch Index even though it is technically an adapted version. Validity of the SAI for quantifying standing foot morphology was previously established by comparison with the reference standard Chippaux-Smirak arch index (r = 0.99, p< 0.001) (20), and with goniometric measurements of standing rearfoot eversion (r = 0.5, p< 0.01) (21). Test-retest reliability of the SAI has been shown to be high (r = 0.96, p< 0.001), particularly when measurements are derived from standing rather than walking footprints (20).
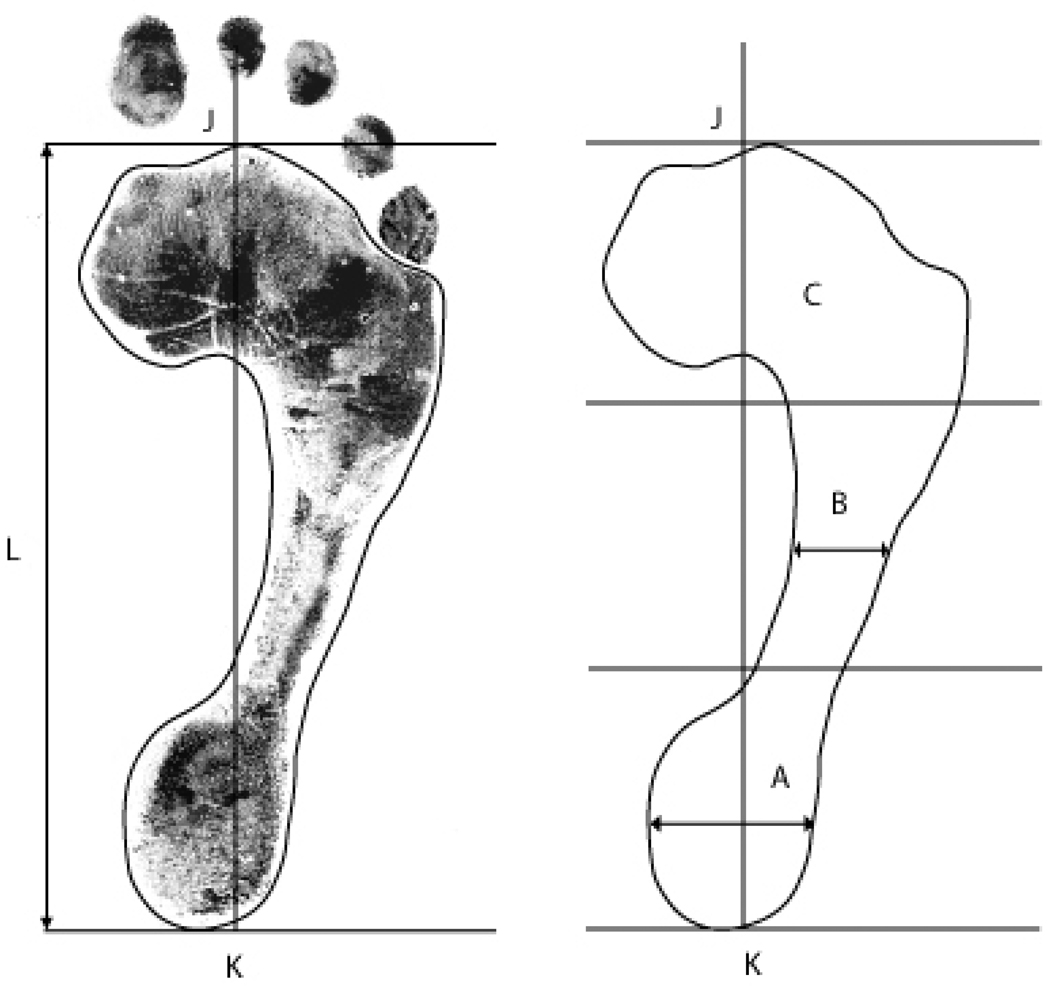
A single trained investigator (RHG) applied Matlab software to calculate the SAI from the dimensions of the acquired digital footprints. From the most distal extent of the forefoot (toes excluded) to the most proximal extent of the heel, the length of each footprint was divided into thirds in order to define the rearfoot (heel), midfoot (arch), and forefoot regions. The SAI is defined as the ratio of the smallest medial to lateral width of the arch region divided by the greatest medial to lateral width of the heel region (Figure 2) (19). Measurements were recorded to the nearest .01 centimeter. The value of the SAI increases with increasingly planus foot morphology and takes on a value of zero with cavus foot morphology.
Figure 2.
Measurement of foot morphology using the Staheli Arch Index (SAI). From the most distal extent of the forefoot (J) to the most proximal extent of the heel (K), the length of each footprint (L) is divided into thirds in order to define the heel (A), arch (B), and forefoot (C) regions. The SAI is defined as the ratio of the smallest medial to lateral width of the arch region divided by the greatest medial to lateral width of the heel region.
Knee Measures
1) Knee Pain: A written questionnaire asked participants to indicate whether they felt “pain, aching, or stiffness on most days” in either of their knees. If a participant answered affirmatively, then he or she was asked to indicate whether the symptoms were felt in the right, left, or both knees.
2) Knee Cartilage Damage: All imaging was performed with a 1.5 Tesla MRI scanner (Siemens, Mountain View, CA) using a phased array knee coil. Imaging sequences included: sagittal (TR 3610 ms, TE 40 ms, 3.5 mm slice thickness, 0 mm interslice gap, 32 slices, 256 × 256 matrix, 139 mm2 FOV, echo train length 7), axial (TR 3610 ms, TE 40 ms, 3.0 mm slice thickness, 0 mm interslice gap, 20 slices, 256 × 256 matrix, 139 mm2 FOV, echo train length 6), and coronal (TR 3610 ms, TE 40 ms, 3.5 mm slice thickness, 0 mm interslice gap, 30 slices, 256 × 256 matrix, 139 mm2 FOV, echo train length 7) intermediate-weighted turbo spin echo sequences with fat suppression. Because of cost constraints, MRIs were generally read for the right knee only.
Two experienced musculoskeletal radiologists (AG and FWR) used the Whole-Organ Magnetic Resonance Imaging Scale (WORMS) (22) to identify the presence of at least minimal cartilage damage (WORMS score ≥2 on a 0–6 ordinal scale) in each of 5 plates (anterior, central, and posterior tibia; and central and posterior femur) defining the medial and lateral TF compartments, and each of 2 plates (patella and trochlea femoris) defining the medial and lateral PF compartments. Among a randomly selected sub-sample of 170 knees across all 14 plates, inter-rater reliability in the identification cartilage damage was high (kappa = 0.75 (95% CI: 0.72, 0.78)).
Covariates
1) Age, Gender, BMI: Age, gender, and body mass index (BMI) were assessed in all study participants. BMI was calculated as weight in kilograms divided by height in square meters. Weight was measured, following removal of shoes and heavy clothing, to the nearest 0.50 pound (0.20 kilogram) using a balance beam scale. Height was measured to the nearest 0.25 inch (6.35 millimeter) using a stadiometer.
2) Knee Alignment: We obtained digitized bilateral long limb radiographs using an established protocol (4). Among a subgroup of participants who had been previously identified for inclusion in a planned case-control study of knee OA (23), a single experienced reader (ICC= 0.99 for intra-rater reliability) applied standardized methods (24) to measure the frontal plane alignment of the knee’s mechanical axis (Hip-Knee-Ankle or HKA alignment). Measurements were made to the nearest 1.0 degree using Efilm software (Merge Healthcare, Inc. Milwaukee, WI). Varus measurements were recorded as positive values, while valgus measurements were assigned negative values.
Analytic Methods
We created four categories of SAI. Category 1 included only cavus feet (SAI = 0), while categories 2–4 reflected the tertile distribution of SAI among non-cavus feet (SAI > 0). We used logistic regression to estimate the odds of ipsilateral knee pain and compartment-specific knee cartilage damage among feet with the most planus morphology (category 4) relative to all other feet (categories 1–3), and then to estimate the odds of knee pain and compartment-specific knee cartilage damage across categories of increasingly planus foot morphology (categories 2–4) relative to cavus feet only (category 1, SAI=0). We used the median SAI value within each category to perform a test for linear trend in the odds of each knee measure. In all analyses, we adjusted for age, sex, and BMI. Generalized Estimating Equations (GEE) were used to account for non-independence between two knees of a subject and multiple cartilage plates from a single knee compartment.
To determine whether any relation of foot morphology to knee measures was mediated by frontal plane knee malalignment, we conducted secondary analyses using the subset of limbs in which HKA alignment had been measured from long limb radiographs. In these analyses, we made additional adjustment for the presence of either varus (HKA > 2 degrees) or valgus (HKA < −2 degrees) knee malalignment.
Results
1903 participants in the Framingham Foot and OA Studies contributed 3782 foot morphology measurements, 3764 knee pain assessments, and 1099 knee cartilage damage assessments to the primary set of analyses. Among the 1903 participants, 56% were female. Their mean age was 65 ± 9 years. 22% of knees were reported as painful most days, while cartilage damage of at least minimal severity (WORMS ≥ 2) was present in 45% of medial TF, 27% of lateral TF, 58% of medial PF, and 42% of lateral PF compartments. The SAI ranged from 0 to 1.20 and had a median of 0.43 (interquartile range 0.14, 0.57). Study sample characteristics were comparable among those contributing information about knee pain and those contributing information about knee cartilage damage (Table 1).
Table 1.
Characteristics of the Study Population
| Knee Pain | Knee Cartilage Damage | |
|---|---|---|
| n = 1903 | n = 1100 | |
| Age mean ± sd | 64.6 ± 9.1 | 63.9 ± 8.8 |
| BMI mean ± sd | 28.6 ± 5.6 | 28.8 ± 5.8 |
| Sex % female | 55.7 | 57.6 |
| SAI median | 0.43 | 0.43 |
| (interquartile range) | (0.14, 0.57) | (0.19, 0.57) |
| N of knees with outcome (%) | 836 / 3764 (22.3) | TF medial 499 / 1099 (45.4) |
| lateral 299 / 1099 (27.2) | ||
| PF medial 639 / 1094 (58.4) | ||
| lateral 462 / 1096 (42.2) |
Compared with all other feet (SAI <0.57), feet with the most planus morphology (SAI ≥0.57) had 1.3 (95% CI: 1.1, 1.6) times the odds of frequent ipsilateral knee pain (p=0.009), and 1.4 (95% CI: 1.1, 1.8) times the odds of medial TF cartilage damage (p=0.002) (Table 2). Nearly a third (30%) of feet with the most planus morphology had ipsilateral knee pain on most days, and 29% had medial TF cartilage damage on MRI. In contrast, there was no association between planus foot morphology and cartilage damage in the lateral TF (p = 0.75), medial PF (p = 0.81), or lateral PF compartments (p = 0.77).
Table 2.
Knee pain, medial and lateral tibiofemoral (TF) and patellofemoral (PF) cartilage damage among the most planus feet and among all other feet
| Other Feet SAI = 0–0.56 |
Planus Feet 0.57–1.20 |
p-value | |
|---|---|---|---|
| Knee Pain | |||
| # of knees | 2699 | 1047 | |
| % painful | 20.6 | 30.4 | 0.009 |
| Adjusted OR* | 1.00 | 1.31 | |
| (95% CI) | (reference) | (1.07, 1.60) | |
| Cartilage Damage | |||
| Medial TF | |||
| # of plates | 3658 | 1786 | |
| % WORMS ≥2 | 18.9 | 29.0 | 0.002 |
| Adjusted OR* | 1.00 | 1.43 | |
| (95% CI) | (reference) | (1.14, 1.81) | |
| Lateral TF | |||
| # of plates | 3659 | 1781 | |
| % WORMS ≥2 | 10.1 | 12.1 | 0.75 |
| Adjusted OR* | 1.00 | 1.05 | |
| (95% CI) | (reference) | (0.78, 1.40) | |
| Medial PF | |||
| # of plates | 1427 | 689 | |
| % WORMS ≥2 | 44.4 | 49.1 | 0.81 |
| Adjusted OR* | 1.00 | 0.97 | |
| (95% CI) | (reference) | (0.77, 1.23) | |
| Lateral PF | |||
| # of plates | 1450 | 708 | |
| % WORMS ≥2 | 28.8 | 34.0 | 0.77 |
| Adjusted OR* | 1.00 | 0.96 | |
| (95% CI) | (reference) | (0.74, 1.24) |
Adjusted for age, sex, BMI
Across categories of increasingly planus foot morphology, there were linear increases in the odds of ipsilateral knee pain (p for linear trend = 0.05) and in the odds of ipsilateral medial TF cartilage damage (p for linear trend = 0.001), indicating a possible dose-response relationship between these measured foot and knee variables (Table 3). When comparing feet with the most planus morphology to feet with the least planus morphology (cavus feet, SAI=0), the odds of frequent knee pain were 1.4 times (95% CI: 1.1, 1.8) greater, and the odds of medial TF cartilage damage were nearly twice as great (1.8 times, 95% CI: 1.2, 2.5). In contrast, there was no linear association between increasingly planus foot morphology and the odds of cartilage damage in the lateral TF (p for linear trend = 0.98), medial PF (p for linear trend = 0.98), or lateral PF (p for linear trend = 0.66) compartments.
Table 3.
Knee pain, medial and lateral tibiofemoral (TF) and patellofemoral (PF) cartilage damage by categories of increasingly planus foot morphology
| Foot Morphology (Staheli Arch Index (SAI)) |
|||||
|---|---|---|---|---|---|
| Cavus SAI = 0 |
Planus 0.57–1.20 |
p for trend |
|||
| 0.01–0.33 | 0.34–0.56 | ||||
| Knee Pain | |||||
| # of knees | 745 | 1013 | 941 | 1047 | |
| % painful | 18.0 | 21.4 | 21.8 | 30.4 | 0.05 |
| Adjusted OR* | 1.00 | 1.14 | 1.03 | 1.39 | |
| (95% CI) | (reference) | (0.88, 1.47) | (0.78, 1.37) | (1.05, 1.84) | |
| Cartilage Damage | |||||
| Medial TF | |||||
| # of plates | 885 | 1351 | 1422 | 1786 | |
| % WORMS ≥1 | 15.0 | 18.2 | 21.9 | 29.0 | 0.001 |
| Adjusted OR* | 1.00 | 1.19 | 1.37 | 1.76 | |
| (95% CI) | (reference) | (0.82, 1.73) | (0.96, 1.96) | (1.24, 2.50) | |
| Lateral TF | |||||
| # of plates | 885 | 1344 | 1430 | 1781 | |
| % WORMS ≥1 | 10.2 | 9.0 | 11.0 | 12.1 | 0.98 |
| Adjusted OR* | 1.00 | 0.80 | 0.94 | 0.94 | |
| (95% CI) | (reference) | (0.51, 1.25) | (0.60, 1.49) | (0.62, 1.44) | |
| Medial PF | |||||
| # of plates | 350 | 520 | 557 | 689 | |
| % WORMS ≥1 | 40.9 | 45.6 | 45.4 | 49.1 | 0.98 |
| Adjusted OR* | 1.00 | 1.13 | 1.03 | 1.03 | |
| (95% CI) | (reference) | (0.81, 1.57) | (0.73, 1.45) | (.73, 1.45) | |
| Lateral PF | |||||
| # of plates | 354 | 533 | 563 | 708 | |
| % WORMS ≥1 | 27.1 | 28.3 | 30.2 | 34.0 | 0.66 |
| Adjusted OR* | 1.00 | 0.94 | 0.94 | 0.92 | |
| (95% CI) | (reference) | (0.66, 1.36) | (0.66, 1.35) | (0.64, 1.32) | |
Adjusted for age, sex, BMI
18% of participants in this study (337 / 1903) underwent bilateral assessment of standing frontal plane knee alignment (radiographic HKA alignment). Members of this subgroup were of similar mean age (65.1 vs. 64.4 years) and gender composition (56.7% vs. 55.5% female), but had a slightly higher mean BMI (29.2 vs. 28.5, p = 0.03) when compared to participants whose knee alignment was not assessed. The subgroup contributed 656 limbs to a secondary analysis of the relation of planus foot morphology to knee pain, and 312 limbs to a secondary analysis of the relation of planus foot morphology to knee cartilage damage, with additional adjustment in each analysis for the presence of either varus (HKA > 2 degrees, present in 48% of 656 limbs) or valgus (HKA < −2 degrees, present in 8% of 656 limbs) knee malalignment. In all instances, additional adjustment for knee malalignment effected little or no dilution of the observed associations. The association of planus foot morphology with cartilage damage in the medial TF compartment remained highly linear (p for linear trend = 0.002), with feet in the highest category of planus morphology (SAI > 0.57) having 1.6 times (95% CI: 1.1, 2.2) the odds of ipsilateral cartilage damage as all other feet, and 3.0 times (95% CI: 1.6, 5.7) the odds of cartilage damage as feet with the least planus morphology (SAI = 0). A comparable, though statistically non-significant, trend was evident in the relationship of planus foot morphology to frequent knee pain. After additional adjustment for knee malalignment, the relative odds of frequent knee pain continued to rise across categories of planus foot morphology (p for linear trend = 0.22), with feet in highest category of planus morphology having 1.3 times (95% CI: 0.8, 1.96) the odds of frequent knee pain as all other feet, and 1.6 times (95% CI: 0.8, 3.3) the odds of knee pain as feet with the least planus morphology.
Discussion
These findings indicate that, among older adults, planus foot morphology is associated with a moderately increased prevalence of frequent knee pain and medial tibiofemoral cartilage damage. Because the mechanical consequences of flat feet are often correctable using foot orthoses (25, 26), these findings may have implications for the prevention and/or treatment of knee pain and cartilage damage in the older adult population.
It is important to note, however, that this study has limitations. The cross-sectional design of the study restricts our ability to infer the direction of causation in the observed associations. It is conceivable that structural damage in the knee, including cartilage damage and diminished medial TF joint space, is the primary cause of both planus foot morphology and frequent knee pain. Previous theorists (27, 28) have argued that the loss of medial TF joint space leads to varus knee malalignment, which in turn creates a demand for compensatory foot flattening in order to allow full plantar contact of the weight bearing foot with a horizontal ground. Contrary to this claim however, the results of this study’s secondary analyses indicate that the association of planus foot morphology with cartilage damage in the medial TF compartment is not dependent on the presence of varus knee malalignment. Moreover, trends in the data suggest that the association of planus foot morphology with frequent knee pain may be similarly independent of the presence of either varus or valgus knee malalignment. Overall, these findings do not support the proposed mechanism of reverse causation, wherein knee OA leads to knee malalignment and subsequent planus foot morphology as a compensatory posture.
Instead, these findings are more closely consistent with the predictions of biomechanical models (10, 11, 29) that underscore the transverse plane linkages between flat footedness and adverse knee loading. According to these models, excessive flattening of the weight bearing foot has potential to constrain the adjacent tibia to rotate internally (13, 14), possibly bringing about increased rotational stress on the load bearing tissues of the tibiofemoral joint (Figure 1). Because the greatest proportion of transverse plane rotation at the TF joint occurs in the medial compartment (30), if the predictions of these speculative models are correct, shear strain on the cartilage is likely to be greatest at this site.
On the other hand, the results of this study do not confirm the anticipated association of planus foot morphology with cartilage damage in the lateral PF compartment. Such an association might be expected if the postural alterations that accompany planus foot morphology also result in a tendency for the femur to accompany the tibia in internal rotation (14). Excessive femoral internal rotation, if it occurs, could cause the lateral trochlea femoris to abut against the lateral patella (Figure 1). However, it is also possible that any tendency for increased contact between the articulating surfaces of the lateral PF joint only results in excessive joint stress and cartilage damage when planus foot morphology is accompanied by more proximal bony malalignment. The combination of planus foot morphology with femoral anteversion, external tibial torsion, and an excessively valgus quadriceps angle (Q-angle) has been dubbed “miserable malalignment” (31, 32) because of the presumed risk that this combination of malalignments is supposed to imply for mechanical overload of the lateral PF joint. In the current study however, neither femoral anteversion, nor tibial torsion, nor Q-angle was assessed, making it impossible to discern whether planus foot morphology might confer additional risk for PF cartilage damage in the presence of any of these more proximal malalignments.
Compared with other indices of foot morphology (33), the Staheli Arch Index (SAI) has the advantage of a longer history of clinical and investigative use among males and females of various ages (21, 34, 35). However, the original SAI was developed using the chalk footprints of younger adults and children (19) while our use of the SAI was adapted in order to quantify foot morphology from the digital footprints of older adults. Among the older adults in the present study, the mean BMI was 29 kg/m2. Although the validity of the original SAI for quantifying foot morphology was established among younger and less overweight populations (20, 21), validity information is still lacking among older and more overweight adults. It is possible that the SAI is simply not able to accurately distinguish between the “fat feet” of overweight elders with excessive soft tissue in the midfoot region and the “flat feet” of more slender individuals whose foot morphology may confer an independent risk for suboptimal limb mechanics and knee pain. Although the present study’s analytic methods controlled for the possibility of confounding by BMI, no other effort was made to identify systematic errors in the measurement of foot morphology using the SAI.
A related concern is that the SAI, like other arch indices, does not provide a direct measure of rearfoot eversion which is an important element of foot pronation. While the mechanism of rearfoot eversion and lower limb internal rotation has been described by many, it is not altogether clear that people with planus foot morphology also demonstrate greater rearfoot eversion. It may be possible to exhibit midfoot pronation without excessive rearfoot eversion. On the other hand, validity of the SAI for quantifying foot morphology was previously established by comparison with both a reference standard arch index (20) and with goniometric measurements of standing rearfoot eversion (21). The results of the latter validity study indicate a moderate and highly significant correlation (r = 0.5, p<0.01) between SAI measurements of foot morphology and goniometric measurements of rearfoot eversion.
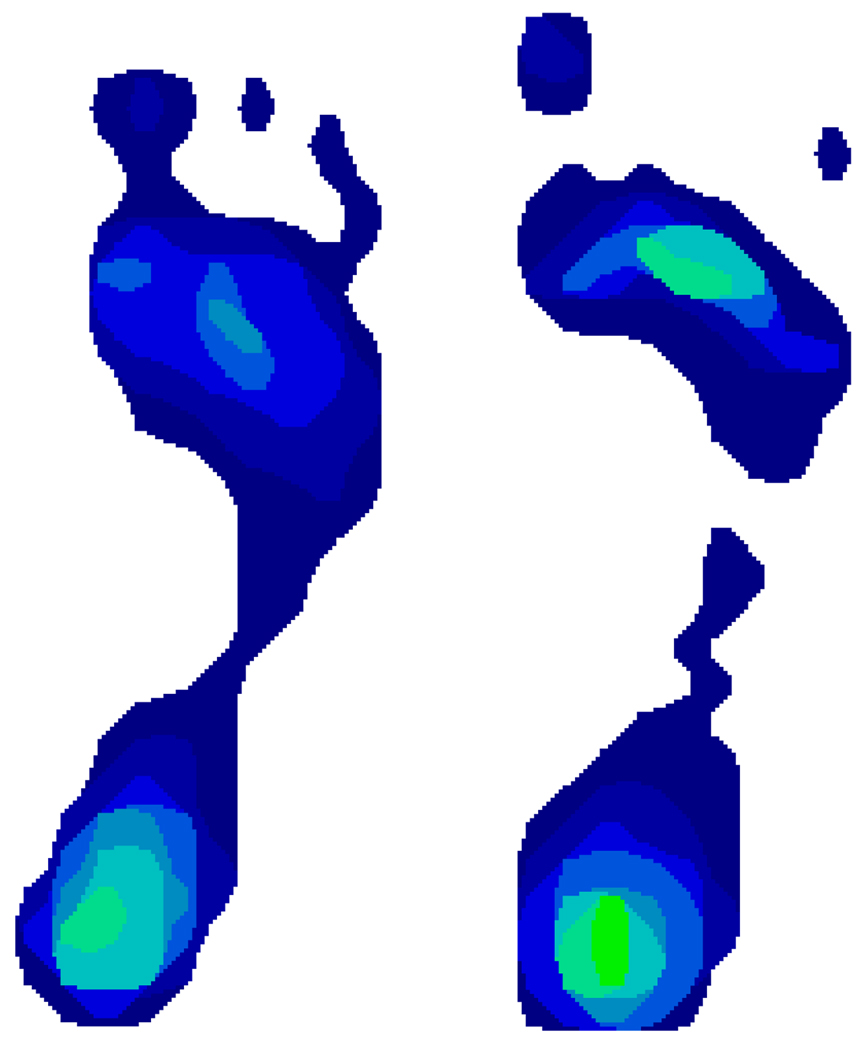
A final limitation of the SAI is that it records a zero value at the onset of cavus foot morphology (when, at the narrowest width of the midfoot, no pressure sensors have been stimulated above threshold) (Figure 3). Because of this “floor effect” in the measurement of foot morphology using the SAI, the data in this study cannot usefully inform an assessment of the possible risk for knee pain and cartilage damage that may be conferred by an excessively “high-arched” or cavus foot. It is conceivable that a more discriminating foot morphology index could reveal that the actual relationship of foot morphology to knee pain and cartilage damage is “J-shaped” rather than linear. In other words, this study is not able to discern whether there is an increased risk of knee pain and cartilage damage among both extremely planus and extremely cavus feet.
Figure 3.
The high-arched foot on the left still exerts sufficient pressure in the narrowest portion of the arch to activate several sensors. This foot was categorized among those whose SAI was between 0.01 and 0.33. In contrast, the narrowest portion of the arch on the right includes not even a single activated sensor. This cavus foot was categorized among those with an SAI equal to zero.
A strength of this study was that it leveraged information from a large sample of the general population of older adults in order to obtain precise estimates of the association between planus foot morphology and prevalent knee outcomes. Moreover, the arch index (SAI) used to quantify foot morphology in this study was, as indicated previously, originally proposed for use with chalk footprints. Therefore, it is possible that the index could lend itself well to adapted use in clinical settings in which digital pedobarographic devices are not available. Where a highly planus foot morphology is identified in an older adult whose ipsilateral knee is at-risk for frequent pain or cartilage damage, clinicians are advised to take this study’s findings into consideration.
While more definitive advice must await longitudinal confirmation of this study’s cross-sectional findings, the results nevertheless pique an interest in the possible role of supportive shoes, corrective arch supports, and compensatory foot orthoses in the prevention and/or treatment of knee disorders among flat-footed older adults. Given the comparative safety and affordability of these non-pharmacological, non-invasive interventions to correct planus foot morphology, future trials are urgently needed.
In summary, we have shown an association of planus foot morphology with frequent knee pain and medial tibiofemoral cartilage damage in older adults.
Acknowledgments
The Framingham Osteoarthritis Study was supported by the NIH grants AR47785 and AG18393. The Framingham Foot Study was supported by NIH grant AR47853. This work was derived from the Framingham Heart Study of the National Heart Lung and Blood Institute of the NIH (Contract No. N01-HC-25195) and Boston University School of Medicine. Dr. Gross’s contribution to this study was funded by a New Investigator Award from the Arthritis Foundation.
References
- 1.Lawrence R, Helmick C, Arnett F, Deyo R, Felson D, Giannini E, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Felson DT. Preventing knee and hip osteoarthritis. Bull Rheum Dis. 1998;47(7):1–4. [PubMed] [Google Scholar]
- 3.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93(1):1–24. xv. doi: 10.1016/j.mcna.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Sharma L, Song J, Felson DT, Cahue S, Shamiyeh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. Jama. 2001;286(2):188–195. doi: 10.1001/jama.286.2.188. [DOI] [PubMed] [Google Scholar]
- 5.Cahue S, Dunlop D, Hayes K, Song J, Torres L, Sharma L. Varus-valgus alignment in the progression of patellofemoral osteoarthritis. Arthritis Rheum. 2004;50(7):2184–2190. doi: 10.1002/art.20348. [DOI] [PubMed] [Google Scholar]
- 6.Kalichman L, Zhang Y, Niu J, Goggins J, Gale D, Zhu Y, et al. The association between patellar alignment on magnetic resonance imaging and radiographic manifestations of knee osteoarthritis. Arthritis Res Ther. 2007;9(2):R26. doi: 10.1186/ar2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter DJ, Zhang Y, Niu J, Tu X, Amin S, Goggins J, et al. Structural factors associated with malalignment in knee osteoarthritis: the Boston osteoarthritis knee study. J Rheumatol. 2005;32(11):2192–2199. [PubMed] [Google Scholar]
- 8.Willliams DSMI, Hamill J, Buchanan TS. Lower Extremity Kinematic and Kinetic Differences in Runners With High and Low Arches. Journal of Applied Biomechanics. 2001;17(2):153–163. [Google Scholar]
- 9.Williams DS, 3rd, McClay IS, Hamill J. Arch structure and injury patterns in runners. Clin Biomech (Bristol, Avon) 2001;16(4):341–347. doi: 10.1016/s0268-0033(01)00005-5. [DOI] [PubMed] [Google Scholar]
- 10.Tiberio D. The effect of excessive subtalar joint pronation on patellofemoral mechanics: a theoretical model. J Orthop Sports Phys Ther. 1987;9:160–165. doi: 10.2519/jospt.1987.9.4.160. [DOI] [PubMed] [Google Scholar]
- 11.Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639–646. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- 12.Reilly A, Barker L, Shamley D, Sandall S. Influence of foot characteristics on the site of lower limb osteoarthritis. Foot Ankle Int. 2006;27(3):206–211. doi: 10.1177/107110070602700310. [DOI] [PubMed] [Google Scholar]
- 13.Nester CJ, Hutchins S, Bowker P. Shank rotation: A measure of rearfoot motion during normal walking. Foot Ankle Int. 2000;21(7):578–583. doi: 10.1177/107110070002100709. [DOI] [PubMed] [Google Scholar]
- 14.Souza TR, Pinto RZ, Trede RG, Kirkwood RN, Fonseca ST. Temporal couplings between rearfoot-shank complex and hip joint during walking. Clin Biomech (Bristol, Avon) 2010;25(7):745–748. doi: 10.1016/j.clinbiomech.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Lafortune MA, Cavanagh PR, Sommer HJ, 3rd, Kalenak A. Foot inversion-eversion and knee kinematics during walking. J Orthop Res. 1994;12(3):412–420. doi: 10.1002/jor.1100120314. [DOI] [PubMed] [Google Scholar]
- 16.Nigg BM, Cole GK, Nachbauer W. Effects of arch height of the foot on angular motion of the lower extremities in running. J Biomech. 1993;26(8):909–916. doi: 10.1016/0021-9290(93)90053-h. [DOI] [PubMed] [Google Scholar]
- 17.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5(4):297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 19.Staheli LT, Chew DE, Corbett M. The longitudinal arch. A survey of eight hundred and eighty-two feet in normal children and adults. J Bone Joint Surg Am. 1987;69(3):426–428. [PubMed] [Google Scholar]
- 20.Mathieson I, Upton D, Birchenough A. Comparison of footprint parameters calculated from static and dynamic footprints. The Foot. 1999;9(3):145–149. [Google Scholar]
- 21.Mathieson I, Upton D, Prior TD. Examining the validity of selected measures of foot type: a preliminary study. J Am Podiatr Med Assoc. 2004;94(3):275–281. doi: 10.7547/0940275. [DOI] [PubMed] [Google Scholar]
- 22.Amin S, LaValley MP, Guermazi A, Grigoryan M, Hunter DJ, Clancy M, et al. The relationship between cartilage loss on magnetic resonance imaging and radiographic progression in men and women with knee osteoarthritis. Arthritis Rheum. 2005;52(10):3152–3159. doi: 10.1002/art.21296. [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ, Niu J, Felson DT, Harvey WF, Gross KD, McCree P, et al. Knee alignment does not predict incident osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2007;56(4):1212–1218. doi: 10.1002/art.22508. [DOI] [PubMed] [Google Scholar]
- 24.Cooke TD, Sled EA, Scudamore RA. Frontal plane knee alignment: a call for standardized measurement. J Rheumatol. 2007;34(9):1796–1801. [PubMed] [Google Scholar]
- 25.Razeghi M, Batt ME. Biomechanical analysis of the effect of orthotic shoe inserts: a review of the literature. Sports Med. 2000;29(6):425–438. doi: 10.2165/00007256-200029060-00005. [DOI] [PubMed] [Google Scholar]
- 26.Nawoczenski DA, Cook TM, Saltzman CL. The effect of foot orthotics on three-dimensional kinematics of the leg and rearfoot during running. J Orthop Sports Phys Ther. 1995;21(6):317–327. doi: 10.2519/jospt.1995.21.6.317. [DOI] [PubMed] [Google Scholar]
- 27.Riegger-Krugh C, Keysor JJ. Skeletal malalignments of the lower quarter: correlated and compensatory motions and postures. J Orthop Sports Phys Ther. 1996;23(2):164–170. doi: 10.2519/jospt.1996.23.2.164. [DOI] [PubMed] [Google Scholar]
- 28.Gross KD, Hillstrom HJ. Noninvasive devices targeting the mechanics of osteoarthritis. Rheum Dis Clin North Am. 2008;34(3):755–776. doi: 10.1016/j.rdc.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Michaud TC. Foot orthoses and other forms of conservative foot care. 2nd ed. Newton, Massachusetts: self-published; 1997. [Google Scholar]
- 30.Freeman MA, Pinskerova V. The movement of the normal tibio-femoral joint. J Biomech. 2005;38(2):197–208. doi: 10.1016/j.jbiomech.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Earl JE, Vetter CS. Patellofemoral pain. Phys Med Rehabil Clin N Am. 2007;18(3):439–458. viii. doi: 10.1016/j.pmr.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Magee DJ. Orthopedic physical assessment. 4th ed. Philadelphia: Saunders; 2002. [Google Scholar]
- 33.Hawes MR, Nachbauer W, Sovak D, Nigg BM. Footprint parameters as a measure of arch height. Foot Ankle. 1992;13(1):22–26. doi: 10.1177/107110079201300104. [DOI] [PubMed] [Google Scholar]
- 34.Staheli LT. Evaluation of planovalgus foot deformities with special reference to the natural history. J Am Podiatr Med Assoc. 1987;77(1):2–6. doi: 10.7547/87507315-77-1-2. [DOI] [PubMed] [Google Scholar]
- 35.Staheli LT. Planovalgus foot deformity. Current status. J Am Podiatr Med Assoc. 1999;89(2):94–99. doi: 10.7547/87507315-89-2-94. [DOI] [PubMed] [Google Scholar]