Abstract
Staphylococcus aureus is an important human pathogen that causes skin and soft tissue abscesses. Abscess formation is not unique to staphylococcal infection and purulent discharge has been widely considered a physiological feature of healing and tissue repair. Here we present a different view, whereby S. aureus deploys specific virulence factors to promote abscess lesions that are distinctive for this pathogen. In support of this model, only live S. aureus are able to form abscesses, requiring genes that act at one or more of four discrete stages during the development of these infectious lesions. Protein A and coagulases are distinctive virulence attributes for S. aureus, and humoral immune responses specific for these polypeptides provide protection against abscess formation in animal models of staphylococcal disease.
Staphylococci and Staphylococcus aureus
Sir Alexander Ogston observed ‘micrococci’ in pus from surgical wound infections and named the bacterium Staphylococcus. Using eggs to isolate pure cultures for the inoculation of rabbits, he demonstrated that staphylococci cause the development of abscesses in infected animals. During the Ninth Surgical Congress in Berlin (1880), Ogston reported his results, firmly establishing staphylococci as the etiological agent of suppurative abscesses. To date, 36 species and 18 subspecies of the genus Staphylococcus have been distinguished1, 2. Ogston’s microbe, Staphylococcus aureus, remains the most prominent member among this group, as it is the most frequent cause of human skin or soft tissue abscesses3. In addition to abscesses, S. aureus strains can cause pneumonia, toxic-shock syndrome, exfoliative skin disease, and enteritis3. Several unique biological traits are associated with S. aureus, including the ability to bind to immunoglobulin, agglutinate and coagulate blood or plasma (Figure 1)3.
Figure 1.
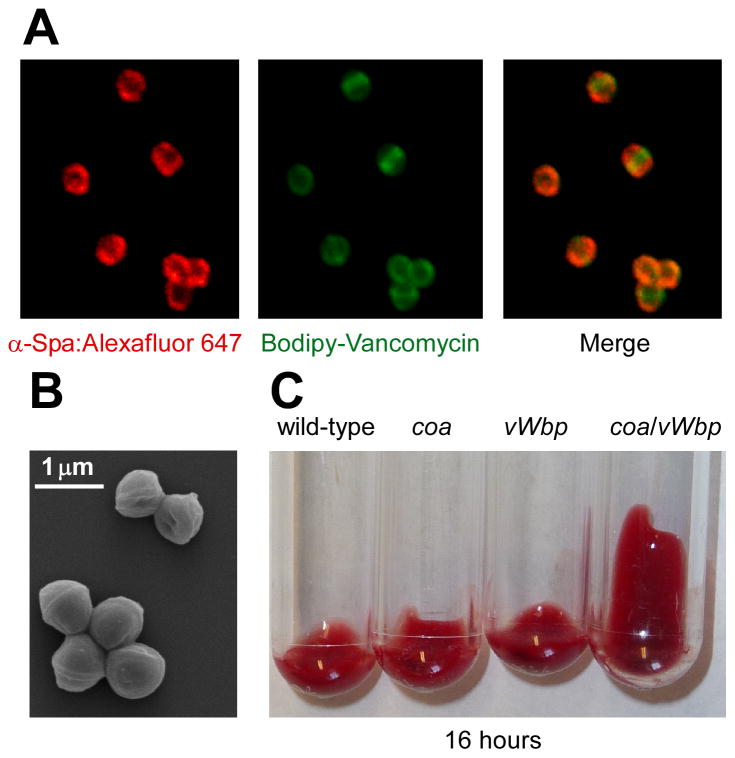
Staphylococcus aureus characteristics and distinguishing features. (a) The immunoglobulin binding properties of staphylococcal protein A were revealed by confocal laser scanning immunofluoresence microscopy as previously described80 . Alexafluor-647 conjugated immunoglobulin (red) binds to protein A (left), Bodipy-conjugated vancomycin (green) stains staphylococcal peptidoglycan (middle), and a merger of the two data sets reveals the deposition of immunoglobulin on the surface of staphylococci (right). (b) Shape and clustering of S. aureus cells imaged by scanning electron microscopy (SEM; image courtesy of Matt Frankel, University of Chicago). (c) S. aureus coagulates blood, a feature conferred by two secreted coagulases: staphylocoagulase (Coa) and von Willebrand factor binding protein (vWbp)16. Lepirudin anti-coagulated mouse blood was incubated with 105 cells of wild-type S. aureus Newman or isogenic mutants lacking coa, vWbp, or coa/vWbp and examined over time for the formation of a blood clot. Blood containing staphylococci lacking both coagulase genes do not form a clot following a 16 hour observation period.
S. aureus and several other staphylococcal species colonize the human skin, nails, and nares and disseminate among recipient host populations via physical contact and aerosols3. The massive consumption of antibiotics over the past 50 years has led to the selection of drug-resistant strains, designated MRSA for methicillin-resistant S. aureus4. Although no longer in clinical use in the United States, methicillin was initially used for the treatment of penicillin-resistant strains; penicillinase is unable to cleave methicillin. MRSA isolates are now widespread5. The basis for their resistance is a penicillin-binding protein that cannot be inhibited by methicillin or oxacillin. As a result, vancomycin has been used as the last-resort antibiotic for MRSA infections. However, S. aureus isolates that have vancomycin-resistant (VRSA) or vancomycin-intermediate (VISA) phenotypes have emerged by generating altered substrates for cell wall synthesis and no longer associate with vancomycin or related glycopeptide drugs6–8. MRSA are a frequent cause of hospital-acquired infection in the United States9. Over the past decade, community-acquired infections (CA-MRSA) increased in abundance and severity9. One such clone has swept over the United States and currently colonizes a large segment of the population10. An FDA approved vaccine that can prevent S. aureus infections caused by MSSA (methicillin-sensitive S. aureus), MRSA, CA-MRSA, VISA or VRSA strains is not yet available.
Breaches in local defense, for example skin cuts or hair follicle trauma, provide opportunities for staphylococcal invasion, which typically manifests as abscess formation: the accumulation of pus accompanied by severe inflammation of surrounding tissues3. Staphylococci in pus disseminate onto skin surfaces or enter circulating lymph and blood, which leads to the formation of abscesses at new sites3. S. aureus is a frequent cause of sepsis, often the consequence of invasive disease originating from one or more abscesses. About one-fifth of staphylococcal skin and soft tissue infections that improve under antibiotic therapy flare up again at a later time11. This phenomenon, as well as clinical data on S. aureus infected individuals, suggests that some humans do not acquire protective immunity following infection3. Here we review what is known about S. aureus abscess formation and the molecular mechanisms that enable the pathogen to form these lesions and suppress the immune responses of its host.
Abscess formation in stages
Staphylococci that disseminate through the bloodstream enter peripheral tissues and seed infectious lesions, initially inducing inflammatory responses that attract neutrophils, macrophages, and other phagocytes3. The responsive invasion of immune cells to the site of infection is accompanied by liquefaction necrosis and the release of pus with replicating staphylococci into circulating body fluids12–14. Defensive host responses to staphylococcal invasion include fibrin deposits to shield healthy tissues from the disseminating microbe. Upon abscess drainage or surgical excision of infected lesions, lost organ tissues can be replaced with fibrotic scars. However, these events cannot be considered specific host responses to S. aureus invasion; they are induced even with chemical trauma, physical insult or injection of sterile biological materials into organ tissues15.
We suggest that abscess formation and staphylococcal entry into purulent material is a pathogen driven process that usurps the default responses of its infected host to enhance microbial replication and dissemination. In support of this hypothesis, several pathogen-specific genes are required for the ability of S. aureus to establish abscess lesions (vide infra)14, 16. Following entry into organ tissues, staphylococci attract immune cells and replicate in their presence to eventually replace physiological epithelia with distinctive lesions14. Within four days of infection, the pathogen has organized itself as a staphylococcal abscess community (SAC) at the center of the lesion, shielded from host immune cells by a surrounding pseudocapsule comprised of fibrin deposits16. These lesions grow through the arrival of immune cells and the destruction of healthy tissues associated with neutrophil necrosis. Staphylococci disseminate from these lesions with the draining pus into circulating body fluids.
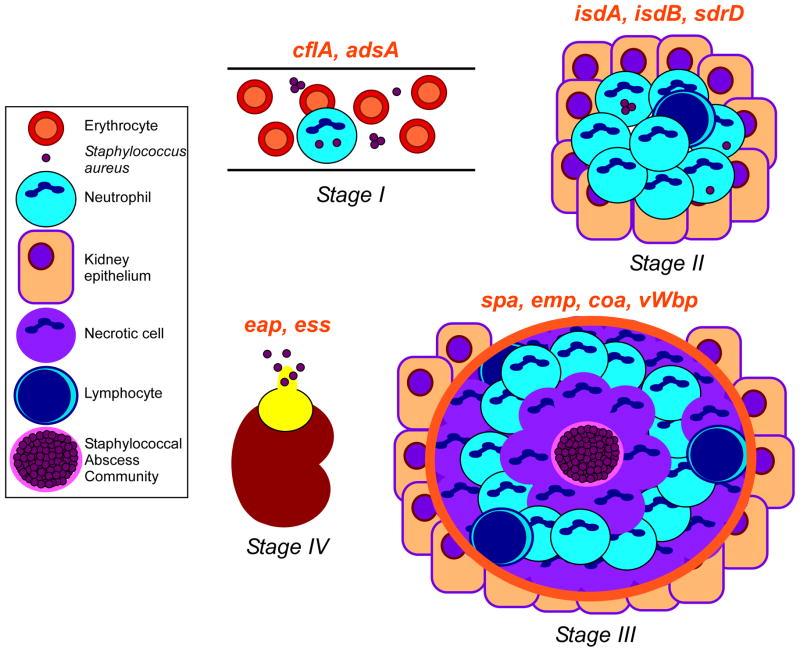
Using C57BL/6 and BALB/c mice in an intravenous challenge model, infected animals produced abscess lesions in all tissues examined similar to those observed in humans14. We liken the pathogenesis of staphylococcal disease to a play that is staged in four acts (Figure 2). Following intravenous injection, S. aureus survives in the bloodstream as an extracellular pathogen or when phagocytosed by neutrophils (Stage I). Upon distribution of staphylococci to peripheral tissues, neutrophils as well as other immune cells are recruited to the site of infection (Stage II). S. aureus reprograms infected lesions to replicate as a SAC at their center, separated from surrounding immune cells by a pseudocapsule with fibrin deposits (Stage III). Movement of lesions to organ capsules and their subsequent rupture releases S. aureus with purulent exsudate to other sites, where the pathogen repeats this infectious program (Stage IV). Without antimicrobial therapy, staphylococci cannot be cleared by host immune responses from abscess lesions and infected animals typically succumb to disease within 30–60 days14.
Figure 2.
Working model for staphylococcal abscess formation and persistence in host tissues. Stage I: following intravenous inoculation, S. aureus survives in the bloodstream and disseminates via the vasculature to peripheral organ tissues. Stage II: staphylococci in renal tissue attract a massive infiltrate of polymorphonuclear leukocytes and other immune cells. Stage III: abscesses mature showing a central accumulation of the pathogen (SAC) surrounded by a pseudocapsule of fibrin deposits (pink rim), and zones of necrotic and healthy polymorphonuclear neutrophils (PMNs; purple and light blue cells, respectively), and finally a rim of eosinophilic material (orange rim). Stage IV: abscesses mature and rupture on the organ surface to initiate new rounds of infections. Genes required for specific stages of staphylococcal abscess development are in red above the corresponding stage of infection. Figure adapted with the authors’ permission from an article published by Cheng and colleagues14.
Protagonists of the plot
Stage I: survival of staphylococci in blood
Following intravenous inoculation of mice with a sublethal dose of bacteria (~107 colony forming units, CFU), 99.9% of the original inoculum disappears from the vasculature within 6 h (Figure 3a). Dissemination to organ tissue occurs within 3 h followed by rapid decline (3–12 h) and measurable replication after 24 h (Figure 3a)14. Unless staphylococci survive or replicate in blood and circulating lymph fluids, this pathogen could not disseminate to new sites within an infected host. Thus, the first stage of the plot is to describe the actors in the play that lead to disease crisis and dramatic endings of S. aureus infections. To survive in blood, S. aureus must escape a variety of innate immune mechanisms, including antimicrobial peptides, complement, and phagocytic killing17, 18. The principal defense against S. aureus is provided by neutrophilic polymorphonuclear leukocytes, which comprise 60–70% of human white blood cells19. Reactive oxygen species, along with the acidic vacuolar environment, prove toxic to many microbes, and help curtail bacterial spread. S. aureus produces the carotenoid staphyloxanthin20, 21, a membrane-bound pigment that scavenges reactive oxygen species and protects S. aureus from neutrophil killing22. Further, staphylococci deploy a group of secreted peptides, termed phenol-soluble modulins (PSMs), to target human neutrophils for destruction by disrupting the integrity of their plasma membrane23.
Figure 3.
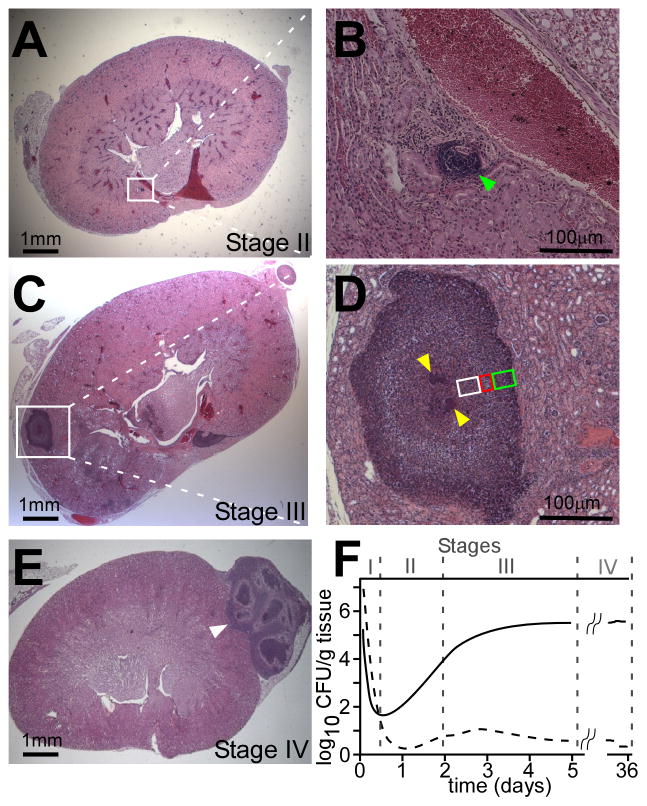
Monitoring and visualizing Staphylococcus aureus abscess formation. (a)–(e) Visualization of lesions in kidneys following infection at Stages II, III and IV as previously described [14]. Paraffin embedded kidneys were thin-sectioned, stained with hematoxylineosin, mounted on glass slides and examined by light microscopy for the formation of staphylococcal abscesses. Micrographs on the left (a, c, e) show sections of entire kidneys. Micrographs on the right (b, d) represent magnifications of the area framed in the white box in (a) and (c). (b) Stage II: Site of inflammation with immune cell infiltrates (green arrowhead). (d) Stage III: staphylococci are clearly distinguishable as central nidus, staphylococcal abscess community (SAC), within the maturing abscess (yellow arrowheads). The SAC is surrounded by an amorphous, eosinophilic capsule (see Figure 4) followed by a zone of dead PMNs (polymorphonuclear neutrophils; white box), apparently healthy PMNs (red box), and necrotic PMNs (green box). The entire abscess region is separated from healthy kidney tissue by a second eosinophilic layer, which appears to increase in size over time. (e) Stage IV: Abscesses migrate to the periphery of organ tissue. The organization of SACs and immune cells during Stage III is abolished prior to rupture of the lesion onto the organ surface. (f) Representative distribution of bacteria in blood (dashed line) and kidney tissue (solid line) over time following intravenous infection of mice with 1 × 107 colony forming units (CFU) of S. aureus. Bacterial loads are represented as CFU per g of blood or renal tissue over time following infection.
The complement cascade plays a central role in innate host defenses to S. aureus24–26. By binding to microbial surfaces, C3 and C5 convertases deposit complement and mediate phagocytic killing of bacteria. The associated release of C3 and C5 fragments further serves as a chemoattractant for phagocytes. S. aureus subverts these mechanisms by secreting factors inhibitory for complement activation and neutrophil chemotaxis27, 28. Although not part of the core S. aureus genome, these prophage-encoded factors are expressed by many virulent strains29.
In addition to secreted factors, staphylococci rely on cell wall-anchored surface proteins for their survival in blood30. Clumping factor (ClfA), a sortase-anchored product whose N-terminal domain is displayed on the bacterial surface, binds fibrinogen31. This biochemical attribute triggers staphylococcal agglutination in the presence of fibrinogen or fibrin, i.e. in lymph fluids or blood31. How agglutination ensures staphylococcal survival is not entirely clear. On one hand, the extremely large size of agglutinated staphylococci can be thought to interfere with phagocytosis32, 33. On the other, ClfA enables staphylococci to adhere to fibrin deposits on the vascular endothelium, providing a niche for pathogen replication at the periphery of circulating fluids. The latter mechanism is certainly essential for the pathogenesis of endocarditis34. Adenosine synthase A (AdsA), another sortase-anchored product, harbors a 5’-nucleotidase signature sequence. AdsA cleaves ADP or AMP to form adenosine. When inoculated into blood, S. aureus synthesizes large amounts of adenosine from AMP using AdsA35. Adenosine is a key regulator of innate and acquired immune responses and is generated at sites of inflammation, hypoxia, organ injury, and traumatic shock36. Host inflammatory responses, e.g. in response to bacterial infection, are regulated to prevent excessive tissue damage during infection; production of adenosine is the key signal to end host inflammatory response36. Thus, staphylococci evade immune responses by signaling to the host that its inflammatory activities must be canceled.
As the pathogen has endowed itself with such a multitude of defense mechanisms, it comes as no surprise that S. aureus, although quickly taken up by polymorphonuclear neutrophils (PMNs) or monocytes, survives within these leukocytes19, 37. Gresham and colleagues demonstrated that PMNs harboring S. aureus can indeed seed infectious lesions when transferred into naïve mice, suggesting that staphylococci use PMNs as vehicles to enter the tissues of an infected host38.
Stage II: preparing a site for the infectious lesion
Although staphylococci arrive within 1–3 hours in peripheral organs where abscess lesions are being established (Figure 3a), a histopathological correlate for this activity is not detectable until about 48 hours after intravenous challenge (Figure 3)14. At that time, sufficiently large numbers of immune cells (predominantly neutrophils) have accumulated in a small area to replace physiological epithelia with microscopically discernable lesions (Figure 3b and 3c)14. During Stage II of abscess maturation, early lesion sites contain a low abundance of staphylococci. It seems unlikely that the dramatic recruitment of immune cells can be explained by the massive replication of bacteria – micro- or macro-colonies of staphylococci are not observed early during infection. Molecular genetic studies on lipoprotein maturation revealed a fascinating phenotype for the S. aureus lgt mutant (lipoprotein diacyl-glyceride transferase), which is unable to link diacyl-glycerol to the cysteine-thiol of lipoprotein precursors39. The lgt mutant multiplied in host tissues with minimal recruitment of immune cells and did not form typical abscess lesions40. Thus, in addition to producing formylated peptides that are perceived by the immune system, S. aureus could actively release lipoproteins for recognition via Toll-like receptor 2 (TLR2)40. The ensuing pro-inflammatory signals could then recruit large numbers of immune cells to the site of infection, thereby contributing to the destruction of organ tissues.
Staphylococcal replication in organ tissues must meet the pathogen’s nutritional requirement for iron41. A key pathway for the scavenging of iron at this stage involves the hemoglobin hemophore IsdB, as well as two other sortase anchored products, IsdA and IsdC, which pass the heme-moiety across the cell wall envelope for subsequent transport across the staphylococcal membrane42. Depending on the site of infection, too much import of heme is not a good thing. The associated production of oxygen radicals proves toxic for staphylococci and immune cells. Mutations in S. aureus hrtA, at the center of a pathway that perceives heme and transports superfluous heme out of bacteria, cause heme-toxicity in mutants but also interfere with neutrophil recruitment, resulting in liver-specific hypervirulence43. HrtA and HrtB form an ABC-type transport system that is essential for alleviating hemin toxicity. In the absence of hrtA, hemin toxicity triggers overexpression of the orphan permease HrtB. This triggers membrane stress, thereby augmenting the secretion of a subset of immunomodulatory proteins that are directed to inhibit neutrophil migration to the site of infection44. Although this is not yet known in detail, we surmise that staphylococci require several unique biosynthetic pathways during this developmental program, in particular because the pathogen must carry out its metabolism in an environment that restricts the availability of zinc45. SdrD, another sortase-anchored surface protein, is also required at Stage II14. Its specific activity during host infection is not yet known.
Stage III: crisis and disease
The molecular and cellular events that promote the formation of a staphylococcal abscess can be imagined when studying the histopathological features of maturing lesions. Within four or five days following infection, a large mass of the invading pathogen (SAC), replicates at the center of the lesion (Figure 3d and 3e). The SAC is separated from surrounding immune cells by an eosinophilic pseudocapsule that is composed of fibrin deposits14. This pseudocapsule is best seen by electron microscopy (Figure 4). In the periphery, staphylococcal abscesses are characterized by layers of immune cells: a ring of largely necrotic neutrophils in the immediate vicinity of SACs, followed by a layer of healthy appearing immune cells and eventually a layer of necrotic immune cells, whose border with healthy tissues is demarcated by a layer of fibrin or extracellular matrix deposits (Figure 3d and 3e)14.
Figure 4.
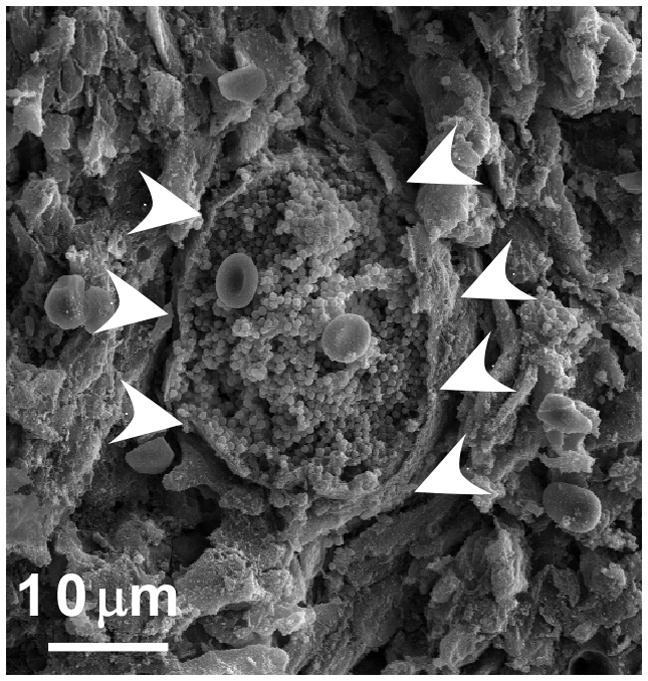
SACs during Stage III. At the center of an abscess lesion, S. aureus cells are found as tightly associated mass covered with a granular, electron-dense substance. Bacteria are enclosed by an amorphous pseudocapsule (white arrowheads) that separates the SAC from neighboring immune cells. Image was captured from the kidney of an animal infected with S. aureus Newman. Organ tissue was thin sectioned, fixed, dehydrated and sputter-coated with platinum/palladium and analyzed by scanning electron microscopy.
What are the molecular events that enable this astonishing metamorphosis? S. aureus secrete coagulase (Coa), which associates with and non-enzymatically activates prothrombin46, 47. The staphylocoagulase-prothrombin complex cleaves fibrinogen to generate fibrin peptides, promoting clot formation48, 49. One hundred years after the discovery of Coa, a second coagulase was identified50. von Willebrand factor-binding protein (vWbp) was initially discovered to interact with its namesake host protein, a key factor in the recruitment of platelets to blood clots51, however vWbp also activates prothrombin to promote fibrin coagulation52. Although mutants lacking either coa or vWbp continue to form abscesses, variants lacking both genes were unable to form these lesions and coagulate blood (Figure 1c)16. Further, prothrombin, fibrin and coagulases localize to the eosinophilic pseudocapsule of staphylococcal abscesses16. A simple explanation for these observations is that staphylococci usurp the clotting cascade via secreted coagulases to form fibrin deposits in organ tissues. The deposits function as a barrier for immune cells preventing their penetration into SACs, where the pathogen replicates without interference while manipulating inflammatory responses, the fate of immune cells in the periphery and the progression of the entire infectious lesion from a safe distance. Additional evidence for the key contribution of coagulases to virulence and abscess formation was obtained through the discovery that S. aureus strains adapted to ruminants or horses require additional vWbp homologs to coagulate blood or plasma of these species and establish staphylococcal disease53.
Mutations in two other genes affect the maturation of staphylococcal abscesses. spa encodes staphylococcal protein A (SpA, Figure 1a), a sortase-anchored product that is released from the bacterial envelope in large amounts. SpA impedes phagocytosis by binding the Fcγ component of immunoglobulin54, 55, activates platelet aggregation via its binding to von Willebrand factor56, and functions as a B-cell superantigen by crosslinking the Fab region of VH3 bearing immunoglobulin M (IgM)57–59. Also, through its activation of tumor necrosis factor receptor 1 (TNFR1), SpA initiates staphylococcal pneumonia60. It is unknown which of the many biochemical attributes of SpA contributes to abscess formation, however, spa mutants appear unable to form abscesses14. Emp is an envelope associated protein of staphylococci, whose virulence attributes have been associated with the ability to promote adherence to endothelia61 and biofilm formation62. Numerous studies have attempted to correlate staphylococcal virulence with biofilm formation; the evidence is equivocal63, 64. SACs are reminiscent of biofilm communities formed in vitro, however several genes that contribute to biofilm formation, e.g. those specifying capsular polysaccharide or poly N-acetylglucosamine synthesis65, 66, are dispensable for abscess formation14. Immunofluorescence microscopy revealed Emp on the surface of staphylococci at the center of abscess lesions14, suggesting that this protein contributes to abscess formation by promoting growth as biofilm-like SACs.
Stage IV: persistence and an end to the drama
Staphylococcal abscesses mature over weeks and, following rupture and release into the peritoneal cavity, lead to new infectious lesions (Stage IV). Variants lacking eap (extracellular adhesion protein) or a non-canonical protein secretion pathway designated Ess (ESAT-6 like secretion system) are defective in persistence and are, therefore, assigned to Stage IV14, 67. Ess secretion is reminiscent of the ESX-1 dependent secretion of Mycobacterium tuberculosis68. ESAT-6 and CFP10, products of the ESX-1 locus, have been shown to have immune evasive effects, including TLR2 downregulation and macrophage phagosome lysis. Deletion of the ESX-1 locus results in an attenuated strain (BCG) that forms irregular granulomas and is ultimately unable to persist within human hosts69, 70. Therefore, it appears that this secretion system secretes immunomodulatory factors that are required for persistence. S. aureus has only one ESX-1-like locus and mutations in the ess cluster survive stages I-III of infection but fail to persist at stage IV67, suggesting that this pathway is also involved in prolonged staphylococcal survival within the abscess71.
All S. aureus isolates appear to secrete Eap, although there is significant strain variation72. Structural studies showed that Eap and the C-terminal half of TSST1 (toxic shock syndrome toxin 1) as well as SEB (staphylococcal enterotoxin B) display homology, and Eap was thus shown to have superantigenic activity and to induce pro-inflammatory (IL6, TNFα) cytokine production by monocytes73. However, Eap has also been shown to exert numerous anti-inflammatory effects by inhibiting neutrophil adherence to the endothelium74, leukocyte recruitment in a murine bacterial peritonitis model75, T cell activation and recruitment to the dermis76.
Distinctive or universal: lessons learned from S. aureus abscess formation
Abscesses, defined as inflammatory lesions releasing purulent material, are standard responses for many different biological, chemical or physical insults to host tissues. Using a mouse model, we describe the concept that S. aureus infection may modify this default program of tissue repair by building complex lesions that ensure the pathogen’s persistence in a hostile environment, its dissemination throughout many different organ systems and its spread to new hosts. In support of our model we note that the injection of heat-killed staphylococci does not trigger abscess formation in mice14. This is unlike other biological materials, for example purified capsular polysaccharide of Bacteroides fragilis, that alone can activate host immune systems and promote abscess formation in a murine peritoneal challenge model77, 78. Further, some, but certainly not all, genes that contribute to the pathogenesis of different S. aureus diseases are also required for abscess formation14. Of those, we note that coagulases and SpA are unique to S. aureus isolates. A reciprocal argument is that the absence of protein A and coagulase genes from S. epidermidis correlates with the inability of this microbe to cause abscesses of the kind that S. aureus generates. The obvious conclusion for these findings is that S. aureus abscesses could indeed be distinctive. Whatever could perturb their formation may also benefit the outcome of infections. The benefit of such research could be significant, for example through the development of vaccines that prevent S. aureus skin and soft tissue infections. In accord with this, antibodies raised against either protein A or staphylococcal coagulases can each prevent abscess formation in the aforementioned mouse model16, 79. Future work will need to determine whether these genes are key virulence factors and vaccine antigens in other animal models of S. aureus infection, for example rats, guinea pigs, rabbits or non-human primates. If so, SpA and coagulases could represent important candidates for the development of a human vaccine. Recently, Kim et al. reported immunization of animals with a non-toxigenic variant of SpA (SpAKKAA) that cannot bind Fcγ and Fab79. The resulting immune responses promoted opsonophagocytic clearance and protected animals against abscess formation79. Further, animals immunized with SpaKKAA were able to mount antibody responses against numerous S. aureus antigens upon challenge79. Typically, infection with S. aureus generates a modest immune response that does not afford protection against subsequent infections79. Thus, protective immunity against S. aureus infection could be achieved via the neutralization of protein A with specific antibodies.
Acknowledgments
Work described herein was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID), Infectious Diseases Branch (AI52474 to O.S. and AI75258 to D.M.M.) and by Novartis Vaccines and Diagnostics (Siena, Italy). A.G.C. was a trainee of the NIH Medical Scientist Training Program at The University of Chicago (GM07281). D.M.M. and O.S. acknowledge membership within and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH Award 1-U54-AI-057153).
Footnotes
Conflict of Interest
Alice Cheng, Andrea DeDent, Olaf Schneewind and Dominique Missiakas are named inventors on a patent owned by The University of Chicago, which is the subject of a license agreement with Novartis Vaccines and Diagnostics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kloos W, et al. The genus Staphylococcus. In: Balows A, et al., editors. The prokaryotes. Springer-Verlag; 1992. pp. 1369–1420. [Google Scholar]
- 2.Götz F, et al. The Genera Staphylococcus and Macrococcus. In: Dworkin M, et al., editors. The Prokaryotes. Springer; New York: 2006. pp. 5–75. [Google Scholar]
- 3.Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 4.Brumfitt W, Hamilton-Miller J. Methicillin-resistant Staphylococcus aureus. N Engl J Med. 1989;320:1188–1199. doi: 10.1056/NEJM198905043201806. [DOI] [PubMed] [Google Scholar]
- 5.DeLeo FR, Chambers HF. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 7.Fridkin SK. Vancomycin-intermediate and -resistant Staphylococcus aureus: what the infectious disease specialist needs to know. Clin Infect Dis. 2001;32:108–115. doi: 10.1086/317542. [DOI] [PubMed] [Google Scholar]
- 8.Weigel LM, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 9.Klevens RM, et al. The impact of antimicrobial-resistant, health care-associated infections on mortality in the United States. Clin Infect Dis. 2008;47:927–930. doi: 10.1086/591698. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy AD, et al. Epidemic community-associated methicillin-resistant Staphylococcus aureus: recent clonal expansion and diversification. Proc Natl Acad Sci USA. 2008;105:1327–1332. doi: 10.1073/pnas.0710217105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers HF. Community-associated MRSA--resistance and virulence converge. N Engl J Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 12.Lam GT, et al. Abscess forming factors produced by Staphylococcus aureus. II. Abscess formation and immunity by Staphylococcus and its mutants. J Bacteriol. 1963;86:87–91. doi: 10.1128/jb.86.1.87-91.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorbach SL. Good and laudable pus. J Clin Invest. 1995;96:2545. doi: 10.1172/JCI118316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng AG, et al. Genetic requirements for Staphylococcus aureus abscess formation and persistence in host tissues. FASEB J. 2009;23:3393–3404. doi: 10.1096/fj.09-135467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tzianabos A, et al. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysachcarides. Proc Natl Acad Sci USA. 2001;98:9365–9370. doi: 10.1073/pnas.161175598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng AG, et al. Contribution of coagulases towards Staphylococcus aureus disease and protective immunity. PLoS pathogens. 2010;6:e1001036. doi: 10.1371/journal.ppat.1001036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005;3:948–958. doi: 10.1038/nrmicro1289. [DOI] [PubMed] [Google Scholar]
- 18.Peschel A, Sahl HG. The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol. 2006;4:529–536. doi: 10.1038/nrmicro1441. [DOI] [PubMed] [Google Scholar]
- 19.Voyich JM, et al. Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils. J Immunol. 2005;175:3907–3919. doi: 10.4049/jimmunol.175.6.3907. [DOI] [PubMed] [Google Scholar]
- 20.Marshall JH, Wilmoth GJ. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: evidence from a study of mutants. J Bacteriol. 1981;147:914–919. doi: 10.1128/jb.147.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelz A, et al. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J Biol Chem. 2005;280:32493–32498. doi: 10.1074/jbc.M505070200. [DOI] [PubMed] [Google Scholar]
- 22.Clauditz A, et al. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang R, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1418–1420. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson BJ, et al. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun. 1978;20:388–392. doi: 10.1128/iai.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verbrugh HA, et al. The role of Staphylococcus aureus cell-wall peptidoglycan, teichoic acid and protein A in the processes of complement activation and opsonization. Immunolgy. 1979;37:615–621. [PMC free article] [PubMed] [Google Scholar]
- 26.Neth O, et al. Enhancement of complement activation and opsonophagocytosis by complexes of mannose-binding lectin with mannose-binding lectin-associated serine protease after binding to Staphylococcus aureus. J Immunol. 2002;169:4430–4436. doi: 10.4049/jimmunol.169.8.4430. [DOI] [PubMed] [Google Scholar]
- 27.de Haas CJ, et al. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J Exp Med. 2004;199:687–695. doi: 10.1084/jem.20031636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rooijakkers SH, et al. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat Immunol. 2005;6:920–927. doi: 10.1038/ni1235. [DOI] [PubMed] [Google Scholar]
- 29.van Wamel WJ, et al. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on b-hemolysin-converting bacteriophages. J Bacteriol. 2006;188:1310–1315. doi: 10.1128/JB.188.4.1310-1315.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mazmanian SK, et al. Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections. Proc Natl Acad Sci USA. 2000;97:5510–5515. doi: 10.1073/pnas.080520697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDevitt D, et al. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 32.Palmqvist N, et al. Expression of staphylococcal clumping factor A impedes macrophage phagocytosis. Microb Infect. 2004;6:188–195. doi: 10.1016/j.micinf.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 33.Higgins J, et al. Clumping factor A of Staphylococcus aureus inhibits phagocytosis by human polymorphonuclear leucocytes. FEMS Microbiol Lett. 2006;258:290–296. doi: 10.1111/j.1574-6968.2006.00229.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreillon P, et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun. 1995;63:4738–4743. doi: 10.1128/iai.63.12.4738-4743.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thammavongsa V, et al. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206:2417–2427. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thiel M, et al. The critical role of adenosine A2A receptors in downregulation of inflammation and immunity in the pathogenesis of infectious diseases. Microb Infect. 2003;5:515–526. doi: 10.1016/s1286-4579(03)00068-6. [DOI] [PubMed] [Google Scholar]
- 37.Rogers DE, Tompsett R. The survival of staphylococci within human leukocytes. J Exp Med. 1952;95:209–230. doi: 10.1084/jem.95.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gresham HD, et al. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J Immunol. 2000;164:3713–3722. doi: 10.4049/jimmunol.164.7.3713. [DOI] [PubMed] [Google Scholar]
- 39.Stoll H, et al. Staphylococcus aureus deficient in lipidation of prelipoproteins is attenuated in growth and immune activation. Infect Immun. 2005;73:2411–2423. doi: 10.1128/IAI.73.4.2411-2423.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bubeck Wardenburg J, et al. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Nat Acad Sci USA. 2006;103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Skaar EP, et al. Iron-source preference of Staphylococcus aureus infections. Science. 2004;305:1626–1628. doi: 10.1126/science.1099930. [DOI] [PubMed] [Google Scholar]
- 42.Mazmanian SK, et al. Passage of heme-iron across the envelope of Staphylococcus aureus. Science. 2003;299:906–909. doi: 10.1126/science.1081147. [DOI] [PubMed] [Google Scholar]
- 43.Torres VJ, et al. A Staphylococcus aureus regulatory system that responds to host heme and modulates virulence. Cell Host Microbe. 2007;1:109–119. doi: 10.1016/j.chom.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attia AS, et al. Membrane damage elicits an immunomodulatory program in Staphylococcus aureus. PLoS pathogens. 2010;6:e1000802. doi: 10.1371/journal.ppat.1000802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corbin BD, et al. Metal chelation and inhibition of bacterial growth in tissue abscesses. Science. 2008;319:962–965. doi: 10.1126/science.1152449. [DOI] [PubMed] [Google Scholar]
- 46.Friedrich R, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–539. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 47.Panizzi P, et al. The staphylocoagulase family of zymogen activator and adhesion proteins. Cell Mol Life Sci. 2004;61:2793–2798. doi: 10.1007/s00018-004-4285-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Duthie ES, Lorenz LL. Staphylococcal coagulase: mode of action and antigenicity. J Gen Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 49.Rammelkamp CH, et al. Specific coagulases of Staphylococcus aureus. J Exp Med. 1950;91:295–307. doi: 10.1084/jem.91.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bjerketorp J, et al. The von Willebrand factor-binding protein (vWbp) of Staphylococcus aureus is a coagulase. FEMS Microbiol Lett. 2004;234:309–314. doi: 10.1016/j.femsle.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 51.Bjerketorp J, et al. A novel von Willebrand factor binding protein expressed by Staphylococcus aureus. Microbiology. 2002;148:2037–2044. doi: 10.1099/00221287-148-7-2037. [DOI] [PubMed] [Google Scholar]
- 52.Kroh HK, et al. von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proc Natl Acad Sci USA. 2009;106:7786–7791. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viana D, et al. Adaptation of Staphylococcus aureus to ruminant and equine hosts involves SaPI-carried variants of von Willebrand factor-binding protein. Mol Microbiol. 2010;77:1583–1594. doi: 10.1111/j.1365-2958.2010.07312.x. [DOI] [PubMed] [Google Scholar]
- 54.Forsgren A, Sjöquist J. Protein A from S. aureus. I. Pseudo-immune reaction with human gamma-globulin. J Immunol. 1966;97:822–827. [PubMed] [Google Scholar]
- 55.Forsgren A, Quie PG. Effects of staphylococcal protein A on heat labile opsonins. J Immunol. 1974;112:1177–1180. [PubMed] [Google Scholar]
- 56.Hartleib J, et al. Protein A is the von Willebrand factor binding protein of Staphylococcus aureus. Blood. 2000;96:2149–2156. [PubMed] [Google Scholar]
- 57.Forsgren A, et al. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976;6:207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- 58.Roben PW, et al. Human IgM antibodies bind domain D of staphylococcal protein A. J Immunol. 1995;154:6437–6445. [PubMed] [Google Scholar]
- 59.Goodyear CS, Silverman GJ. Death by a B cell superantigen: In vivo VH-targeted apoptotic supraclonal B cell deletion by a staphylococcal toxin. J Exp Med. 2003;197:1125–1139. doi: 10.1084/jem.20020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomez MI, et al. Staphylococcus aureus protein A induces airway epithelial inflammatory responses by activating TNFR1. Nat Med. 2004;10:842–848. doi: 10.1038/nm1079. [DOI] [PubMed] [Google Scholar]
- 61.Chavakis T, et al. Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb Haemost. 2005;94:278–285. doi: 10.1160/TH05-05-0306. [DOI] [PubMed] [Google Scholar]
- 62.Johnson M, et al. Iron-regulated biofilm formation in Staphylococcus aureus Newman requires ica and the secreted protein Emp. Infect Immun. 2008;76:1756–1765. doi: 10.1128/IAI.01635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kristian SA, et al. The ability of biofilm formation does not influence virulence of Staphylococcus aureus and host response in a mouse tissue cage infection model. Microb Pathog. 2004;36:237–245. doi: 10.1016/j.micpath.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 64.Kropec A, et al. Poly-N-acetylglucosamine production in Staphylococcus aureus is essential for virulence in murine models of systemic infection. Infect Immun. 2005;73:68686–66876. doi: 10.1128/IAI.73.10.6868-6876.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cramton SE, et al. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–5433. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu Quoc PH, et al. Isolation and characterization of biofilm formation-defective mutants of Staphylococcus aureus. Infect Immun. 2007;75:1079–1088. doi: 10.1128/IAI.01143-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Burts ML, Missiakas DM. EsaC substrate for the ESAT-6 secretion pathway and its role in persistent infections of Staphylococcus aureus. Mol Microbiol. 2008;69:736–746. doi: 10.1111/j.1365-2958.2008.06324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burts ML, et al. EsxA and EsxB are secreted by an ESAT-6-like system that is required for the pathogenesis of Staphylococcus aureus infections. Proc Natl Acad Sci USA. 2005;102:1169–1174. doi: 10.1073/pnas.0405620102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis JM, Ramakrishnan L. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell. 2009;136:37–49. doi: 10.1016/j.cell.2008.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Russell DG, et al. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10:943–948. doi: 10.1038/ni.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anderson M, et al. EsaD, a secretion factor for the Ess pathway in Staphylococcus aureus. J Bacteriol. 2011 doi: 10.1128/JB.01096-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hussain M, et al. eap gene as novel target for specific identification of Staphylococcus aureus. J Clin Microbiol. 2008;46:470–476. doi: 10.1128/JCM.01425-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Scriba TJ, et al. The Staphyloccous aureus Eap protein activates expression of proinflammatory cytokines. Infect Immun. 2008;76:2164–2168. doi: 10.1128/IAI.01699-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Haggar A, et al. The extracellular adherence protein from Staphylococcus aureus inhibits neutrophil binding to endothelial cells. Infect Immun. 2004;72:6164–6167. doi: 10.1128/IAI.72.10.6164-6167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chavakis T, et al. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat Med. 2002;8:687–693. doi: 10.1038/nm728. [DOI] [PubMed] [Google Scholar]
- 76.Wang H, et al. Extracellular adherence protein of Staphylococcus aureus suppresses disease by inhibiting T-cell recruitment in a mouse model of psoriasis. J Invest Dermatol. 2009;130:743–754. doi: 10.1038/jid.2009.310. [DOI] [PubMed] [Google Scholar]
- 77.Onderdonk AB, et al. Use of a model of intraabdominal sepsis for studies of the pathogenicity of Bacteroides fragilis. Rev Infect Dis. 1984;6:S91–95. doi: 10.1093/clinids/6.supplement_1.s91. [DOI] [PubMed] [Google Scholar]
- 78.Tzianabos AO, et al. Polysaccharide-mediated protection against abscess formation in experimental intra-abdominal sepsis. J Clin Invest. 1995;96:2727–2731. doi: 10.1172/JCI118340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim HK, et al. Non-toxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections. J Exp Med. 2010;207:1863–1870. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeDent AC, et al. Distribution of protein A on the surface of Staphylococcus aureus. J Bacteriol. 2007;189:4473–4484. doi: 10.1128/JB.00227-07. [DOI] [PMC free article] [PubMed] [Google Scholar]