Abstract
Acute kidney injury (AKI) may result from ischemia or by the use of nephrotoxic agents. The incidence of AKI is variable, depends on comorbidities, and ranges from 5 to 35% in all hospitalized patients. The mechanisms of kidney injury exist within a large network of signaling pathways driven by interplay of inflammatory cytokines/chemokines, reactive oxygen species (ROS), and apoptotic factors. The effects and progression of injury overlap extensively with the remarkable ability of the kidney to repair itself both by intrinsic and extrinsic mechanisms that involve specific cell receptors/ligands as well as possible paracrine influences. The fact that kidney injury is usually part of a generalized comorbid condition makes it all the more challenging in terms of assessment of severity. In this review, we attempt to analyze the mechanisms of ischemic injury and repair in acute and chronic kidney disease from the perspectives of both preclinical and human studies.
AKI AND THE COMPLEX SPECTRUM OF DISEASE
Renal injuries exist across a wide spectrum of disease encompassed by the descriptive term acute kidney injury (AKI). This disease spectrum includes all injuries of the kidney ranging from minor dysfunction to a need for dialysis, and therefore is not defined merely by acute kidney failure or acute tubular necrosis.1 Consequently, AKI has historically been complicated to classify in terms of utilizing accurate grading criteria and depiction of clinical outcome. To date, the RIFLE (risk-injury-failure-loss-end-stage kidney disease) classification, along with modifications made by Acute Kidney Injury Network (AKIN) have been used to define severity of AKI.2–4 Waikar et al.5 proposed a definition of AKI using absolute increases in magnitude of serum creatinine (SCr) levels at specific time intervals. This differs from AKIN in that 0.3 mg/dL increases in SCr are only considered significant within a 24-h time interval, instead of up to 48 h. Also, the period of SCr observational changes is limited to 48 h. In patients with AKI who have underlying chronic kidney disease with a higher baseline SCr, percentage reductions in calculated creatinine clearance were found to be inadequate to detect subtle incremental increases in SCr.5
AKI occurs in 5–35% of all hospitalized patients and is associated with a two- to fivefold increased mortality risk.6 Ischemia and toxins account for the majority of AKI. The kidneys’ vulnerability is attributed to their being a major recipient of cardiac output, having a high level of metabolic activity, the complexity of multiple enzyme pathways, actions of biotransformation enzymes, intricate endothelial cell transport, a large capacity for reabsorption, and a high oxygen (O2) consumption of the outer medulla.7 Patients with AKI often have comorbidities such as sepsis and are often immunocompromised.8 Regions of the kidney most prone to ischemic injury are the S3 segment of the proximal tubule and the medullary thick ascending limb of the loop of Henle (mTAL), as these tubular areas exist physiologically in relatively lower oxygen conditions.9 The S3 segment, in particular, has a much lower capability of producing energy through glycolysis under anaerobic conditions than the mTAL.9 Ischemic reduction in the microvascular blood flow to the outer medulla also occurs in comparison to a normal cortical blood flow under the same conditions, thereby compounding injury to the S3 segment.10 The target of most nephrotoxicants is the proximal tubule. Aminoglycoside antibiotics and cadmium chloride target the S1, S2 segments.11 Gentamicin, cisplatin, cyclosporine, mercuric chloride, the halogenated hydrocarbon dichlorovinylcysteine and its conjugates, as well cysteine conjugates of organic cations, such as benzylquinolinium, all induce direct tubular toxicity to the S3 segment because they are metabolized and concentrated in this region.11
TISSUE INJURY CLASSIFICATION
Primary Injury
The two most common causes of primary kidney injury are ischemia and toxicant induced; however, other causes may include congenital, autoimmune, infectious, neoplastic, and obstructive diseases, as well as injury by iatrogenic or non-iatrogenic causes. Autoimmune diseases include the small vessel vasculitides, which have the most impact on kidney physiology due to the large number of small vessels present, especially the glomerular capillaries. Epithelial cell injury with disruption of the filtration slit diaphragms results in proteinuria and/or hematuria, and consequent glomerulopathy. Infectious conditions of the kidney include pyelonephritis and renal abscess complication. Many cases of obstructive uropathy may progress to urinary tract infection and kidney failure from chronic back pressure. Glomerulonephritis may be caused by bacterial, viral, fungal, or parasitic infections and may lead to nephritis or nephrotic syndrome.
Secondary Injury
Secondary causes of kidney injury may be due to non-renal organ injury and generalized severe illness, including shock states and chronic malnutrition. Sepsis, for example, causes multiorgan failure including AKI and has a mortality rate up to 70%.12 Another example would be diabetes mellitus, in which hyperglycemia triggers stimulation of the renin-angiotensin system (RAS), which promotes inflammation through the angiotensin-1 (AT1) receptor. The formation of superoxides in states of hyperglycemia has been found to be an initiating factor contributing to DNA damage. These ROS have been demonstrated to down regulate the actions of the angiotensin-2 (AT2) receptor, which plays a protective role.13 The AT2 receptor seems to exert a hypotensive effect by decreasing the sensitivity of AT1 receptors to Angiotensin II (Ang II), thereby counteracting the hypertensive actions of AT1.14 AT2 null mice show an increase in blood pressure and vascular sensitivity to Ang II.14
DETECTION OF KIDNEY TISSUE INJURY
The SCr and blood urea nitrogen (BUN) that are most commonly used to detect kidney toxicity/injury in preclinical and clinical studies and in routine clinical care have severe limitations relating to their sensitivity and specificity.15 Both are suboptimal markers following injury since levels are often not reflective of glomerular filtration rate (GFR) due to a number of renal and non-renal influences.16 Furthermore, BUN and SCr concentration render a very delayed signal even after considerable kidney injury and this delay in the diagnosis of AKI prevents timely patient-management decisions, such as withdrawal or reduction in dose of the offending agent or administration of agents to mitigate the injury.17 In a large, multisite, preclinical rodent toxicology study involving 11 structurally and mechanistically different models of renal tubular injury in rats it was clearly demonstrated that BUN and SCr, are effective only with more severe histopathological injury.18 The results obtained by the Predictive Safety Testing Consortium, a collaboration of the pharmaceutical industry, the United States Food and Drug Administration, the European Medicines Agency, and academia have led to qualification of a subset of ‘second generation’ biomarkers for preclinical studies that outperform or add significant value to the conventional biomarkers. There is enthusiasm that these seven qualified urinary biomarkers (albumin, β2-microglobulin, Clusterin, Cystatin C, kidney injury molecule-1, trefoil factor 3, and total protein) will aid in the evaluation of preventative strategies and putative interventions aimed at ameliorating AKI in preclinical and clinical studies.18–21 Additional biomarkers such as neutrophil gelatinase-associated lipocalin,22 interleukin (IL)-18,23 and fatty acid binding protein—liver type24 are recognized to be promising candidates for evaluation of AKI in humans. Qualification of these biomarkers for clinical applications will involve a systematic evaluation of their diagnostic performance in well-controlled observational and/or interventional clinical protocols using both standard-of-care agents with known nephrotoxic properties and/or exploratory agents with renal safety concerns.17,25
MECHANISMS AND PATHOPHYSIOLOGY OF KIDNEY INJURY
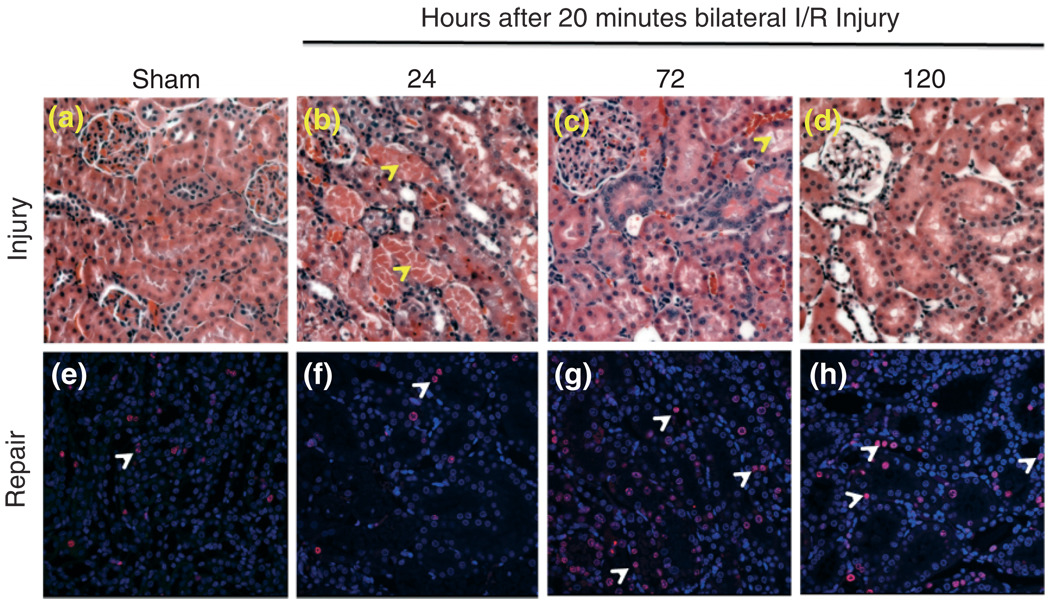
AKI is comprised of both functional and histological changes in the kidney2 that lead to the expression of disease and disease complications, therefore it is very important to understand the mechanisms of kidney injury. As the medical field heads toward more tailored forms of specialized care, there will be greater need for therapies that more specifically target areas of injury. This need is especially great in the face of an increasing incidence of AKI, as well as an increase in the necessity for dialysis over the last 15 years. In clinical AKI, the net effect of acute deterioration in kidney function is usually the result of several, subsequent and accumulative injuries. Reproduction of AKI in animal models has been widely performed in the setting of a single insult, using unilateral or bilateral ischemia followed by reperfusion or administration of drugs or environmental contaminants that induce acute kidney tubular toxicity.18 Widespread damage in the form of dilated, flattened, and congested epithelium, and loss of tubular epithelial cells with luminal cast formation in the S3 segment of the proximal tubules and the mTAL, is especially noted in ischemic and nephrotoxic forms of AKI in animal models (Figure 1(a)–(d)). Most notable as a result of toxicant injury, these casts of epithelial cell, integrin-bound aggregates increase intraluminal pressure and decrease GFR, leading to ‘back-leak’ of filtrate into the tubular cells, which causes a vicious circle of further GFR decline.11
FIGURE 1.
Kidney tissue injury (a–d) and repair (e–h) over time following 20 min of bilateral renal ischemia/reperfusion injury. Male Wistar rats were subjected to sham or bilateral ischemia by clamping the renal pedicles for 20 min and then removing the clamps and confirming reperfusion. Rats were euthanized at various times and kidney tissues were collected. Representative photomicrographs of H&E-stained paraffin-embedded kidney sections (at 200 × magnification) and immunohistochemistry for Ki67 (at 400 × magnification) are presented from the following time points: (a, e) Sham surgery; (b, f) 24 h; (c, g) 72 h; and (d, h) 120 h. All fields were chosen from the cortex and outer medulla. Arrows in panels b and c indicate sloughing of cells, tubular dilation and necrosis. Arrows in panels e–h show Ki67 positive nuclei as an indicator of tubular epithelial cell proliferation.
Inflammatory Molecules
Inflammatory mediators play a central role in the pathogenesis of toxic and ischemia-reperfusion (I/R) injury in the kidney. Due to the relatively larger consumption of O2 in the outer medulla, this region undergoes severe vascular congestion.10 Leukocyte and endothelial cell interactions post-I/R have been described to play a role in the inflammatory progression of acute renal failure (ARF).10 I/R injury causes endothelial cell damage by upregulating adhesion molecules such as intercellular adhesion molecule 1 and 2 (ICAM-1 and -2), CD99, and the junctional adhesion molecule family (JAM), as well as recruiting leukocytes.26 To examine the role of inflammation in the progression of initial injury to acute renal failure, Kelly et al.27 induced bilateral I/R injury to aggravate injury in diabetic obese rats.27 Follow-up revealed a poor renal tissue histology of the diabetic obese ischemic rats as compared to the control groups, as well as a higher SCr. This provided a unique insight into acute on top of chronic injury and showed evidence of a key role for inflammation in leading to sustained injury.27 The interferon (IFN) regulatory factor (IRF-1) is released by S3 segment cells (upon ischemia-induced ROS stimulation) to initiate inflammation in the early stages of injury.28 In transgenic knockout mice of IRF-1, there was improvement of renal function and attenuation of inflammation post-I/R. Although leukocyte IRF-1 protein was not seen by immunohistology, it is a possibility that it is essential for leukocyte differentiation along several cell lines, including T cells, natural killer (NK) cells, macrophages, and dendritic cells.28
Immune Modulators
The early phase of AKI involves activation of kidney dendritic cells, which leads to an amassing of CD4+ T cells, macrophages, B cells, neutrophils, and NK cells.29 Chemokines form gradients by which leukocytes are guided toward the site of injury. This occurs with the help of chemokine receptors, which are expressed by effector T cells.30 Th1 and Th2 T cells are involved in the early stages of tissue destruction post-I/R, as well as in other forms of injury, including autoimmune glomerulonephritis. A new population of T cell secreting IL-17 (Th17) cells demonstrated a role in the evolution of autoimmune disease, the pathophysiology of which is an inflammatory cell-mediated immune response.30 In a study by Satpute et al.,31 T cell deficient nude mice (nu/nu) were examined against the wild-type mice for the effects of T-cell receptor (TCR) repertoire on kidney injury post-I/R. There are two mechanisms by which T cells wield their injurious effects (1) antigen-independent by way of cytokine secretion and ROS/complement activation and (2) antigen-dependent TCR upregulation. TCR repertoire is vital to obtain a full blown tissue injury and may contribute to ischemic pathology without antigen-specific activation.
The late phase of AKI is regulated by CD4+ CD25+ FOX3+ regulatory T cells (Tregs) and macrophages. To demonstrate the protective role of Tregs in I/R-induced AKI, lymph node cells from FOX3+ deficient Scurfy mice were used to reconstitute recombination activating gene knockout (RAG-1−/−) mice. This resulted in a direct inhibition of innate immunity by Tregs.26 Also, an interleukin-10 knockout (IL-10−/−) Treg model showed enhanced I/R injury, highlighting the importance of IL-10 in Treg activity.32 The latter finding is compounded by Tregs’ known role in inhibiting effector T-cell growth and function, and identifies this T-cell subtype as a strong mechanism in the post injury ‘clean house’ that may pave the way for intrinsic repair.
Apoptotic Pathways
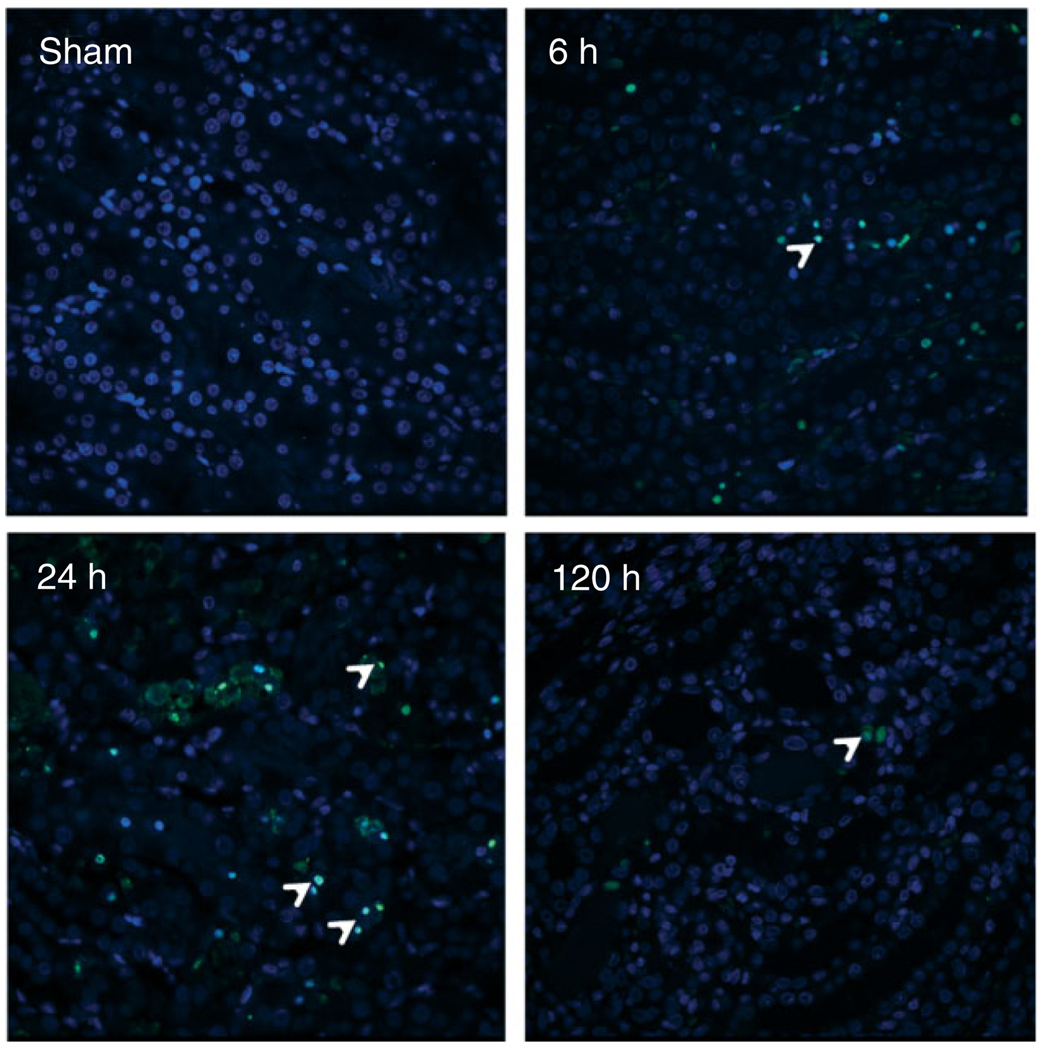
In a 20-min bilateral I/R injury model in rats, we can see an initial increase in apoptotic cells at 6 h as stained by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) which peaks at 24 h and then decreases by 120 h (Figure 2). There exist several interacting pathways and regulators for which the outcome is DNA fragmentation and apoptosis of injured cells. GTP depletion33 is one potential independent modifying factor for apoptosis through the p53 molecule. P53 translocates from the cytosol to the mitochondria, interacting with members of the anti-(Bcl-2, Bcl-xL, Mcl-1) and pro- (Bax, Bak, Bad) apoptotic Bcl-2 family. p53, Bid, and Bim stimulate Bax to induce lipid pores in the outer mitochondrial membrane, thereby triggering membrane permeability and the release of cytochrome c, caspases,34 and apoptosis inducing factor. This sudden and sustained increase in membrane porosity results from oxidative stress-induced mitochondrial Ca2+ overload, with Ca2+ remaining elevated 24 h after reperfusion injury.35 In ischemic damage, both apoptosis and oncosis occur, and therefore preventing either form of cell death in this setting may confer protection against renal function decline.36 Cyclophilin D may play a major role in the instigation of non-defined oncotic signaling pathways through a calcium-triggered loss of membrane potential, with consequent ATP synthesis disruption and cell death.36 In a study using Amphotericin B against cells of a porcine renal tubular epithelial cell line (LLC-PK1), it was found that depolarization of the mitochondrial membrane was induced by elevations in intracellular Na2+ and Ca2+, which, acting in parallel to activation of the mitogen-activated protein kinase (MAPK) pathways, resulted in tubular necrosis.37
FIGURE 2.
Apoptosis in the kidney tissue over time following 20 min of bilateral renal ischemia/reperfusion injury. Male Wistar rats were subjected to sham or bilateral ischemia by clamping the renal pedicles for 20 min and then removing the clamps and confirming reperfusion. Rats were euthanized at various times, kidney tissues were collected and transferase dUTP nick end labeling (TUNEL) immunostaining was performed to label apoptotic cells. Representative photomicrographs at 400 × magnification are presented. Arrows in panels show TUNEL positive nuclei as an indicator of tubular epithelial cell apoptosis.
p53 also exerts apoptotic activity in the cytosol, where it is governed by soluble Bcl-xL, to which p53 has a high affinity. Upon stress activation, Puma rescues p53 from Bcl-xL, and p53 activates cytosolic Bax.34 Tumor necrosis factor (TNF-α) activates the extrinsic pathway through death receptor mediated apoptosis and is responsible for nuclear factor-B (NF-κB) activation, which potentiates leukocyte migration and expression of cytokines and adhesion molecules.38 A link between extrinsic and intrinsic apoptotic pathways occurs through TNF-α action on Bid, resulting in caspase-dependent activated truncated Bid (tBid) incorporation into the mitochondrial cell39 (Figure 3).
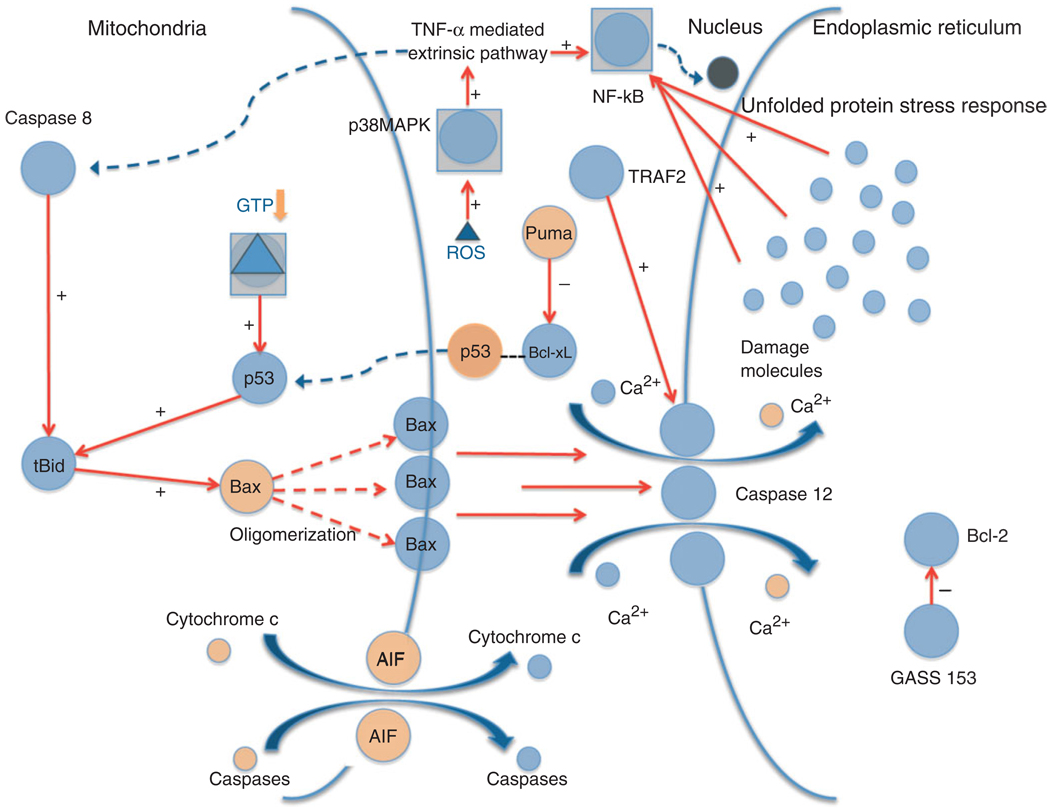
FIGURE 3.
Apoptosis: intrinsic, extrinsic, and endoplasmic reticulum (ER) stress mechanisms. The diagram shows the various signaling pathways leading to apoptosis and the generation of ROS. Upon stress activation, p53 translocates to the mitochondria where it interacts with the BcL-2 family members, including anti-apoptotic BcL-xL and pro-apoptotic Bax and Bak. P53 disrupts inhibitory complexes formed with tBid and Bim, which effectively activate Bax and Bak to oligomerize and form lipid pores in the outer mitochondrial membrane, a process called mitochondrial outer membrane permeabilization (MOMP). The Bax/Bak pathway subsequently activates caspase 12 mediated apoptosis through the release of Ca2+ into the cytoplasm. Cytoplasmic p53 also plays a role in transcription independent apoptosis by being liberated from BcL-xL by Puma, and then activating cytosolic Bax. TNF-α is responsible for extrinsic death receptor mediated apoptosis, which is proposed to be activated by ROS stimulation through the p38 MAPK pathway. This leads to NF-κB and caspase 8 activation, which link the extrinsic pathway to the intrinsic apoptotic mechanism initiated by p53. The ER stress response mechanism might be triggered by the accumulation of ‘damage molecules’ in the ER, stimulating the unfolded protein response (UPR) and activating NF-κB. GADD-153 activates pro-apoptotic factors and downregulates anti-apoptotic BcL-2. Tumor necrosis factor receptor-associated factor-2 (TRAF-2) and caspase 12 stimulation contribute to apoptosis and cell death.
The link between apoptosis and inflammatory progression was exhibited by pharmacological caspase inhibition, which showed anti-inflammatory effects along with preventing apoptosis.38 This concept was also tested by pretreating renal endothelial cells with a broad-spectrum caspase inhibitor, as well as specific caspase 3, 8, and 9 inhibitors, and then exposing cells to TNF-α.38 Apoptosis diminished with all pretreatments, leading to the conclusion that the mitochondrial apoptotic mechanism was being stimulated by TNF-α through caspase 8, which cleaves Bid to its truncated form. The exact mechanism as to how caspase 8 regulates T-cell proliferation and homeostasis of cell-mediated immunity remains elusive. It may occur through stimulation of TCR, B cell, Toll-like receptors (TLR) and/or activation by stress factors like TNF-α and lipopolysaccharides (LPS).38 In humans, a deficiency of caspase 8 results in lymphoproliferative disorders, as well as immunodeficiency secondary to an incapacity to stimulate leukocyte receptors. Mice with a T-cell deletion of caspase 8 (tcasp8−/−) are defective in activation of T-cell proliferation and function, and consequently develop lymphatic hyperplasia and splenomegaly.40
The endoplasmic reticulum (ER) is sensitive to environmental stresses, including I/R injury. This leads to defective folding of proteins that amass inside the lumen of the ER, resulting in aberrations of function known as ER stress. This intrinsic ‘stress system’ provokes transcription of genes such as glucose-related protein-78 (GRP-78) or BiP, with the aim of re-establishing normal function. Pereira et al.41,42 demonstrated that the calcineurin inhibitor, cyclosporine (CsA), may trigger upregulation of the ER stress marker, BiP, in kidney transplant biopsies.41,42 If stress is overwhelming or prolonged, apoptotic pathways activate CHOP or GADD-153, inducing DNA damage and downregulating Bcl-2 while enhancing the actions of tumor necrosis factor receptor-associated factor-2 (TRAF-2) and caspase 12.43 Markan et al.44 examined kidney biopsies of patients with primary glomerular disease to assess levels of GRP-78, GADD-153, and Bcl-2. Patients with proliferative glomerulopathy, which typically progresses to kidney failure, had higher levels of stress proteins than in non-proliferating forms (Figure 3).
Reactive Oxygen Species (ROS) and Oxidative Damage
Robust models of both ischemia- and toxicant-induced generation of ROS have identified major sources of ROS including: NADPH oxidase, damaged mitochondrial electron transport system, lipoxygenases, cyclooxygenases, and xanthine reductase. A disproportion of both ROS sources and protective free radicals (reduced glutathione, catalase, and super-oxide dismutase) result in AKI and chronic kidney disease (CKD).45 In generalized sepsis, kidney failure is common and potentially fatal, and is thought to be due mainly to oxidative damage.46 Oxidative stress activates transcription of inflammatory mediators, including NF-κB. Antioxidants administered to septic rats exert an anti-inflammatory effect by decreasing neutrophil migration in the kidney.46
CKD is a low-grade inflammatory condition, mediated by TNF-α, C-reactive protein, adhesion molecules, and IL-6, among other inflammatory molecules.45 The progression from AKI to CKD is thought to be largely driven by ROS. Histological conversion of kidney tubular epithelial cells and macrophages to collagen over a period of 16 days has been shown in I/R-induced AKI with collagen deposition seen predominantly in the interstitial outer medulla at 8- and 16-day time intervals following I/R injury.47 Pretreatment with a molecule mimicking superoxide dismutase function showed a decrease in kidney fibrosis post-I/R.47 More importantly, indoxyl sulfate (IS), the most robust generator of free radicals among the uremic toxins, effectively drives oxidative stress by increased oxygen consumption in damaged tubular cells.48,49 Proximal tubular cells isolated from Sprague-Dawley rats and humans, and incubated with varying concentrations of IS, showed an increase in oxygen consumption measurements by tubular cells over a significant range of pathophysiological IS concentrations.49 Previous studies also confirmed progression of renal dysfunction and tubulointerstitial damage in rats in correlation with increased IS levels, contributing to the onset of end-stage renal disease (ESRD).49
Chemokines/Chemoattractants
Many signaling factors are involved in the inflammatory process leading AKI into full blown interstitial fibrosis. NF-κB is activated by p38 MAPK and regulates chemokine/chemokine receptor interactions. ROS would presumably induce TNF-α through phosphorylation of p38 MAPK, activating NF-κB to drive oxidative stress-induced inflammation. NF-κB is also activated by a family of pattern recognition receptors (PRR’s) capable of detecting ‘endogenous, endothelial released danger molecules,’ including heat shock proteins and fibrinogen.50,51 The Toll-like receptor family (TLR 1–10), are members of the PRR’s. With the exception of TLR-3, they all initiate stimulation of NF-κB through the myeloid differentiation primary response protein 88 (MyD-88).51 These ‘danger molecules’ accumulate in large amounts and/or uncharacteristic cellular sites.52 It is possible that they may initiate ER stress by unnaturally accumulating in the ER,52 thereby inducing apoptosis through caspases 8 and 12, and a hypothetical T-cell proliferative response through TLR stimulation. TLR-2 may potentially regulate the progression of kidney injury.52 The expression of TLR-2 in murine TLR-2−/− and TLR-2+/+ models of unilateral ureteric obstruction revealed an upregulation of TLR-2 specific danger ligands in TLR-2+/+, as well as a decrease in chemokine and neutrophil levels in TLR-2−/− mice. A decrease in transforming growth factor (TGF)-β in TLR-2 deficient mice, seen as a reduction in tubular apoptotic cells, suggests that there may be a pro-apoptotic mechanism for which TLR-2 is responsible.
KIDNEY TISSUE REPAIR
Recovery from renal injury depends on a number of factors including a prompt and sufficient tissue repair.53 Studies using well-established models of proximal tubular damage have established that rodents mount a compensatory tissue repair response following the nephrotoxic insult leading to recovery of sublethally injured cells, removal of necrotic cells and intratubular casts, and regeneration of renal cells to restore the normal continuity and function of the tubular epithelium.9,53–56 Several studies suggest that there is a very delicate and dynamic relationship between tissue repair and progression or regression of renal injury.57–59 A delay or inhibition of nephrogenic tissue repair appears to lead to progression of nephrotoxic injury. Timely tissue repair appears to restrain progression of injury leading to regression of injury, thereby paving the way for recovery. Structural and functional recovery from acute tubular damage is largely governed by the simultaneous proliferation of the surviving epithelial cells to adapt to the loss of adjacent cells as shown by a robust increase in proliferating cells assessed by the proliferation marker Ki67 at 72 and 120 h following 20 min bilateral I/R injury in rats (Figure 1(e)–(h)). The molecular basis of recovery from AKI is accompanied by a complex pattern of gene expression that bears a variety of molecular regulators including various growth factors, adhesion molecules, transcription factors, immediate early genes, and proto-oncogenes known to regulate the renal tubule repair.
Growth Factors, Adhesion Molecules, and Cell Cycle Regulators
Growth factors and adhesion molecules represent two major classes of genes that are expressed during kidney repair and influence cell division, differentiation, and cell survival or apoptotic processes thought to be essential for tissue repair. A number of mediators such as epidermal growth factor (EGF), heparin binding epidermal growth factor-like growth factor (HB-EGF), TGF-α, insulin-like growth factor-1 (IGF-1), and hepatocyte growth factor (HGF) are upregulated after AKI and exert their actions directly on the kidney.60,61 Many of these growth factors are produced in the renal tissue and participate via autocrine or paracrine processes. Others, such as HGF, act via the endocrine pathway and interact with the kidney through circulation. These polypeptide messengers stimulate cells in the G0 phase of the cell cycle via G1 to initiate DNA synthesis and subsequently to undergo mitosis. After a transient injury, resilient quiescent epithelial cells of the proximal tubule and the thick ascending limb of Henle’s loop reenter the cell cycle and the injured nephron segments become mitogenically highly active. Binding of the growth factors to specific receptors, which reside on the cell surface, induces activation of the intracellular signaling pathway and the growth promoting signals commit the cell to a new phase of the cell cycle (G1).
The Wingless (Wnt) signaling pathway has been shown by Lin et al.62 to guide cells past the G2 cell cycle check point. This effectively avoids apoptosis, resulting in basement membrane regeneration. β-Catenin, a structural component of the intercellular junction, migrates to the nucleus during metabolic stress and stimulates the Wnt signaling mammalian counterpart to induce cell proliferation and repair. The mechanism behind β-Catenin/Wnt pro-survival signaling is through regulation of Bax phosphorylation (inhibition of Bax mitochondrial translocation) by Akt, a robust anti-apoptotic protein.63 Post-injury kidney macrophages have increased expression of Wnt signaling ligands, which interact with specific receptors, including Fzd4, in the proximal tubular epithelium.64 Receptors for pro-survival and repair are highly expressed on the proximal tubular epithelium and are receptive to factors secreted by the distal tubular epithelium.53 CSF-1, a hematopoietic growth factor, has been proposed to exert a paracrine/autocrine influence on the proliferation. The CSF-1 receptor is expressed on macrophages as well as tubular epithelial cells (TECs), co-expressing with CSF-1 in acute injury to enhance TEC proliferation and decrease apoptosis. It is also theorized to be involved in a macrophage-dependent repair mechanism.65
In contrast to the role of the cell cycle as an initiator for regeneration, cell cycle dysregulation in the setting of severe acute kidney damage may lead to excessive fibrosis.66 Yang et al.67 used various approaches to show an increased number of G2/M arrested proximal tubular epithelial cells expressing high levels of TGF-β post-I/R injury. Conditioned media from these arrested cells stimulated collagen and fibroblast synthesis in vitro.66
The role of IGF-1 has been largely investigated in post ischemia experimental animal models, and results have shown increases in the GFR via direct action on the glomerular vessels. In rats, infusion of IGF-1 decreases both afferent and efferent glomerular arteriolar resistances and consequently increases the glomerular ultrafiltration coefficient.68 IGF-1 given post-operatively to patients in whom blood flow to the kidneys had been interrupted effectively eliminated the drop in GFR that occurred in placebo-treated patients. This study was limited in conclusiveness, however, due to the low number of patients displaying ARF, and a second similar trial proved to be of little value due to the heterogeneity of ARF etiologies in the patient population.68 In ESRD, the question of whether or not these patients are responsive or have uremia-induced resistance to the renal effects of IGF-1, has remained controversial also. In a double-blinded, placebo-controlled randomized trial, IGF-1 was administered to individuals whose baseline inulin clearances were at a level where dialysis would be considered. Clearances of both inulin and p-aminohippurate were at levels comparable to the effects of dialysis.68,69 Although promising, to date, no single growth factor has proven to be efficacious in promoting recovery of the postischemic kidney in man.69,70
Differences in the effects of growth factors between animal and human models may be accounted for by several factors. The multifactorial picture of ARF in clinical studies is one explanation for this discrepancy and requires further exploration before we are able to fully appreciate the potential impact that growth factors such as IGF-1 will have on clinical outcome. It may also be that timing of growth factor administration is critical, as they are usually given directly following injury in animal models, whereas in clinical studies, growth factors are administered when AKI is somewhat advanced.71 The epidermal growth factor (EGF) has been found to be highly upregulated in both ischemia and toxicant-induced injury, and is capable of stimulating proliferation of kidney epithelial and progenitor cells through the EGF receptor, which is upregulated by IGF-1.71 Interestingly, in an EGF KO model, protection from injury was demonstrated in the initial phases of ischemia as evidenced by severe tubular necrosis in WT mice as compared to KO mice at 6 h.72 Likewise, the expression of the vascular endothelial growth factor (VEGF) was also shown to be reduced in the initial postischemic phase in models of progressive kidney disease.73 Leonard et al73 utilized a rat I/R model exposed to an elevated salt diet, to demonstrate attenuation in progression to CKD as a result of recombinant VEGF administration. Interstitial scarring and albuminuria were effectively eliminated in rats given VEGF during the initial 2 weeks post injury, however this effect was not observed when VEGF was delayed until day 21. Another possible explanation for differences in response to growth factors may be the fact that several types of cells, including epithelial, endothelial, glomerular, and mesangial cells are involved in kidney injury and tissue repair. It is no simple matter to account for the extent of influence of a specific growth factor when varying types and severities of cellular damage are involved.
Angiogenesis
In human and animal studies, deficits occur in the outer medulla during I/R injury.74 Microvascular permeability occurs by leukocyte-endothelial interactions with shedding of the glycocalyx, alterations of cell-cell contact by breakdown of the actin cytoskeleton, and disruption of the perivascular matrix. This results in hemoconcentration and vascular congestion.74 Under these induced hypoxic conditions, the kidney responds by secreting a number of vasoactive as well as angiogenic factors in order to maintain vascular flow and promote oxygenation of tissue. These vasoactive factors include the RAS, which promotes blood volume/pressure control in vivo. The activation of endogenous RAS in murine proximal epithelial tubular cells in vitro leads to increased extracellular synthesis of angiotensin II, which consequently exerts autocrine actions to generate the VEGF.75 VEGF promotes vascular proliferation and endothelial cell repair,76 and has been shown to be strongly expressed in podocytes as well as proximal tubular epithelium in both mouse and human kidneys.75 Administering exogenous VEGF lead to an improvement in the renal blood flow and microvascular density in both the cortex and medulla, as well as an increase in downstream angiogenic signaling factors, including angiopoietins and endothelial nitric oxide synthase.77,78
Endothelial and vascular smooth muscle cells have been identified as expressors of the Nox 1, 2, and 4 subunits of NADPH oxidase, which functions as a vascular proliferative factor. This reparative function is a relatively novel one for NADPH, as it has classically been understood to enhance oxidative stress and inflammatory endothelial damage, which lead to fibrosis and ESRD.79 VEGF stimulates NADPH production of ROS, encouraging cell proliferation, migration, and differentiation for angiogenesis. The complexity of the Nox isoforms, and their roles in modulating between vascular repair and fibrosis, is not fully understood. The link between NADPH and the differentiation and regulation of embryonic stem cells in the myocardium is another aspect of repair that has not yet been examined directly in kidney cells.79
Renal Progenitors
Regeneration of damaged TECs has been found to occur mainly from populations of surviving epithelial cells post injury. These surviving TECs may proliferate and produce new cells or dedifferentiate and reenter the cell cycle.53 Foxc2, a transcription factor, acts as one of the moderators for this dedifferentiation and redifferentiation in favor of repair and survival.80 When murine glomerular parietal-epithelial cells undergoing epithelial–mesenchymal transition were implanted in uninephrectomised mice, the cells formed immature glomeruli, thereby showing a capability for transfer of renal progenitor traits by this mechanism.81 Adult renal stem/progenitor cells (ARPCs) CD133+/CD24+/PAX2+ have been proposed to reside mainly in the Bowman’s capsule and proximal tubules, and may be capable of differentiating into various cell lines.82 Sallustio et al82 used a microarray analysis to recover significant gene expression of TLR-2 by ARPCs, advocating the potential activation of these cells through TLR-2/‘danger molecule’ interaction. To investigate whether adult stem/progenitor cells contribute to epithelial cell renewal following kidney injury, Humphreys et al.83 generated a transgenic mouse in which majority of tubular epithelial cells, but no interstitial cells, were labeled with either β-galactosidase (lacZ) or red fluorescent protein (RFP). Upon I/R injury to these mice despite an extensive tubular epithelial cell proliferation, no dilution of either cell-fate marker was observed, emphasizing that the predominant mechanism of adult kidney tissue repair is through surviving tubular epithelial cells.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) have been highlighted to play a major role in both repair of acute tubular injury after I/R and cisplatin injury, as well as improving function in chronic kidney failure.84 MSCs release microvesicles (MVs) that may contain mRNA transmitting the differentiative properties of MSCs, as well as regulatory factors of transcription and proliferation, conferring a resistance to apoptosis. These MVs are also thought to communicate inter-cellularly and influence the function of progenitor cells to stimulate angiogenesis and other reparative processes. One proposed hypothesis is that MVs express cell line specific surface adhesion molecules (CD44, CD29), and may utilize ligand/receptor interactions to induce vascular permeability modification and endothelial as well as epithelial cell proliferation.85 When human MSC-derived MVs were given intravenously, they exerted similar effects attributed to MSCs in the recovery of glycerol-induced AKI damage in immunodeficient mice.85 It is obvious that MVs may have a significant therapeutic role to play in kidney repair in the future. Furthermore, they are capable of not only sharing genetic information between cells but are also capable of remaining in a stable state, unlike MSCs, which may maldifferentiate.84,85
CONCLUSION
Kidney injury and tissue repair are dynamic events in the spectrum of kidney disease progression and regression. The factors that govern this delicate balance between injury and repair may need to be analyzed extensively for future therapies to have a hope of setting back the ‘ticking clock’ of AKI progression into terminal disease (Figure 4). The inflammatory process, and its role in the progression of AKI to fibrosis and eventual CKD, has huge implications for targeted therapy. Involvement of apoptotic pathways in inflammation, as revealed by caspase inhibition, may be the key to unlocking one of the earliest mechanisms of injury. Studies examining the role of TLRs and danger molecule ligands in the ER stress response, as well as the reparative properties of TLR-2 will hopefully provide more information on the common pathways between apoptosis and inflammation. With a focus on the kidneys’ intrinsic capability for repair, it is not difficult to assume momentum will be directed at discerning mechanisms of this internal repair process. The role of NADPH in promoting differentiation of epithelial cells is of great interest with respect to CKD development. The eventual use of microvessels to ‘program’ cells for repair is a very exciting possibility, as well as the use of siRNA to offset pro-apoptotic and therefore inflammatory pathways.
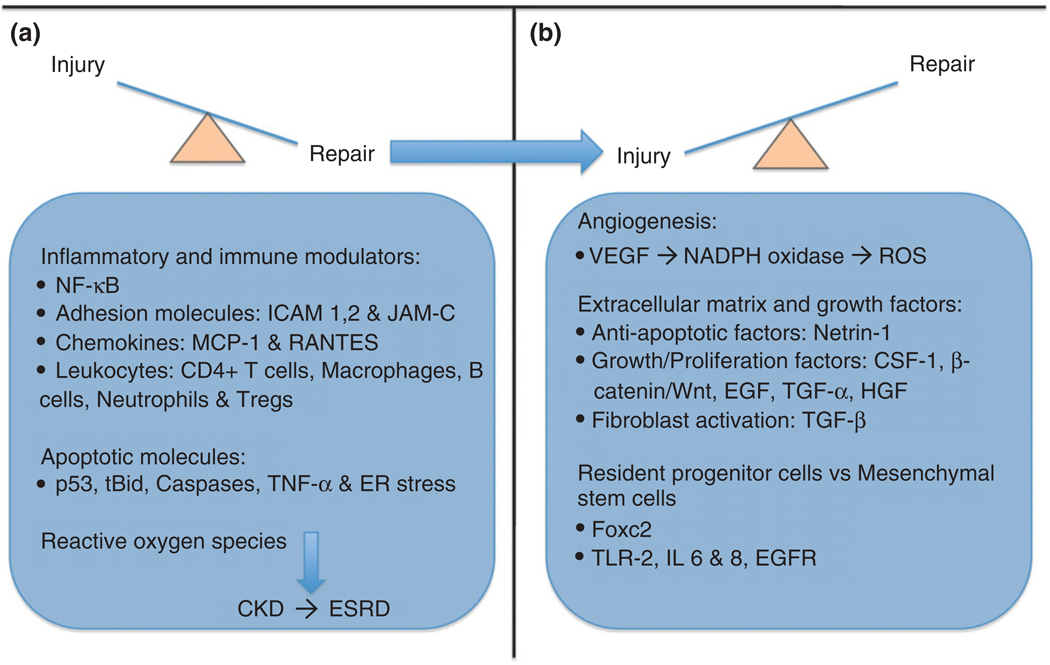
FIGURE 4.
Overview of injury and repair mechanisms. In the early stages of kidney injury, there is involvement of numerous inflammatory and immune modulators, including NF-κB, adhesion molecules such as ICAM and junctional adhesion molecule (JAM)-C, chemokines, neutrophils, and CD4+ T cells. Apoptotic molecules including p53, caspases, and tBid play a corresponding role along with these inflammatory factors. The late stages of injury involve ‘clean house’ molecules including mainly Tregs and macrophages in preparation for tissue recovery. If the insult is severe and/or prolonged it may progress to chronic kidney disease (CKD), driven by the production of free radicals and activated fibroblasts. Reparative mechanisms include angiogenic factors such as vascular endothelial growth factor (VEGF), anti-apoptotic factors like Netrin-1, and growth/proliferation factors such as Wingless (Wnt)/β-catenin, and transforming growth factor (TGF)-α and -β. Transcription factors including the Fox family, as well as Toll-like receptors (TLR)-2 and interleukin (IL)-6, -8 are involved in the influences of adult/renal progenitor stem cells and mesenchymal cells on repair.
ACKNOWLEDGEMENTS
The authors would like to acknowledge Victoria Ramirez and Aparna Krishnamoorthy for critical reading of this manuscript and help with microscopy and imaging. Vaidya’s laboratory is supported by the National Institutes of Health grant ES016723 and a pilot grant from Harvard Catalyst, The Harvard Clinical and Translational Science Center (NIH Grant #1 UL1 RR 025758-02).
REFERENCES
- 1.Kellum JA. Acute kidney injury. Crit Care Med. 2008;36(4 suppl):S141–S145. doi: 10.1097/CCM.0b013e318168c4a4. [DOI] [PubMed] [Google Scholar]
- 2.Soni SS, Ronco C, Katz N, Cruz DN. Early diagnosis of acute kidney injury: the promise of novel biomarkers. Blood Purif. 2009;28:165–174. doi: 10.1159/000227785. [DOI] [PubMed] [Google Scholar]
- 3.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: a prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–1376. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Murray PT, Devarajan P, Levey AS, Eckardt KU, Bonventre JV, Lombardi R, Herget-Rosenthal S, Levin A. A framework and key research questions in AKI diagnosis and staging in different environments. Clin J Am Soc Nephrol. 2008;3:864–868. doi: 10.2215/CJN.04851107. [DOI] [PubMed] [Google Scholar]
- 5.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol. 2009;20:672–679. doi: 10.1681/ASN.2008070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanbay M, Kasapoglu B, Perazella MA. Acute tubular necrosis and pre-renal acute kidney injury: utility of urine microscopy in their evaluation—a systematic review. Int Urol Nephrol. 2010;42:425–433. doi: 10.1007/s11255-009-9673-3. [DOI] [PubMed] [Google Scholar]
- 7.Dieterle F, Marrer E, Suzuki E, Grenet O, Cordier A, Vonderscher J. Monitoring kidney safety in drug development: emerging technologies and their implications. Curr Opin Drug Discov Devel. 2008;11:60–71. [PubMed] [Google Scholar]
- 8.Thadhani R, Pascual M, Bonventre JV. Acute renal failure. N Engl J Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 9.Bonventre JV. Molecular response to cytotoxic injury: role of inflammation, MAP kinases, and endoplasmic reticulum stress response. Semin Nephrol. 2003;23:439–448. doi: 10.1016/s0270-9295(03)00115-3. [DOI] [PubMed] [Google Scholar]
- 10.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 11.Lash LH, Cummings BS. Mechanisms of toxicant-induced acute kidney injury. In: Schnellmann RG, editor. Comprehensive Toxicology–Renal Toxicology. Oxford: Elsevier; 2010. pp. 81–116. [Google Scholar]
- 12.Doi K, Leelahavanichkul A, Yuen PS, Star RA. Animal models of sepsis and sepsis-induced kidney injury. J Clin Invest. 2009;119:2868–2878. doi: 10.1172/JCI39421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Steckelings UM, Rompe F, Kaschina E, Unger T. The evolving story of the RAAS in hypertension, diabetes and CV disease: moving from macrovascular to microvascular targets. Fundam Clin Pharmacol. 2009;23:693–703. doi: 10.1111/j.1472-8206.2009.00780.x. [DOI] [PubMed] [Google Scholar]
- 14.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- 15.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 16.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 17.Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nat Biotechnol. 2010;28:436–440. doi: 10.1038/nbt0510-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaidya VS, Ozer JS, Dieterle F, Collings FB, Ramirez V, Troth S, Muniappa N, Thudium D, Gerhold D, Holder DJ. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozer JS, Dieterle F, Troth S, Perentes E, Cordier A, Verdes P, Staedtler F, Mahl A, Grenet O, Roth DR. A panel of urinary biomarkers to monitor reversibility of renal injury and a serum marker with improved potential to assess renal function. Nat Biotechnol. 2010;28:486–494. doi: 10.1038/nbt.1627. [DOI] [PubMed] [Google Scholar]
- 20.Yu Y, Jin H, Holder D, Ozer JS, Villarreal S, Shughrue P, Shi S, Figueroa DJ, Clouse H, Su M, et al. Urinary biomarkers trefoil factor 3 and albumin enable early detection of kidney tubular injury. Nat Biotechnol. 2010;28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 21.Dieterle F, Perentes E, Cordier A, Roth DR, Verdes P, Grenet O, Pantano S, Moulin P, Wahl D, Mahl A, et al. Urinary clusterin, cystatin C, beta2-microglobulin and total protein as markers to detect drug-induced kidney injury. Nat Biotechnol. 2010;28:463–469. doi: 10.1038/nbt.1622. [DOI] [PubMed] [Google Scholar]
- 22.Devarajan P. Review: neutrophil gelatinase-associated lipocalin: a troponin-like biomarker for human acute kidney injury. Nephrology (Carlton) 2010;15:419–428. doi: 10.1111/j.1440-1797.2010.01317.x. [DOI] [PubMed] [Google Scholar]
- 23.Siew ED, Ikizler TA, Gebretsadik T, Shintani A, Wickersham N, Bossert F, Peterson JF, Parikh CR, May AK, Ware LB. Elevated urinary IL-18 levels at the time of ICU admission predict adverse clinical outcomes. Clin J Am Soc Nephrol. 2010;5:1497–1505. doi: 10.2215/CJN.09061209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferguson MA, Vaidya VS, Waikar SS, Collings FB, Sunderland K, Gioules C, Bonventre JV. Urinary liver-type fatty acid binding protein is predictive of adverse outcomes in acute kidney injury. Kidney Int. 2010;77:708–714. doi: 10.1038/ki.2009.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. In: Schnellmann RG, editor. Comprehensive Toxicology–Renal Toxicology. Oxford: Elsevier; 2010. pp. 197–211. [Google Scholar]
- 26.Scheiermann C, Colom B, Meda P, Patel NS, Voisin MB, Marrelli A, Woodfin A, Pitzalis C, Thiemermann C, Aurrand-Lions M, et al. Junctional adhesion molecule-C mediates leukocyte infiltration in response to ischemia reperfusion injury. Arterioscler Thromb Vasc Biol. 2009;29:1509–1515. doi: 10.1161/ATVBAHA.109.187559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly KJ, Burford JL, Dominguez JH. Postischemic inflammatory syndrome: a critical mechanism of progression in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F923–F931. doi: 10.1152/ajprenal.00205.2009. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, John R, Chen J, Richardson JA, Shelton JM, Bennett M, Zhou XJ, Nagami GT, Zhang Y, Wu QQ, et al. IRF-1 promotes inflammation early after ischemic acute kidney injury. J Am Soc Nephrol. 2009;20:1544–1555. doi: 10.1681/ASN.2008080843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87:859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 30.Steinmetz OM, Turner JE, Paust HJ, Lindner M, Peters A, Heiss K, Velden J, Hopfer H, Fehr S, Krieger T, et al. CXCR3 mediates renal Th1 and Th17 immune response in murine lupus nephritis. J Immunol. 2009;183:4693–4704. doi: 10.4049/jimmunol.0802626. [DOI] [PubMed] [Google Scholar]
- 31.Satpute SR, Park JM, Jang HR, Agreda P, Liu M, Gandolfo MT, Racusen L, Rabb H. The role for T cell repertoire/antigen-specific interactions in experimental kidney ischemia reperfusion injury. J Immunol. 2009;183:984–992. doi: 10.4049/jimmunol.0801928. [DOI] [PubMed] [Google Scholar]
- 32.Kinsey GR, Sharma R, Huang L, Li L, Vergis AL, Ye H, Ju ST, Okusa MD. Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J Am Soc Nephrol. 2009;20:1744–1753. doi: 10.1681/ASN.2008111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagher PC. Apoptosis in ischemic renal injury: roles of GTP depletion and p53. Kidney Int. 2004;66:506–509. doi: 10.1111/j.1523-1755.2004.761_7.x. [DOI] [PubMed] [Google Scholar]
- 34.Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–420. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang HL, Sedlic F, Bosnjak Z, Nilakantan V. SOD1 and MitoTEMPO partially prevent mitochondrial permeability transition pore opening, necrosis, and mitochondrial apoptosis after ATP depletion recovery. Free Radic Biol Med. 2010;49:1550–1560. doi: 10.1016/j.freeradbiomed.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devalaraja-Narashimha K, Diener AM, Padanilam BJ. Cyclophilin D gene ablation protects mice from ischemic renal injury. Am J Physiol Renal Physiol. 2009;297:F749–F759. doi: 10.1152/ajprenal.00436.2003. [DOI] [PubMed] [Google Scholar]
- 37.Yano T, Itoh Y, Kawamura E, Maeda A, Egashira N, Nishida M, Kurose H, Oishi R. Amphotericin B-induced renal tubular cell injury is mediated by Na+ influx through ion-permeable pores and subsequent activation of mitogen-activated protein kinases and elevation of intracellular Ca2+ concentration. Antimicrob Agents Chemother. 2009;53:1420–1426. doi: 10.1128/AAC.01137-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu X, Guo R, Chen P, Wang Q, Cunningham PN. TNF induces caspase-dependent inflammation in renal endothelial cells through a Rho- and myosin light chain kinase-dependent mechanism. AmJ Physiol Renal Physiol. 2009;297:F316–F326. doi: 10.1152/ajprenal.00089.2009. [DOI] [PubMed] [Google Scholar]
- 39.Campbell MT, Dagher P, Hile KL, Zhang H, Meldrum DR, Rink RC, Meldrum KK. Tumor necrosis factor-alpha induces intrinsic apoptotic signaling during renal obstruction through truncated bid activation. J Urol. 2008;180:2694–2700. doi: 10.1016/j.juro.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salmena L, Hakem R. Caspase-8 deficiency in T cells leads to a lethal lymphoinfiltrative immune disorder. J Exp Med. 2005;202:727–732. doi: 10.1084/jem.20050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira BJ, Castro I, Burdmann EA, Malheiros DM, Yu L. Effects of sirolimus alone or in combination with cyclosporine A on renal ischemia/reperfusion injury. Braz J Med Biol Res. 2010;43:737–744. doi: 10.1590/s0100-879x2010007500058. [DOI] [PubMed] [Google Scholar]
- 42.Pallet N, Rabant M, Xu-Dubois YC, Lecorre D, Mucchielli MH, Imbeaud S, Agier N, Hertig A, Thervet E, Legendre C, et al. Response of human renal tubular cells to cyclosporine and sirolimus: a toxicogenomic study. Toxicol Appl Pharmacol. 2008;229:184–196. doi: 10.1016/j.taap.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 43.Inagi R. Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol. 2009;112:e1–e9. doi: 10.1159/000210573. [DOI] [PubMed] [Google Scholar]
- 44.Markan S, Kohli HS, Joshi K, Minz RW, Sud K, Ahuja M, Anand S, Khullar M. Up regulation of the GRP-78 and GADD-153 and down regulation of Bcl-2 proteins in primary glomerular diseases: a possible involvement of the ER stress pathway in glomerulonephritis. Mol Cell Biochem. 2009;324:131–138. doi: 10.1007/s11010-008-9991-2. [DOI] [PubMed] [Google Scholar]
- 45.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int Suppl. 2008;111:S4–S9. doi: 10.1038/ki.2008.516. [DOI] [PubMed] [Google Scholar]
- 46.Andrades M, Ritter C, de Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F. Antioxidant treatment reverses organ failure in rat model of sepsis: role of antioxidant enzymes imbalance, neutrophil infiltration, and oxidative stress. J Surg Res. 2009 doi: 10.1016/j.jss.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 47.Kim J, Seok YM, Jung KJ, Park KM. Reactive oxygen species/oxidative stress contributes to progression of kidney fibrosis following transient ischemic injury in mice. Am J Physiol Renal Physiol. 2009;297:F461–F470. doi: 10.1152/ajprenal.90735.2008. [DOI] [PubMed] [Google Scholar]
- 48.Barreto FC, Barreto DV, Liabeuf S, Meert N, Glorieux G, Temmar M, Choukroun G, Vanholder R, Massy ZA. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin J Am Soc Nephrol. 2009;4:1551–1558. doi: 10.2215/CJN.03980609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palm F, Nangaku M, Fasching A, Tanaka T, Nordquist L, Hansell P, Kawakami T, Nishijima F, Fujita T. Uremia induces abnormal oxygen consumption in tubules and aggravates chronic hypoxia of the kidney via oxidative stress. Am J Physiol Renal Physiol. 2010;299:F380–F386. doi: 10.1152/ajprenal.00175.2010. [DOI] [PubMed] [Google Scholar]
- 50.Pulskens WP, Teske GJ, Butter LM, Roelofs JJ, van der Poll T, Florquin S, Leemans JC. Toll-like receptor-4 coordinates the innate immune response of the kidney to renal ischemia/reperfusion injury. PLoS One. 2008;3:e3596. doi: 10.1371/journal.pone.0003596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Opitz B, Eitel J, Meixenberger K, Suttorp N. Role of Toll-like receptors, NOD-like receptors and RIG-I-like receptors in endothelial cells and systemic infections. Thromb Haemost. 2009;102:1103–1109. doi: 10.1160/TH09-05-0323. [DOI] [PubMed] [Google Scholar]
- 52.Leemans JC, Butter LM, Pulskens WP, Teske GJ, Claessen N, van der Poll T, Florquin S. The role of Toll-like receptor 2 in inflammation and fibrosis during progressive renal injury. PLoS One. 2009;4:e5704. doi: 10.1371/journal.pone.0005704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benigni A, Morigi M, Remuzzi G. Kidney regeneration. Lancet. 2010;375:1310–1317. doi: 10.1016/S0140-6736(10)60237-1. [DOI] [PubMed] [Google Scholar]
- 54.Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14 suppl 1:S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- 55.Humphreys BD, Bonventre JV. Mesenchymal stem cells in acute kidney injury. Annu Rev Med. 2008;59:311–325. doi: 10.1146/annurev.med.59.061506.154239. [DOI] [PubMed] [Google Scholar]
- 56.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaidya VS, Shankar K, Lock EA, Bucci TJ, Mehendale HM. Renal injury and repair following S-1, 2 dichlorovinylL-cysteine administration to mice. Toxicol Appl Pharmacol. 2003;188:110–121. doi: 10.1016/s0041-008x(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 58.Vaidya VS, Shankar K, Lock EA, Bucci TJ, Mehendale HM. Role of tissue repair in survival from s-(1,2-dichlorovinyl)-L-cysteine-induced acute renal tubular necrosis in the mouse. Toxicol Sci. 2003;74:215–227. doi: 10.1093/toxsci/kfg111. [DOI] [PubMed] [Google Scholar]
- 59.Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. doi: 10.1172/JCI117214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hammerman MR. Growth factors and apoptosis in acute renal injury. Curr Opin Nephrol Hypertens. 1998;7:419–424. doi: 10.1097/00041552-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 61.Schena FP. Role of growth factors in acute renal failure. Kidney Int Suppl. 1998;66:S11–S15. [PubMed] [Google Scholar]
- 62.Lin SL, Li B, Rao S, Yeo EJ, Hudson TE, Nowlin BT, Pei H, Chen L, Zheng JJ, Carroll TJ, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci U S A. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Havasi A, Gall JM, Mao H, Schwartz JH, Borkan SC. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009;20:1919–1928. doi: 10.1681/ASN.2009030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Menke J, Iwata Y, Rabacal WA, Basu R, Yeung YG, Humphreys BD, Wada T, Schwarting A, Stanley ER, Kelley VR. CSF-1 signals directly to renal tubular epithelial cells to mediate repair in mice. J Clin Invest. 2009;119:2330–2342. doi: 10.1172/JCI39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wynn TA. Fibrosis under arrest. Nat Med. 16:523–525. doi: 10.1038/nm0510-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 16:535–543. doi: 10.1038/nm.2144. 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hammerman MR. The growth hormone-insulin-like growth factor axis in kidney re-revisited. Nephrol Dial Transplant. 1999;14:1853–1860. doi: 10.1093/ndt/14.8.1853. [DOI] [PubMed] [Google Scholar]
- 69.Hammerman MR. Insulin-like growth factor I treatment for end-stage renal disease at the end of the millennium. Curr Opin Nephrol Hypertens. 2000;9:1–3. doi: 10.1097/00041552-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 70.Hammerman MR. Potential role of growth factors in the prophylaxis and treatment of acute renal failure. Kidney Int Suppl. 1998;64:S19–S22. [PubMed] [Google Scholar]
- 71.Liu KD, Brakeman PR. Renal repair and recovery. Crit Care Med. 2008;36(4 suppl):S187–S192. doi: 10.1097/CCM.0b013e318168ca4a. [DOI] [PubMed] [Google Scholar]
- 72.Mulder GM, Nijboer WN, Seelen MA, Sandovici M, Bos EM, Melenhorst WB, Trzpis M, Kloosterhuis NJ, Visser L, Henning RH, et al. Heparin binding epidermal growth factor in renal ischaemia/reperfusion injury. J Pathol. 2010;221:183–192. doi: 10.1002/path.2698. [DOI] [PubMed] [Google Scholar]
- 73.Leonard EC, Friedrich JL, Basile DP. VEGF-121 preserves renal microvessel structure and ameliorates secondary renal disease following acute kidney injury. Am J Physiol Renal Physiol. 2008;295:F1648–F1657. doi: 10.1152/ajprenal.00099.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sutton TA. Alteration of microvascular permeability in acute kidney injury. Microvasc Res. 2009;77:4–7. doi: 10.1016/j.mvr.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Feliers D, Kasinath BS. Mechanism of VEGF expression by high glucose in proximal tubule epithelial cells. Mol Cell Endocrinol. 314:136–142. doi: 10.1016/j.mce.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iliescu R, Fernandez SR, Kelsen S, Maric C, Chade AR. Role of renal microcirculation in experimental renovascular disease. Nephrol Dial Transplant. 25:1079–1087. doi: 10.1093/ndt/gfp605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hendel RC, Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Bonow RO. Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation. 2000;101:118–121. doi: 10.1161/01.cir.101.2.118. [DOI] [PubMed] [Google Scholar]
- 78.Henry TD, Rocha-Singh K, Isner JM, Kereiakes DJ, Giordano FJ, Simons M, Losordo DW, Hendel RC, Bonow RO, Eppler SM, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–880. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 79.Chan EC, Jiang F, Peshavariya HM, Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Ther. 2009;122:97–108. doi: 10.1016/j.pharmthera.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 80.Hader C, Marlier A, Cantley L. Mesenchymal-epithelial transition in epithelial response to injury: the role of Foxc2. Oncogene. 29:1031–1040. doi: 10.1038/onc.2009.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swetha G, Chandra V, Phadnis S, Bhonde R. Glomerular parietal epithelial cells of adult murine kidney undergo EMT to generate cells with traits of renal progenitors. J Cell Mol Med. 2009 doi: 10.1111/j.1582-4934.2009.00937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sallustio F, De Benedictis L, Castellano G, Zaza G, Loverre A, Costantino V, Grandaliano G, Schena FP. TLR2 plays a role in the activation of human resident renal stem/progenitor cells. FASEB J. 2010;24:514–525. doi: 10.1096/fj.09-136481. [DOI] [PubMed] [Google Scholar]
- 83.Humphreys BD, Valerius MT, Kobayashi A, Mugford JW, Soeung S, Duffield JS, McMahon AP, Bonventre JV. Intrinsic epithelial cells repair the kidney after injury. Cell Stem Cell. 2008;2:284–291. doi: 10.1016/j.stem.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 84.Semedo P, Correa-Costa M, Antonio Cenedeze M, Maria Avancini Costa Malheiros D, Antonia dos Reis M, Shimizu MH, Seguro AC, Pacheco-Silva A, Saraiva Camara NO. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 85.Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, Morando L, Busca A, Falda M, Bussolati B, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20:1053–1067. doi: 10.1681/ASN.2008070798. [DOI] [PMC free article] [PubMed] [Google Scholar]
FURTHER READING
- Brenner BM, Rector FC. Brenner & Rector’s the Kidney. 8th ed. Philadelphia, PA: Saunders Elsevier; 2008. [Google Scholar]
- Schrier RW. Manual of Nephrology. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009. [Google Scholar]