Abstract
Objectives
Hypoxia has been theorized to play a role in overactive bladder (OAB) symptoms. This study was designed to test this hypothesis by studying how in vitro stretch of primary cultured bladder urothelial cells (BUC) from OAB subjects and asymptomatic subjects (NB) altered the expression of angiogenic factors: hypoxia-inducible factor 1 alpha subunit (HIF-1α), hypoxia-inducible factor 2 alpha subunit (HIF-2α), and vascular endothelial growth factor (VEGF).
Methods
HIF-1α, HIF-2α and VEGF mRNA expression were analyzed using real-time quantitative PCR (qPCR). Fluorescence activated cell sorting (FACS) was used to measure protein expression. Release of VEGF in the supernatant of stretched OAB and NB BUC was measured with ELISA.
Results
Stretching of OAB BUC increased expression of mRNA for HIF-1α, HIF-2α, and VEGF by 1.5 (p<0.01), 1.5 (p<0.01) and 3.5 fold (p<0.001) over unstretched OAB BUC. This augmentation was not detected comparing stretched NB with unstretched NB BUC. Using FACS quantitation, only HIF-2α found to be significantly increased (p<0.01). Measuring VEGF in supernatant revealed that stretched OAB released significantly more VEGF than non-stretched OAB BUC at multiple time points whereas stretched NB BUC did not release VEGF.
Conclusions
OAB BUC responded to stretch by expressing increased angiogenic markers HIF-1α, HIF-2α and/or VEGF, measured at the transcript and protein levels. This suggested that OAB BUC respond as if they were primed by hypoxia. The knowledge would add to the pathophysiologic understanding of OAB.
Keywords: hypoxia-inducible factor 1 alpha subunit, HIF-1, hypoxia-inducible factor 2 alpha subunit, HIF-2, vascular endothelial growth factor, VEGF, bladder urothelial cells, overactive bladder
INTRODUCTION
Overactive bladder (OAB) is defined by the International Continence Society (ICS) as urgency, with or without urge incontinence, usually with frequency, defined as urinating more than 8 times a day and nocturia. While OAB is a clinical syndrome, detrusor overactivity (DO) refers to involuntary bladder contractions during bladder filling detected with urodynamics [1]. OAB and DO are not necessarily synonymous, but often occur together. OAB is a common and costly problem. Population-based estimates suggest that OAB affects 12–17 % of adults in the United States and Europe [2–4] and compromises health related quality of life [5–7].
The etiology of OAB is still not clear. Traditionally the bladder urothelial cells (BUC), which form the bladder urothelium, are thought to only have barrier function which protects the bladder against the external environment. Nevertheless, recent studies demonstrate an active role for BUC in bladder sensory function [8], which suggests that BUC provide more than a protective function. Recent studies in guinea pigs show that DO reduced bladder blood supply and hypoxia changes the working conditions of the bladder and negatively affects its acute and long-term function [9–12]. In a rabbit model, DO leads to repeated cycles of hypoxia and re-oxygenation in the ischemic bladder and the production of oxidative products [13]. Other studies show that mechanical stretch of human bladder smooth muscle cells activates a gene program involved in paracrine signaling of angiogenesis, and that a mechanical overload changes the capillary density in the rat bladder wall [14].
We hypothesized that the bladder urothelium in OAB may be subject to hypoxia due to increased urinary frequency and/or DO. This symptomatic condition could lead to changes in the urothelium that can be studied in a cultured cell model. We further hypothesized that the OAB BUC may respond to stretch by increased production of several hypoxia growth and transcriptional factors including hypoxia-inducible factor 1 alpha subunit (HIF-1α), hypoxia-inducible factor 2 alpha subunit (HIF-2α) also known as endothelial PAS domain protein 1 (EPAS-1) and vascular endothelial growth factor (VEGF).
We studied the expression of HIF-1α, HIF-2α and VEGF in human OAB BUC in response to stretch. The knowledge that OAB BUC may respond as if they were hypoxic compared to normal BUC would add to the pathophysiologic understanding of OAB. Hopefully, with increased pathophysiologic understanding, novel treatment strategies to treat OAB may be developed.
MATERIALS AND METHODS
1. Definition of Normal and OAB Subjects for Bladder Biopsies and Biopsy Technique
This protocol was approved by our Institutional Review Board. The inclusion criteria for non-neurogenic OAB subjects were: female gender, age ≥ 18 years, urinary frequency >10 in 24 hours, ≥ 1 incontinent episode associated with urge per day, bladder pain and/or discomfort score of 0 on Likert (0–9) scale, and negative urinalysis dip and/or negative urine culture. Exclusion criteria for OAB cohort were: male gender, diagnosis of neurologic disease (stroke, MS, Parkinsons’ disease, spinal cord injury, etc.), bacterial cystitis, bladder calculi, current active ureteral or urethral calculi, genital herpes within past 12 months, history of uterine, cervical, vaginal or urethral cancer, symptomatic urethral diverticulum, history of cyclophosphamide use or any type of chemical cystitis, history of tuberculous cystitis, history of pelvic irradiation, history of benign or malignant bladder tumors, active vaginitis, pregnancy. The inclusion criteria for the asymptomatic normal (NB) subjects were: female gender ≥ 18 years of age, no voiding symptoms (AUA symptom score ≤ 7 out of max 35), no bladder pain and/or discomfort (0 on Likert 0–9 scale), denies any urinary incontinence, requirement for concomitant cystoscopy during pelvic surgery such as ureteroscopy, cystoscopy for microhematuria, retrograde pyelogram, hysterectomy, ureteral reimplantation, and negative urinalysis dip and/or negative urine cultures. The exclusion criteria for the normal (NB) subjects were identical to the OAB cohort exclusion criteria. Three OAB and three NB subjects were enrolled for this study.
Two random biopsies per subject were obtained using the cold cup biopsy technique. The technique of in vitro primary cell culture from cystoscopic biopsies was followed per previous publication [15].
2. Cell Stretch Procedure
The Flexercell International Corporation strain unit FX-4000 was used for the cell stretch experiments. A confluent culture of BUC was grown on special Bioflex culture plates (Flexcell Inc., NC.). The parameters were set as 1 second of stretch and 2 seconds of relaxation at 20% elongation according to previous published paper [15]. In addition, control conditions involved cells grown on Bioflex culture plates that were not stretched. Cell pellet was collected from the Bioflex plates and frozen at −20°C for later analysis.
3. Expression of HIF-1α, HIF2-α and VEGF mRNA using Real Time Quantitative PCR
Real-time quantitative RT-PCR (qPCR) was performed using SYBER®Green I Brilliant® Master Mix (Stratagene, La Jolla, CA) according to manufacturer instructions. Expression ratio was analyzed using 2−ΔΔCt method [16]. The primers used were as follows. HIF-1α: forward primer: 5′-CCCAATGGATGATGACTTCC-3′, reverse primer: 5′-TGGGTAGGAGATGGAGATGC-3′; HIF-2α: forward primer: 5′-GGCTTTTTGCCATCTGTGAT-3′, reverse primer: 5′-GCACTTGAAGGGCTAGCAAC-3′. VEGF: forward primer: 5′-CCTGGTGGACATCTTCCAGGAGTACC-3′, reverse primer: 5′-GAAGCTCATCTCTCCTATGTGCTGGC-3′; The PCR conditions were as follows: 94°C denaturing for 5 minutes, followed by 40 cycles of 94°C for 1 minute, 53°C for 1 minute and 72°C for 1 minute. GAPDH (212bp) served as an internal control, primers for GAPDH were: 5′-CtTCCTGCACCACCAACTGCTTAG-3′ and 5′-GATGACCTTGCCACAGCCTTG-3′. PCR products were visualized on 3.0% agarose gels and stained with ethidium bromide.
4. Flow Cytometry and Fluorescence Activated Cell Sorter (FACS) Analysis
FACS analysis was performed to determine the expression of HIF-1α, HIF-2α and VEGF in whole cell suspension preparations and the techniques followed were as published [17]. The antibodies used for FACS were: anti-HIF-1 α (1:1000), anti-HIF-2α (1:1000) and anti-VEGF (1:100). During FACS analysis, cells were analyzed with CellQuest (Becton Dickinson, Sunnyvale, California) software. For each run 5000 cells were collected. Signals were gated to exclude debris. Histograms of fluorescence intensity were plotted against cell numbers (events). The relative amount of fluorescence density was compared across all experimental conditions.
5. Detection of VEGF in Cell Supernatant using ELISA
Supernatants at 0, 8, 24, 48 and 96 hours during stretch were collected. Supernatant of RT4 cell line (purchased from ATCC) served as a positive control. VEGF ELISA was performed with the Quantikine human VEGF kit from R&D Systems, Inc. (Minneapolis, MN) according to manufacturer’s instructions. The VEGF standard curve was generated by serial dilution of a stock 2000 pg/ml solution of human VEGF under the same culture media conditions as used for the stretch experiment. The standard curve showed no interference of culture media with the detection of VEGF (the culture media contained no VEGF).
6. Statistical Analysis
All data were presented as means ± SEM. Paired and unpaired t-tests were used with p< 0.05 considered as statistically significant.
RESULTS
1. Expression of HIF-1α, HIF-2α and VEGF mRNA Transcripts in OAB and NB BUC
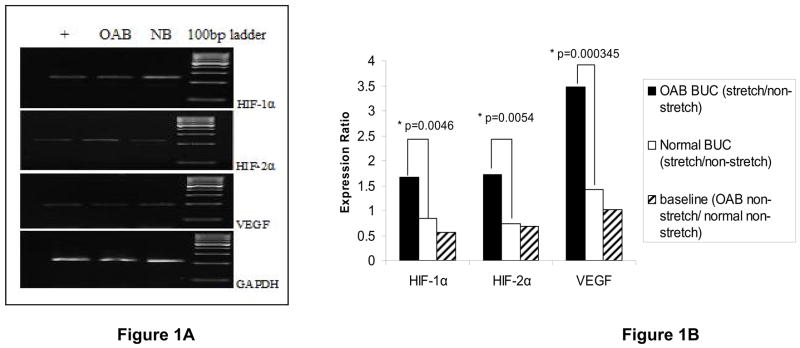
Specificity of RT-PCR products was documented with gel electrophoreses with the detection of the desired length of HIF-1α (240 bp), HIF-2α (223 bp), VEGF (197 bp) (Fig. 1A). Validation was confirmed by DNA sequencing.
Figure 1. Expression of HIF-1α, HIF-2α and VEGF mRNA in OAB and Normal BUC.
Figure 1A. Representative gel electrophoreses images of real-time qPCR products: HIF-1α, 240 bp; HIF-2α, 223 bp; VEGF, 197 bp. RT4 cell line served as positive control (+). Figure 1B: Expression ratio of HIF-1α, HIF-2α and VEGF in stretched and non-stretched normal and OAB BUC. Cell lines from 3 OAB patients and 3 normal controls were examined. OAB BUC showed higher expression than normal BUC, especially for VEGF. Baseline expression showed that there is no difference between non-stretched OAB cells and non-stretched normal cells.
Real-time qPCR was used to quantitate the expression of mRNA for these angiogenic factors. The ratios of stretch to non-stretched angiogenic mRNA levels in OAB BUC were compared to the ratios of stretch to non-stretched angiogenic mRNA levels in NB BUC (Figure 1B: HIF-1α transcript ratio in OAB BUC stretch/non-stretch vs. NB BUC stretch/non-stretch:, p = 0.0046; HIF-2α, p = 0.0054; VEGF, p = 0.000345). Therefore, stretching induced significantly higher increases in OAB BUC expression of the angiogenic mRNA levels compared to stretch in NB BUC.
2. Expression of HIF-1α, HIF-2α and VEGF Protein in OAB and NB BUC
We examined the protein expression of HIF-1α, HIF-2α and VEGF by FACS (Table 1). The percentages shown in Table represent percentage of the fluorescence histogram in the M1 region which is correlated to the amount or quantity of angiogenic protein of interest. Stretch of OAB cells significantly increased HIF-2α compared to un-stretched OAB BUC expressing significantly higher HIF-2α than in the non-stretched OAB BUC (SOAB 24.38% ± 1.53% vs. COAB 13.7% ± 1.75%, p< 0.01). There were no significant differences in the other two angiogenic proteins.
Table 1.
FACS Analyses for Angiogenic Factors With and Without Stretch in OAB and Normal BUC
| HIF-1α | HIF-2α | VEGF | |
|---|---|---|---|
| Stretched -OAB | 24.14% ± 3.15 | 24.38% ± 1.53* | 31.69% ± 7.17 |
| Unstretched -OAB | 20.06% ± 11.74 | 13.7% ± 1.75* | 23.51% ± 9.88 |
| Stretched - Normal | 26.59% ± 5.94 | 20.86% ± 3.11 | 24.46% ± 8.80 |
| Unstretched - Normal | 21.58% ± 12.39 | 16.3% ± 3.15 | 24.52% ± 4.68 |
p=0.01 comparing stretched-OAB with unstretched-OAB
3. Detection of VEGF in Culture Supernatant of OAB and Normal BUC by ELISA
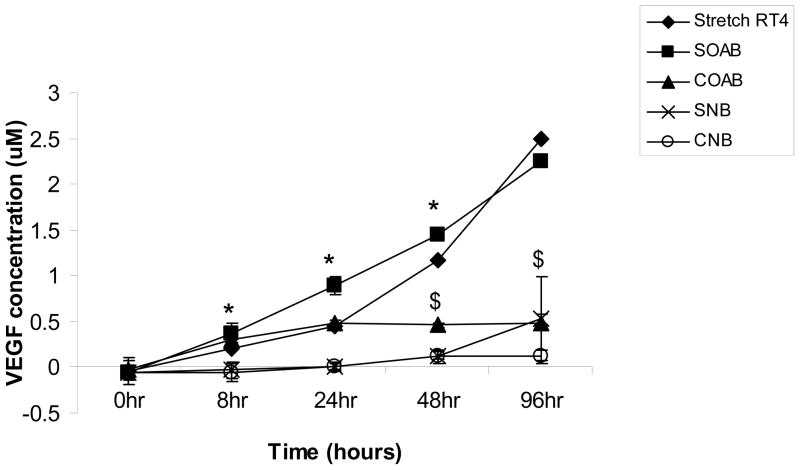
The supernatant sample analysis at 8 hour, 24 hour and 48 hour time points showed significantly more VEGF in stretched OAB BUC (Soab) compared to stretched normal BUC (Snb) (Figure 2). At 48 hour and 96 hour time points, stretched OAB BUC (Soab) released significantly greater VEGF compared to non-stretched OAB BUC (Coab). RT4 bladder urothelial cancer cells were used as a positive control since these cells are known to express VEGF.
Figure 2. Detection of VEGF in Culture Supernatant of OAB and Normal BUC by ELISA.
Level of VEGF in stretched and non-stretched OAB BUC and normal BUC were measured. Stretched RT4 cells served as positive control (◆). Stretched OAB BUC (SOAB ■) showed significant higher VEGF relaese than stretched normal BUC (SNB x) at 8hr, 24hr and 48hr time points (* p<0.05). Stretched OAB BUC (SOAB ■) showed significantly higher VEGF release than non-stretched OAB BUC (COAB ▲) at 48hr and 96hr time points ($: p<0.05).
DISCUSSION
This study demonstrated that OAB BUC responded to stretch very differently than normal BUC in terms of higher expression of different angiogenic factors. Figure 1B showed unstretched OAB BUC expression of HIF-1α, HIF-2α and VEGF mRNA similar to unstretched normal BUC expression of these transcripts; however when stretched, OAB BUC significantly increased expression of all 3 transcripts compared stretched normal BUC. FACS measures cell-associated protein expression including
Investigators have shown in an animal model that bladder ischemia induced detrusor overactivity possibly through ischemia-induced structural damage in the urothelium and neurodegeneration [9, 13]. OAB BUC, compared to normal BUC, reacted to stretch as if they were responding to a more hypoxic state, even though the oxygen content exposure was similar in the experimental conditions. Hypoxic conditions in the OAB bladder could have induced epigenetic changes in the urothelium that recapitulates an abnormal phenotype in vitro. This epigenetic signature phenomenon has been described in prostatic epithelial cells exposed to chronic hypoxia [18] and this similar phenomenon may be occurring in the OAB BUC.
The correlation between hypoxia and the expression of HIF-1α, HIF-2α and VEGF has been well known [19–20]. These studies found that under hypoxia, HIF-1α, HIF-2α and VEGF are upregulated. It is also known that the expression pathways of HIF-1α, HIF-2α and VEGF are complex. Other factors such as inflammatory signaling [21, 22] may influence expression of these factors. Recently, investigators have suggested that OAB may represent an inflammatory phenomenon [23] which may ultimately modulate these angiogenic factors.
The fact that stretch could induce the expression of hypoxic-induced growth and transcription factors is also confirmed by Yang et al [14]. They used FX-4000 unit to measure the gene expression after mechanical strain to the bladder smooth muscle cells. They found that mechanical stretch activates a gene program involved in paracrine signaling of angiogenesis suggesting that mechanical control of angiogenic genes is an integral part of the adaptive and plasticity response to mechanical overload. The findings in this paper suggest that a portion of the pathophysiology of OAB might also occur at the level of the urothelial cells, in addition to the bladder smooth muscle.
While we found insignificant differences in the protein expression of HIF-1α and VEGF, this could be due to the FACS technique. FACS utilizes cell flow cytometry to sort antibody-antigen complex fluorescence signals. There may be inadequate antibody penetration into the cystoplasmic and intranuclear space where transcriptional factors, such as HIF-1α, are found. Because VEGF is often released by cells as a paracrine growth signaling molecule, an additional methodology of measuring supernatant levels of VEGF using ELISA was performed. HIF-1α and HIF-2α are transcription factors which are not expected to be transported to the extracellular space. We found that OAB BUC released significantly higher quantities of VEGF with stretch compared to stretched control BUC and unstretched OAB BUC (Figure 2). This significant increase in VEGF was not due to change in the cell numbers that could have occurred during the stretching because no change in cell numbers were detected (data not shown).
These results demonstrated significant differences in angiogenic factor expression between OAB BUC and NB BUC. More research is necessary to determine if these results can be translated to the clinical arena. This should lead to more insight in specific OAB pathophysiology and might point to novel treatment strategies to treat OAB.
CONCLUSIONS
Stretch of OAB BUC resulted in greater percentage increase of expression of mRNA for HIF-1α, HIF-2α, and VEGF compared to stretch of normal BUC. At the protein level, stretch of OAB BUC resulted in greater HIF-2α and VEGF compared to stretch of normal BUC. These results suggested that OAB BUC reacted to stretch as if they were in hypoxic condition. These findings are consistent with hypothesis that bladder urothelial pathology may partially contribute to OAB etiology and future mechanistic studies at the urothelial cellular level are warranted.
Acknowledgments
Supported by: NIH-R01-DK075728
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P, Ulmsten U, et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn. 2002;21:167. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Abrams P, Cardozo L, Roberts RG, Thuroff J, Wein AJ. How widespread are the symptoms of an overactive bladder and how are they managed? A population-based prevalence study. BJU Int. 2001;87:760. doi: 10.1046/j.1464-410x.2001.02228.x. [DOI] [PubMed] [Google Scholar]
- 3.Stewart WF, Van Rooyen JB, Cundiff GW, Abrams P, Herzog AR, Corey R, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20:327. doi: 10.1007/s00345-002-0301-4. [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Milsom I, Hunskaar S, Reilly K, Kopp Z, Herschorn S, et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC study. Eur Urol. 2006;50:1306. doi: 10.1016/j.eururo.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Coyne KS, Payne C, Bhattacharyya SK, Revicki DA, Thompson C, Corey R, et al. The impact of urinary urgency and frequency on health-related quality of life in overactive bladder: results from a national community survey. Value Health. 2004;7:455. doi: 10.1111/j.1524-4733.2004.74008.x. [DOI] [PubMed] [Google Scholar]
- 6.Coyne KS, Zhou Z, Bhattacharyya SK, Thompson CL, Dhawan R, Versi E. The prevalence of nocturia and its effect on health-related quality of life and sleep in a community sample in the USA. BJU Int. 2003;92:948. doi: 10.1111/j.1464-410x.2003.04527.x. [DOI] [PubMed] [Google Scholar]
- 7.Liberman JN, Hunt TL, Stewart WF, Wein A, Zhou Z, Herzog AR, et al. Health-related quality of life among adults with symptoms of overactive bladder: results from a U.S. community-based survey. Urology. 2001;57:1044. doi: 10.1016/s0090-4295(01)00986-4. [DOI] [PubMed] [Google Scholar]
- 8.Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004;171:448. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- 9.Azadzoi KM, Tarcan T, Kozlowski R, Krane RJ, Siroky MB. Overactivity and structural changes in the chronically ischemic bladder. J Urol. 1999;162:1768. [PubMed] [Google Scholar]
- 10.Brading A, Pessina F, Esposito L, Symes S. Effects of metabolic stress and ischaemia on the bladder, and the relationship with bladder overactivity. Scand J Urol Nephrol Suppl. 2004;(215):84. doi: 10.1080/03008880410015336. [DOI] [PubMed] [Google Scholar]
- 11.de Jong BW, Wolffenbuttel KP, Arentshorst ME, Lodder P, Kok DJ. Detrusor glycogen reflects the functional history of bladders with partial outlet obstruction. BJU Int. 2007;100:846. doi: 10.1111/j.1464-410X.2007.07046.x. [DOI] [PubMed] [Google Scholar]
- 12.de Jong BW, Wolffenbuttel KP, Scheepe JR, Kok DJ. The detrusor glycogen content of a de-obstructed bladder reflects the functional history of that bladder during PBOO. Neurourol Urodyn. 2008;27:454. doi: 10.1002/nau.20567. [DOI] [PubMed] [Google Scholar]
- 13.Azadzoi KM, Yalla SV, Siroky MB. Oxidative stress and neurodegeneration in the ischemic overactive bladder. J Urol. 2007;178:710. doi: 10.1016/j.juro.2007.03.096. [DOI] [PubMed] [Google Scholar]
- 14.Yang R, Amir J, Liu H, Chaqour B. Mechanical strain activates a program of genes functionally involved in paracrine signaling of angiogenesis. Physiol Genomics. 2008;36:1. doi: 10.1152/physiolgenomics.90291.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y, Keay S, De Deyne PG, Chai TC. Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol. 2001;166:1951. [PubMed] [Google Scholar]
- 16.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 May 1;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Y, Chai TC. Up-regulation of P2X3 receptor during stretch of bladder urothelial cells from patients with interstitial cystitis. J Urol. 2004 Jan;171(1):448–52. doi: 10.1097/01.ju.0000099660.46774.3c. [DOI] [PubMed] [Google Scholar]
- 18.Watson JA, Watson CJ, McCrohan AM, Woodfine K, Tosetto M, McDaid J, et al. Generation of an epigenetic signature by chronic hypoxia in prostate cells. Hum Mol Genet. 2009;18:3594. doi: 10.1093/hmg/ddp307. [DOI] [PubMed] [Google Scholar]
- 19.Pagès G, Pouysségur J. Transcriptional regulation of the Vascular Endothelial Growth Factor gene--a concert of activating factors. Cardiovasc Res. 2005 Feb 15;65(3):564–73. doi: 10.1016/j.cardiores.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Jones A, Fujiyama C, Blanche C, Moore JW, Fuggle S, Cranston D, et al. Relation of vascular endothelial growth factor production to expression and regulation of hypoxia-inducible factor-1 alpha and hypoxia-inducible factor-2 alpha in human bladder tumors and cell lines. Clin Cancer Res. 2001;7:1263. [PubMed] [Google Scholar]
- 21.Saban R, Saban MR, Maier J, Fowler B, Tengowski M, Davis CA, et al. Urothelial expression of neuropilins and VEGF receptors in control and interstitial cystitis patients. Am J Physiol Renal Physiol. 2008;295:F1613. doi: 10.1152/ajprenal.90344.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saban MR, Backer JM, Backer MV, Maier J, Fowler B, Davis CA, et al. VEGF receptors and neuropilins are expressed in the urothelial and neuronal cells in normal mouse urinary bladder and are upregulated in inflammation. Am J Physiol Renal Physiol. 2008;295:F60. doi: 10.1152/ajprenal.00618.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyagi P, Barclay D, Zamora R, Yoshimura N, Peters K, Vodovotz Y, et al. Urine cytokines suggest an inflammatory response in the overactive bladder: a pilot study. Int Urol Nephrol. 2009 Sep 26; doi: 10.1007/s11255-009-9647-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]