Abstract
Enhanced external counterpulsation (EECP) is a non-invasive modality for treatment of symptomatic coronary disease (CAD) in patients not amenable to revascularization procedures. However, the mechanism(s) underlying the benefits of EECP remain unknown. We hypothesized that reductions in arterial stiffness and aortic wave reflection are a therapeutic target for EECP. CAD patients with chronic angina pectoris were randomized (2:1 ratio) to either 35 1-hr sessions of EECP (n=28) or Sham-EECP (n=14). Central and peripheral arterial pulse wave velocity (PWV) and aortic wave reflection (augmentation index; AIx) were measured using applanation tonometry before, and after 17 and 35 1-hr treatment sessions. Wasted left ventricular pressure energy and aortic systolic tension time index, markers of left-ventricular myocardial oxygen demand were derived from the synthesized aortic pressure wave. Exercise duration, anginal threshold, and peak oxygen consumption were measured using a graded treadmill test. Central arterial stiffness and AIx were reduced following 17- and 35-sessions in the treatment group. Measures of peripheral arterial stiffness were reduced following 35 sessions in the treatment group. Changes in aortic pressure wave reflection resulted in decreased measures of myocardial oxygen demand and wasted left ventricular energy. No changes in either central or peripheral arterial stiffness were observed in the Sham group. Furthermore, measures of exercise capacity were improved in the EECP group, but unchanged in the Sham group. In conclusion, EECP therapy reduces central and peripheral arterial stiffness, which may explain improvements in myocardial oxygen demand in patients with chronic angina pectoris following treatment.
Keywords: arterial stiffness, angina, exercise capacity, wave reflections
Angina pectoris can be caused by either decreased coronary blood supply or increased myocardial oxygen demand. Enhanced external counterpulsation (EECP) has been demonstrated to be an effective treatment for patients with angina pectoris.1–5 The central hypothesis, in most investigations conducted to elucidate the mechanism of action, is that EECP may promote coronary angiogenesis and improved myocardial perfusion.3,4,6–8 Despite evidence of improved myocardial perfusion in dogs 6, there is a lack of data that supports the central hypothesis in humans. A few small non randomized studies have reported that improvements in anginal class and exercise capacity are a result of improved myocardial perfusion.4,7,8 However these findings have not been entirely consistent3, thus suggesting extra-cardiac factors, such as altered peripheral vascular function and myocardial oxygen demand may be the therapeutic target for EECP. Therefore, in the current randomized, sham-controlled study we tested the hypothesis that EECP treatment would decrease central and peripheral arterial stiffness and improve indices of myocardial oxygen consumption in coronary artery disease (CAD) patients with chronic angina pectoris.
Methods
Forty-two consecutive patients with chronic stable angina referred for EECP treatment were randomized in a 2:1 manner into either an EECP treatment group or a Sham-EECP control group. The patients were enrolled for EECP treatment between November 2004 and June 2009 because they experienced chronic angina for more than 3 months caused by myocardial ischemia in the presence of angiographic multivessel coronary artery disease that could not be controlled by a combination of medical therapy, angioplasty/stent, and/or coronary bypass surgery. Patient recruitment was relatively slow due to the single-center study design and the non-metropolitan regional location of Shands Hospital at the University of Florida. The study was approved by the Institutional Review Board of the University of Florida and written informed consent was obtained from all patients. Patient characteristics are presented in Table 1.
Table 1.
Patient Characteristics
| Variable | EECP (N = 28) | SHAM (N = 14) |
|---|---|---|
| Age (years) | 64 ± 2 | 64 ± 3 |
| Men | 22 (79%) | 12 (86%) |
| Height (cm) | 173 ± 2 | 174 ± 2 |
| Weight (kg) | 92.6 ± 3 | 101 ± 3 |
| Body mass index (kg/m2) | 30.9 ± 0.80 | 33.4 ± 1.1 |
| Left ventricular ejection fraction (%) | 51.6 ± 2.8 | 48.2 ± 3.9 |
| Left ventricular ejection fraction < 40% | 5 (18%) | 2 (14%) |
| Prior coronary artery bypass graft | 19 (68%) | 11 (79%) |
| Prior percutaneous transluminal coronary angioplasty | 23 (82%) | 12 (86%) |
| Prior Myocardial Infarction | 17 (61%) | 6 (43%) |
| Multivessel coronary artery disease | 25 (89%) | 13 (93%) |
| Diabetes mellitus | 21 (75%) | 11 (79%) |
| Hypertension | 23 (82%) | 12 (86%) |
| Hyperlipidemia | 26 (93%) | 14 (100%) |
| Lipid Lowering Agent | 28 (100%) | 14 (100%) |
| Beta-Blocker | 24 (86%) | 11 (79%) |
| Calcium Channel Blocker | 11 (39%) | 6 (43%) |
| Long Lasting Nitrates | 25 (89%) | 12 (86%) |
| ACE Inhibitor/ARB | 26 (93%) | 12 (86%) |
| Insulin | 4 (14%) | 2 (14%) |
Data are expressed as mean ± SEM. Medical history, gender and drug regimen data are presented as number of subjects and percentage of total number within each group. Hypertension was defined as a systolic blood pressure >140 mmHg or a diastolic blood pressure > 90 mmHg. Hyperlipidemia was defined as a total cholesterol > 200 mg/dl and or LDL cholesterol > 130 mg/dl. Baseline characteristics were not significantly different between groups.
Exclusion criteria included absence of ST segment depression (1 mm minimum) during exercise testing, coronary artery bypass graft (CABG) within past 3 months or PCI in past 6 months, arrhythmia that would significantly interfere with triggering of the EECP device, symptomatic heart failure and/or LV ejection fraction < 30%, valvular heart disease, ICD if triggered within past 6 months, history of deep vein thrombosis, uncontrolled hypertension, pregnancy, pulmonary congestion, and systemic hypotension.
Patients in the EECP (n = 28) and Sham (n = 14) groups received 35 1-h daily sessions of EECP for 7 consecutive weeks using cuff inflation pressures of 300 and 70 mm Hg, respectively. It was previously determined that 70 mm Hg inflation pressure is adequate to preserve the appearance and feel of EECP application, but insufficient to alter blood pressure.1 EECP equipment (Vasomedical, Westbury, New York) has been described previously.1
Data were collected and analyzed at study entry and after 17 and 35 1-hour sessions of EECP or Sham. All measurements were performed in a quiet, temperature controlled room (21–23°C) with the subjects in a fasting state (10–12 hours). Subjects were asked to abstain from caffeine and alcohol for at least 24 hours prior to vascular measurements. To avoid potential diurnal variations, all measurements were conducted in the morning (7:00–9:00 a.m.).9 All patients discontinued their medications for 12 hours prior to vascular measurements.
Following 15 minutes of supine rest, heart rate (HR) and brachial blood pressure (BP) measurements were performed in triplicate in the left arm using an automated non-invasive BP cuff (Omron, Inc.). An average of the 3 HR and BP measurements were used for resting values. The assessment of arterial wave reflection characteristics was performed non-invasively using the SphygmoCor system (AtCor Medical, Sydney, Australia) described previously.10 Briefly, peripheral pressure waveforms were recorded from the radial artery at the wrist, using applanation tonometry with a high fidelity micromanometer (Millar Instruments, Houston, Texas). A validated, generalized transfer function was used to generate the corresponding aortic pressure waveform.11
Pulse wave analysis of the aortic pressure waveform provided the following key variables of interest; aortic augmentation index (AIx), round trip travel time of the forward traveling wave from the ascending aorta to the major reflection site and back (Δtp), and wasted LV pressure energy (Ew), which is the component of extra myocardial oxygen requirement due to early systolic wave reflection.10,12 Ew was estimated as [(π/4)*(augmented pressure × Δtr)*1.333], where 1.333 is the conversion factor for mmHg/s to dynes·cm2· s and Δtr is the systolic duration of the reflected wave. Augmented pressure (AP) is defined as the difference between the first (forward wave) and second systolic shoulders of the aortic systolic blood pressure. Additional calculations derived from the synthesized aortic pressure wave were the aortic systolic tension time index (AsTTI), diastolic pressure time index (DPTI), and subendocardial viability ratio (SEVR). AsTTI is a marker of aortic systolic stress and myocardial oxygen demand, and was estimated as the integral of aortic pressure and time during ventricular systole. DPTI is an indirect indicator of diastolic perfusion and was estimated as the integral of the diastolic pressure during ventricular diastole. The SEVR is the ratio of DPTI to TTI and an index of subendocardial perfusion.13 Only high-quality recordings, defined as an in-device quality index of over 80% were accepted for analysis.
To determine PWV, pressure waveforms were recorded at the following 2 sites sequentially: carotid-radial, carotid-femoral, and femoral-dorsalis pedis using a SphygmoCor Pulse Wave Velocity Vx system and SCOR-2000 Version 6.31 software (Atcor, Sydney, Australia). Pressure waveforms were gated with simultaneous ECG and used to calculate PWV between the 2 sites. Foot-to-foot PWV to each peripheral site (radial, femoral, dorsalis pedis) was calculated by determining the delay between the appearance of each pressure waveform foot in the carotid and peripheral sites.14 The distance between recording sites was adjusted for parallel transmission in the aorta and carotid by correcting for the distance between the supra-sternal notch and the carotid. These corrected distances were divided by the respective foot-to-foot transmission delays (carotid-radial, carotid-femoral) to give PWV. Central PWV (in the mostly elastic aorta) was evaluated using the carotid-femoral data and peripheral PWV (in the more muscular conduits) using the femoral-dorsalis pedis and carotid-radial data.
All subjects performed a symptom limited maximum graded exercise test (SL-GXT) on a treadmill using a modified Naughton protocol with a metabolic cart for peak oxygen uptake measurements (VO2peak) at study entry and after 17 and 35h of treatment. Primary measurements included time to angina, total exercise duration, and VO2peak. The Seattle Angina Questionnaire (SAQ) was used to measure anginal symptomatology.15 Additionally, the Canadian Cardiovascular Society (CCS) anginal classification was determined.
All statistical analyses were performed using SPSS version 18.0 for Windows (SPSS, Chicago, Illinois). Group and continuous variable data are presented as mean ± SEMs. All data were tested for normal distribution with the Shapiro-Wilk test for normality. An alpha level of P < 0.05 was required for statistical significance. Repeated measures analysis of variance (ANOVA) was used to evaluate all continuous dependent variables. When a significant group-by-time interaction was observed, within-group comparisons between time points and between-group comparisons at each time point were performed using Tukey post hoc analysis.
Results
All patients completed the entire EECP or Sham treatment sessions. The baseline characteristics for the EECP and Sham subjects are presented in Table 1. The EECP and Sham groups did not differ with respect to age, gender, height, weight, BMI, drug therapy, previous cardiovascular history and/or procedures, or cardiovascular risk factors. None of the patients were current smokers. The patients did not change their daily medication or physical activity during the duration of the study.
Hemodynamic and pulse wave analysis results are presented in Table 2. Baseline (pre) hemodynamic and pulse wave characteristics did not differ between the EECP and Sham group (Table 2). Mean arterial pressure and aortic systolic pressure were decreased after 17 sessions of EECP treatment. Systolic, diastolic, and pulse pressures in both the periphery (brachial artery) and aorta were decreased after 35 sessions of EECP. There were no significant changes in any of the BP components in the Sham group.
Table 2.
Hemodynamic Changes Following Enhanced External Counterpulsation and Sham Treatment.
| Variable | EECP (N =28) | SHAM (N = 14) | ||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| Heart Rate (beats/min) | 59 ± 2 | 60 ± 2 | 61 ± 2 | 60 ± 2 | 62 ± 2 | 61 ± 2 |
| Mean Arterial Pressure (mmHg) | 95 ± 2 | 92 ± 2*§ | 90 ± 2†§ | 97 ± 3 | 99 ± 3 | 97 ± 2 |
| Brachial Systolic Pressure (mmHg) | 135 ± 4 | 131 ± 4§ | 127 ± 3†§ | 137 ± 3 | 139 ± 4 | 135 ± 3 |
| Brachial Diastolic Pressure (mmHg) | 76 ± 2 | 74 ± 1 | 73 ± 2*§ | 78 ± 3 | 79 ± 3 | 78 ± 2 |
| Brachial Pulse Pressure (mmHg) | 59 ± 4 | 57 ± 3 | 54 ± 3†§ | 59 ± 4 | 60 ± 3 | 59 ± 4 |
| Aortic Systolic Pressure (mmHg) | 125 ± 4 | 120 ± 4*§ | 116 ± 3†§ | 127 ± 3 | 128 ± 4 | 126 ± 3 |
| Aortic Diastolic Pressure (mmHg) | 76 ± 2 | 74 ± 1§ | 73 ± 2*§ | 78 ± 3 | 80 ± 3 | 78 ± 2 |
| Aortic Pulse Pressure (mmHg) | 48 ± 3 | 45 ± 3 | 42 ± 3†§ | 48 ± 4 | 47 ± 3 | 47 ± 3 |
| Augmented Pressure (mmHg) | 15 ± 2 | 13 ± 2 | 11 ± 2†‡§ | 15 ± 2 | 14 ± 2 | 15 ± 2 |
| Δtp (msec) | 140 ± 2 | 143 ± 2†§ | 145 ± 2†§ | 139 ± 3 | 139 ± 3 | 138 ± 3 |
| Δtr (msec) | 204 ± 6 | 196 ± 6 | 188 ± 6†‡ | 197 ± 7 | 192 ± 5 | 193 ± 7 |
Data are expressed as mean ± SEM. Δtp = round trip travel time of the pressure wave from the heart to the periphery and back; Δtr = systolic duration of the reflected wave.
P < 0.05 and
P < 0.01 versus pretreatment values,
P < 0.05 versus midway values.
P < 0.05 vs. SHAM group
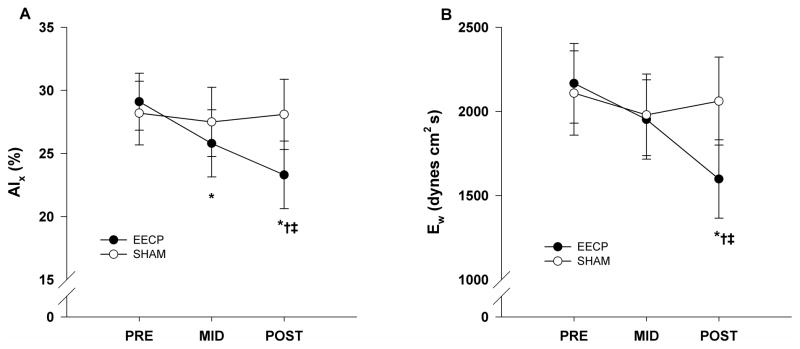
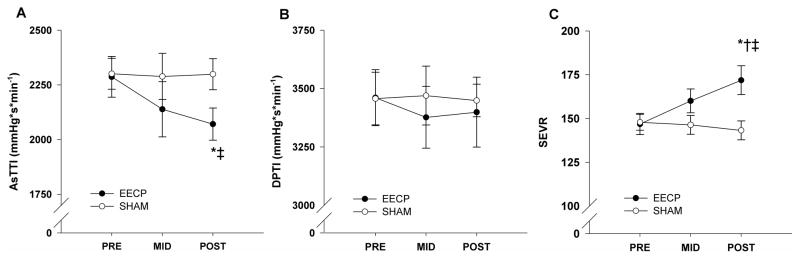
Pulse wave analysis results demonstrated that there was a progressive decrease in AIx after 17 (25.8 ± 2.7%) and 35 (23.3 ± 2.7%) sessions of EECP compared to baseline (29.1 ± 2.3%, P ≤ 0.01; Fig. 1A). Likewise, there was a progressive increase in round trip travel time of the reflected wave (Δtp) after 17 and 35 sessions of EECP compared to baseline (Table 2). Additionally, there were decreases in AP (reflected pressure wave (systolic duration of the reflected wave) following 35 sessions of amplitude) and Δtr EECP (Table 2). In turn, this lead to a substantial reduction in Ew following 35 sessions of EECP compared to pretreatment values (1598 ± 233 vs. 2167 ± 372 dynes·cm2·s, P < 0.01; Fig. 1B). AsTTI was also reduced following 35 sessions of EECP (2071 ± 73 vs. 2287 ± 93, P < 0.05, Fig. 2A). There were no differences in DPTI (3399 ± 150 v 3461 ± 120, P = 0.68, Fig 2B), but the difference in AsTTI resulted in a significantly higher SEVR (172 ± 8 vs. 147 ± 6, P < 0.01, Fig. 2C) following treatment in the EECP group. No changes in BP (peripheral or aortic) and indices of wave reflection were observed in the Sham group.
Figure 1.
Changes in aortic augmentation index (AIx; A) and wasted left ventricular pressure energy (Ew; B) before (PRE) and after 17 (MID) and 35 (POST) sessions of EECP and Sham treatment. Data are means ± SEMs. *P < 0.01 versus PRE; †P < 0.05 versus MID. ‡P < 0.05 versus Sham.
Figure 2.
Changes in aortic systolic tension time index (AsTTI; A) and aortic diastolic pressure time index (DPTI, B) before (PRE) and after 17 (MID) and 35 (POST) sessions of EECP and Sham treatment. Data are means ± SEMs. *P < 0.05 versus PRE; †P < 0.05 versus MID. ‡P < 0.05 versus Sham.
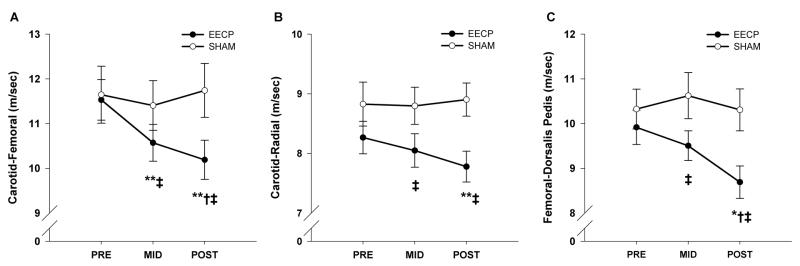
Central and peripheral PWV values at baseline and following 17 and 35 sessions of EECP and Sham are presented in Figure 3. Regional PWV did not differ between groups prior to the respective treatments. Carotid-femoral (central) PWV was decreased after 17 (10.6 ± 0.4 m/sec, P < 0.01) and 35 (10.2 ± 0.4 m/sec, P < 0.01) sessions of EECP compared to pretreatment values (11.5 ± 0.5 m/sec) (Fig. 3A). Both carotid-radial (7.8 ± 0.3 vs. 8.3 ± 0.3 m/sec, P < 0.01; Fig. 3B) and femoral-dorsalis pedis (8.7 ± 0.4 vs. 9.9 ± 0.4 m/sec, P < 0.05; Fig. 3C) PWV (peripheral) were decreased following 35 sessions of EECP compared to pretreatment values. No changes in either central or peripheral PWV were observed in the Sham group.
Figure 3.
Pulse wave velocity (PWV) between the carotid and femoral artery (A), carotid and radial artery (B), and femoral and dorsalis pedis artery (C) before and after 17 (MID) and 35 sessions (POST) of EECP and Sham treatment. Data are means ± SEMs. *P < 0.05 versus PRE; **P < 0.01 versus PRE; †P < 0.05 versus MID. ‡P < 0.05 versus Sham.
At study entry, exercise parameters did not differ between the EECP and Sham group (Table 3). EECP treatment led to progressive increases in VO2peak, total exercise duration, and time to angina following 17 and 35 sessions. No changes in SL-GXT parameters were observed in the Sham group. Pre and post (35-sessions) exercise capacity data for these patients has been previously published by our group.16
Table 3.
Changes in Exercise Capacity Following Enhanced External Counterpulsation and Sham Treatment
| Variable | EECP (N =28) | SHAM (N = 14) | ||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| Peak VO2 (ml/kg/min) | 17.0 ± 1.3 | 18.4 ± 1.4† | 19.4 ± 1.5†‡§ | 16.5 ± 1.3 | 17.0 ± 1.4 | 16.6 ± 1.4 |
| Peak Exercise Duration (s) | 586 ± 41 | 678 ± 52† | 774 ± 65†‡§ | 597 ± 49 | 607 ± 49 | 612 ± 47 |
| Peak Time to Angina (s) | 406 ± 39 | 537 ± 48† | 645 ± 64†‡§ | 449 ± 54 | 466 ± 52 | 471 ± 53 |
| Peak Angina Rating | 2.5 ± 0.2 | 2.0 ± 0.2† | 1.7 ± 0.3†§ | 2.3 ± 0.3 | 2.1 ± 0.4 | 2.3 ± 0.4 |
| Peak Heart Rate (beats/min) | 112 ± 3 | 115 ± 3 | 117 ± 4 | 116 ± 5 | 120 ± 5 | 119 ± 5 |
| Rating of Perceived Exertion | 16.1 ± 0.4 | 16.6 ± 0.4 | 16.8 ± 0.4 | 16.6 ± 0.4 | 16.1 ± 0.4 | 16.4 ± 0.4 |
| Respiratory Exchange Ratio | 0.99 ± 0.02 | 1.03 ± 0.02*§ | 1.04 ± 0.02†§ | 0.98 ± 0.03 | 0.98 ± 0.02 | 0.98 ± 0.03 |
Data are expressed as mean ± SEM.
P < 0.05 and
P < 0.01 versus pretreatment values,
P < 0.05 versus midway values,
P < 0.05 vs. SHAM group
EECP treatment led to an improvement in anginal as evidenced by progressive reductions in the number of anginal episodes and nitroglycerin (NTG) usage per day following 17 and 35 sessions. Consequently, there was an overall improvement in CCS classification for the EECP group (Table 4). There were no changes in any measure of anginal symptom reduction in the Sham group. Pre and post (35-sessions) anginal symptom data for these patients has been previously published by our group.16
Table 4.
Changes in Anginal Symptoms Following Enhanced External Counterpulsation and Sham Treatment
| Variable | EECP (N =28) | SHAM (N = 14) | ||||
|---|---|---|---|---|---|---|
| PRE | MID | POST | PRE | MID | POST | |
| Angina Classification (CCS) | 3.2 ± 0.1 | 2.4 ± 0.1*§ | 1.2 ± 0.1‡§ | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 |
| Anginal Episodes (average per day) | 1.8 ± 0.3 | 0.9 ± 0.2†§ | 0.5 ± 0.1‡§ | 1.7 ± 0.4 | 1.6 ± 0.3 | 1.6 ± 0.3 |
| Nitroglycerine Usage (average per day) | 1.1 ± 0.3 | 0.5 ± 0.2*§ | 0.2 ± 0.1†§ | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 |
Data are expressed as mean ± SEM.
P < 0.05,
P < 0.01, and
P < 0.001 versus pretreatment values,
P < 0.05 vs. SHAM group
Discussion
This is the first randomized sham-controlled study to evaluate the effects of EECP on arterial stiffness and wave reflection characteristics in CAD patients with refractory angina. The major findings of the present study include: 1) EECP reduces central and peripheral arterial stiffness; 2) EECP improves timing and amplitude of aortic wave reflection; and 3) EECP decreases indices of myocardial oxygen demand.
Our current results demonstrate that aortic stiffness is reduced following short- and long-term EECP treatment (17 and 35 sessions, respectively) in patients with CAD. Using ultrasonography techniques, Levenson et al.17 demonstrated that 35 sessions of EECP reduced carotid artery stiffness and resistance in patients with CAD. Although we used a different technique and a different target artery (carotid vs. aorta) to assess central artery stiffness, our findings are in agreement with those of Levenson et al.17 Additionally, we found a significant increase in Δtp, which is inversely related to aortic PWV.10 In aggregate, our data show that EECP reduces central elastic arterial stiffness in patients with CAD. EECP treatment also reduced peripheral arterial stiffness in the upper (arm) and lower (leg) body, thus suggesting that improvements in arterial stiffness are not limited to vascular regions directly exposed to the external mechanical compression.
Results from the current study also demonstrate improved aortic pressure wave characteristics following EECP. To date, 2 other studies have examined the effect of EECP on aortic pressure wave characteristics.12,18 In a pilot uncontrolled study, we previously demonstrated that 34 sessions of EECP resulted in a significant decrease in AIx, Ew, aortic pressures and an increase in Δtp.12 These modifications in wave reflection characteristics were accompanied by an improvement in CCS class and reductions in the average number of anginal episodes. However, Dockery et al.18 found that EECP did not reduce AIx in patients with CAD. One potential explanation for the disparity in findings could be that patients in the Dockery study18 were taking their cardiovascular medications at the time of vascular testing and were likely optimally dilated. In the present study, all baseline and treatment measurements were performed following at least a 12 hour period of withholding medications to help minimize the acute effects of drug therapy on vascular reactivity. Of clinical importance, the improvements in aortic pressure wave characteristics observed in the present study resulted in a substantial decrease in Ew and estimated myocardial oxygen demand (AsTTI). Ew is influenced by the systolic duration (Δtr) and the amplitude (AP) of the reflected wave. In the present study, reduction in each of the components contributed to the reduced Ew.
The stiffness of a vessel is commonly thought to be controlled by the distending (mean arterial) pressure and structural elements within the vessel wall. Changes in distending pressure can impact stiffness in large elastic arteries as well as muscular arteries. Therefore, the possibility exists that the changes in arterial stiffness (i.e. PWV) following EECP are due to the passive effect of a decreased MAP. The change in MAP following EECP treatment in the current study was not related to changes in carotid-femoral, carotid-radial, or femoral dorsalis pedis PWV (r = 0.16, P = 0.45; r = 0.29, P = 0.16; and r = 0.06, P = 0.80, respectively). To further address the influence of pressure changes on the improvements in PWV following treatment we performed repeated measures ANCOVA using the change in MAP as the covariate. After controlling for the change in MAP, the change in carotid-femoral PWV in the EECP group remained highly significant (P < 0.001). However, changes in carotid-radial (P = 0.15) and femoral-dorsalis pedis (P = 0.08) PWV following treatment were no longer significant. This suggests that the improvements in central PWV (i.e. aorta) were independent of BP changes, whereas, decreases in peripheral PWV following EECP were partially due to a reduction in pressure.
The smooth muscle of large arteries can influence the distribution of stresses between the compliant elastin and stiffer collagen fibers of a vessel wall and thus alter arterial stiffness.19 Because circulating and local vasoactive substances influence smooth muscle tone, arterial stiffness may be actively regulated. Along these lines, removal of the vascular endothelium in animals alters large artery stiffness,20 suggesting that endothelial-derived substances regulate arterial stiffness. Furthermore, substances such as nitric oxide (NO) and endothelin-1 (ET-1) have been shown to regulate large artery stiffness in humans.21,22 Additionally, Nigam et al.23 demonstrated a significant correlation between endothelial function, as assessed by brachial FMD, and tonometry measured aortic compliance of the proximal aorta – a more direct index of large artery stiffness.
We recently demonstrated in the same cohort of patients that EECP has a beneficial effect on peripheral artery flow-mediated dilation and endothelial-derived vasoactive agents.16 Therefore, we reasoned that reductions in regional and systemic arterial stiffness following EECP may be explained by improvements in endothelial function. The rapid inflation/deflation cycle of the EECP cuffs causes cyclic strain on leg arteries and systemically increases the rate of shear stress along the arterial wall which could possibly improve the function of endothelial cells and increase NO release. We calculate that with a resting HR of 60 beats/min, the 35 1-hr EECP sessions cause ~150,000 reactive hyperemia episodes (stimuli) in the arteries of the lower extremities. Mechanical forces, such as arterial shear stress and cyclic strain, have been shown to stimulate NO release.24,25 Indeed, improvements in peripheral endothelial function in CAD patients26,27 and endothelial dependent dilation in carotid arteries of pigs28 have been demonstrated following EECP treatment. Furthermore, EECP has a dose related, sustained effect in stimulating endothelial cell production of NO and decreasing the release of the potent vasoconstrictor ET-1.7,29 Together, these studies suggest that EECP treatment improves systemic endothelial function which likely reduces smooth muscle tone and arterial stiffness and consequently alters wave reflection from peripheral arteries. It is also possible that improvements in the capacitive or reservoir function of the aorta following EECP contributed to the improved aortic wave characteristics. Along these lines, the reservoir function of the aorta and other large elastic arteries contributes to the aortic blood pressure waveform and influences AIx.30
Our evidence of decreased arterial stiffness and aortic wave reflection after EECP was paralleled by increases in VO2peak, and decreased angina symptoms. Indeed, central artery stiffness is associated with ischemic threshold during treadmill exercise in patients with CAD.31 The improvements in wave reflection characteristics and aortic stiffness led to decreases in wasted left ventricular energy and are likely responsible for decreases in myocardial oxygen demand. These improvements in arterial function may explain improvements in anginal class and exercise tolerance that are commonly observed in patients with CAD following EECP therapy.
Acknowledgments
This study was supported by NIH R01 HL077571-01 to Dr. Braith
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arora RR, Chou TM, Jain D, Fleishman B, Crawford L, McKiernan T, Nesto RW. The multicenter study of enhanced external counterpulsation (MUST-EECP): effect of EECP on exercise-induced myocardial ischemia and anginal episodes. J Am Coll Cardiol. 1999;33:1833–1840. doi: 10.1016/s0735-1097(99)00140-0. [DOI] [PubMed] [Google Scholar]
- 2.Lawson WE, Hui JC, Cohn PF. Long-term prognosis of patients with angina treated with enhanced external counterpulsation: five-year follow-up study. Clin Cardiol. 2000;23:254–258. doi: 10.1002/clc.4960230406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaels AD, Raisinghani A, Soran O, de Lame PA, Lemaire ML, Kligfield P, Watson DD, Conti CR, Beller G. The effects of enhanced external counterpulsation on myocardial perfusion in patients with stable angina: a multicenter radionuclide study. Am Heart J. 2005;150:1066–1073. doi: 10.1016/j.ahj.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 4.Urano H, Ikeda H, Ueno T, Matsumoto T, Murohara T, Imaizumi T. Enhanced external counterpulsation improves exercise tolerance, reduces exercise-induced myocardial ischemia and improves left ventricular diastolic filling in patients with coronary artery disease. J Am Coll Cardiol. 2001;37:93–99. doi: 10.1016/s0735-1097(00)01095-0. [DOI] [PubMed] [Google Scholar]
- 5.Springer S, Fife A, Lawson W, Hui JC, Jandorf L, Cohn PF, Fricchione G. Psychosocial effects of enhanced external counterpulsation in the angina patient: a second study. Psychosomatics. 2001;42:124–132. doi: 10.1176/appi.psy.42.2.124. [DOI] [PubMed] [Google Scholar]
- 6.Jacobey JA, Taylor WJ, Smith GT, Gorlin R, Harken DE. A new therapeutic approach to acute coronary occlusion. I. Production of standardized coronary occlusion with microspheres. Am J Cardiol. 1962;9:60–73. doi: 10.1016/0002-9149(62)90098-x. [DOI] [PubMed] [Google Scholar]
- 7.Masuda D, Nohara R, Hirai T, Kataoka K, Chen LG, Hosokawa R, Inubushi M, Tadamura E, Fujita M, Sasayama S. Enhanced external counterpulsation improved myocardial perfusion and coronary flow reserve in patients with chronic stable angina; evaluation by(13)N-ammonia positron emission tomography. Eur Heart J. 2001;22:1451–1458. doi: 10.1053/euhj.2000.2545. [DOI] [PubMed] [Google Scholar]
- 8.Stys TP, Lawson WE, Hui JC, Fleishman B, Manzo K, Strobeck JE, Tartaglia J, Ramasamy S, Suwita R, Zheng ZS, Liang H, Werner D. Effects of enhanced external counterpulsation on stress radionuclide coronary perfusion and exercise capacity in chronic stable angina pectoris. Am J Cardiol. 2002;89:822–824. doi: 10.1016/s0002-9149(02)02191-4. [DOI] [PubMed] [Google Scholar]
- 9.Papaioannou TG, Karatzis EN, Papamichael CM, Karatzi KN, Zakopoulos NA, Lekakis JP, Mavrikakis M, Stefanadis C. Circadian variation of arterial pressure wave reflections. Am J Hypertens. 2006;19:259–263. doi: 10.1016/j.amjhyper.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Nichols WW, Singh BM. Augmentation index as a measure of peripheral vascular disease state. Curr Opin Cardiol. 2002;17:543–51. doi: 10.1097/00001573-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 11.Pauca AL, O’Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 12.Nichols WW, Estrada JC, Braith RW, Owens K, Conti CR. Enhanced external counterpulsation treatment improves arterial wall properties and wave reflection characteristics in patients with refractory angina. J Am Coll Cardiol. 2006;48:1208–1214. doi: 10.1016/j.jacc.2006.04.094. [DOI] [PubMed] [Google Scholar]
- 13.Sarnoff SJ, Braunwald E, Welch GH, Jr, Case RB, Stainsby WN, Macruz R. Hemodynamic determinants of oxygen consumption of the heart with special reference to the tension-time index. Am J Physiol. 1958;192:148–156. doi: 10.1152/ajplegacy.1957.192.1.148. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell GF, Izzo JL, Jr, Lacourciere Y, Ouellet JP, Neutel J, Qian C, Kerwin LJ, Block AJ, Pfeffer MA. Omapatrilat reduces pulse pressure and proximal aortic stiffness in patients with systolic hypertension: results of the conduit hemodynamics of omapatrilat international research study. Circulation. 2002;105:2955–2961. doi: 10.1161/01.cir.0000020500.77568.3c. [DOI] [PubMed] [Google Scholar]
- 15.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 16.Braith RW, Conti CR, Nichols WW, Choi CY, Khuddus MA, Beck DT, Casey DP. Enhanced external counterpulsation improves peripheral artery flow-mediated dilation in patients with chronic angina: a randomized sham-controlled study. Circulation. 2010;122:1612–1620. doi: 10.1161/CIRCULATIONAHA.109.923482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levenson J, Simon A, Megnien JL, Chironi G, Gariepy J, Pernollet MG, Craiem D, Iliou MC. Effects of enhanced external counterpulsation on carotid circulation in patients with coronary artery disease. Cardiology. 2007;108:104–110. doi: 10.1159/000095949. [DOI] [PubMed] [Google Scholar]
- 18.Dockery F, Rajkumar C, Bulpitt CJ, Hall RJ, Bagger JP. Enhanced external counterpulsation does not alter arterial stiffness in patients with angina. Clin Cardiol. 2004;27:689–692. doi: 10.1002/clc.4960271206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson IB, Franklin SS, Cockcroft JR. Nitric oxide and the regulation of large artery stiffness: from physiology to pharmacology. Hypertension. 2004;44:112–116. doi: 10.1161/01.HYP.0000138068.03893.40. [DOI] [PubMed] [Google Scholar]
- 20.Boutouyrie P, Bezie Y, Lacolley P, Challande P, Chamiot-Clerc P, Benetos A, de la Faverie JF, Safar M, Laurent S. In vivo/in vitro comparison of rat abdominal aorta wall viscosity. Influence of endothelial function. Arterioscler Thromb Vasc Biol. 1997;17:1346–1355. doi: 10.1161/01.atv.17.7.1346. [DOI] [PubMed] [Google Scholar]
- 21.Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38:1049–1053. doi: 10.1161/hy1101.095329. [DOI] [PubMed] [Google Scholar]
- 22.McEniery CM, Qasem A, Schmitt M, Avolio AP, Cockcroft JR, Wilkinson IB. Endothelin–1 regulates arterial pulse wave velocity in vivo. J Am Coll Cardiol. 2003;42:1975–1981. doi: 10.1016/j.jacc.2003.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Nigam A, Mitchell GF, Lambert J, Tardif JC. Relation between conduit vessel stiffness (assessed by tonometry) and endothelial function (assessed by flow-mediated dilatation) in patients with and without coronary heart disease. Am J Cardiol. 2003;92:395–399. doi: 10.1016/s0002-9149(03)00656-8. [DOI] [PubMed] [Google Scholar]
- 24.Awolesi MA, Widmann MD, Sessa WC, Sumpio BE. Cyclic strain increases endothelial nitric oxide synthase activity. Surgery. 1994;116:439–44. discussion 444–5. [PubMed] [Google Scholar]
- 25.Markos F, Hennessy BA, Fitzpatrick M, O’Sullivan J, Snow HM. Reverse arterial wall shear stress causes nitric oxide-dependent vasodilatation in the anaesthetised dog. Pflugers Arch. 2002;445:51–54. doi: 10.1007/s00424-002-0915-9. [DOI] [PubMed] [Google Scholar]
- 26.Bonetti PO, Barsness GW, Keelan PC, Schnell TI, Pumper GM, Kuvin JT, Schnall RP, Holmes DR, Higano ST, Lerman A. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003;41:1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 27.Shechter M, Matetzky S, Feinberg MS, Chouraqui P, Rotstein Z, Hod H. External counterpulsation therapy improves endothelial function in patients with refractory angina pectoris. J Am Coll Cardiol. 2003;42:2090–2095. doi: 10.1016/j.jacc.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Tao J, Tu C, Yang Z, Zhang Y, Chung XL, Ma H, Zhen ZS. Enhanced external counterpulsation improves endothelium-dependent vasorelaxation in the carotid arteries of hypercholesterolemic pigs. Int J Cardiol. 2006;112:269–724. doi: 10.1016/j.ijcard.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 29.Akhtar M, Wu GF, Du ZM, Zheng ZS, Michaels AD. Effect of external counterpulsation on plasma nitric oxide and endothelin-1 levels. Am J Cardiol. 2006;98:28–30. doi: 10.1016/j.amjcard.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 30.Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Foale RA, Malik IS, Tyberg JV, Parker KH, Mayet J, Hughes AD. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol. 2010;298:H580–H586. doi: 10.1152/ajpheart.00875.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kingwell BA, Waddell TK, Medley TL, Cameron JD, Dart AM. Large artery stiffness predicts ischemic threshold in patients with coronary artery disease. J Am Coll Cardiol. 2002;40:773–779. doi: 10.1016/s0735-1097(02)02009-0. [DOI] [PubMed] [Google Scholar]