Abstract
Recent studies show that thymic stromal lymphopoietin (TSLP) plays a critical role in the upstream phase of the allergic cascade to induce T helper type 2 cell (Th2)-dominant allergic diseases. However, the effect of blocking TSLP signalling with the soluble TSLP receptor (TSLPR), TSLPR-immunoglobulin (Ig), on asthma development needs further investigation. Here, we examined the effects of TSLPR-Ig on asthmatic airway inflammation and dendritic cell (DC) function. TSLPR-Ig (comprising the extracellular domain of murine TSLPR and an IgG2a Fc tail) purified from transfected COS-7 cells reduced the expression of CD40, CD80 and CD86 on TSLP-activated DCs in vitro. We also investigated the mechanisms underlying TSLPR-Ig-mediated amelioration of allergic airway inflammation in a murine asthma model. When TSLP signalling was blocked by intratracheal administration of TSLPR-Ig prior to sensitization, allergen-specific serum IgE levels, airway tissue inflammation, inflammatory cell infiltration and Th2 cytokine levels in the bronchiolar lavage fluid (BALF) were reduced significantly. This was because of the TSLP-Ig-mediated down-regulation of co-stimulatory molecule expression on pulmonary DCs. We also transferred bone marrow-derived mature DCs (mDCs) into the airways of asthmatic mice. Intratracheal administration of TSLPR-Ig prior to the transfer of mDCs reduced eosinophilic airway inflammation and Th2 differentiation significantly. Collectively, these data suggest that local use of TSLPR-Ig prevents airway inflammation, at least in part, by regulating DC function, and that blocking TSLP signalling using TSLPR-Ig may be a novel strategy for the treatment of asthma bronchiale.
Keywords: asthma bronchiale, dendritic cells, ovalbumin, thymic stromal lymphopoietin, soluble receptor
Introduction
Asthma bronchiale is a chronic inflammatory disease characterized by reversible airflow obstruction, over-production of mucus and infiltration of inflammatory cells into the airways. The over-production and activation of T-helper type 2 (Th2) lymphocytes plays a critical role in the pathogenesis of asthma by releasing a variety of cytokines such as interleukin (IL)-4, IL-5, IL-9 and IL-13 [1,2]. These cytokines are thought to promote infiltration of inflammatory cells (such as eosinophils, mast cells, T lymphocytes and neutrophils) into the airways and immunoglobulin (Ig) isotype switching to IgE [3]. However, dysregulated Th2 cell-mediated responses do not explain adequately many of the clinical and molecular aspects of asthma bronchiale. Many therapeutic approaches based on targeting Th2 cells have, so far, been disappointing. The mechanisms underlying Th2 skewing in allergen-induced asthmatic responses have been investigated intensively. Airway dendritic cells (DCs) are one of the most important immune cells in the development of asthma bronchiale because of their ability to activate naive T cells and to maintain, and further polarize, primed Th2 cells in the lung [4,5]. Studies using animal models have highlighted the potential of DCs to direct different Th responses in a manner dependent upon the signals they receive from the antigen and the tissue environment. Governed by the signals received at the time of antigen presentation, DCs can polarize naive T cells into Th1 or Th2 effector cells, and initiate primary immune responses. Increasing numbers of studies show that the activation of innate pattern recognition receptors, such as Toll-like receptors (TLRs) on DCs, plays a key role in Th1 cell differentiation [6]. The mechanisms underlying DC-mediated Th2 differentiation upon encountering clinically relevant aeroallergens has also been well studied [7].
Recent studies show that thymic stromal lymphopoietin (TSLP) plays an important role in the development of allergic disease [8]. TSLP promotes the migration of myeloid DCs to the regional lymph nodes, and TSLP-activated DCs (TSLP-DCs) induce naive T cells to develop into the Th2 cells via OX40 ligand and OX40 co-stimulatory interactions [9]. TSLP-DCs also produce chemokines [e.g. macrophage-derived chemokine (MDC) and thymus- and activation-regulated chemokine (TARC)] which recruit Th2 cells from the regional lymph nodes to the sites of allergen entry, where they induce allergic inflammation. Over-expression of TSLP in dermatitis skin lesions and asthmatic airways, and the induction of allergic airway inflammation by TSLP transgenes, demonstrate an association between TSLP and the development of allergic disease [10]. Another study shows that TSLP receptor (TSLPR)-deficient (tslpr−/−) mice fail to develop an asthmatic response to inhaled allergens [11], supporting a critical role for TSLP signalling in allergic airway inflammation.
TSLP signalling is mediated by a heterodimeric receptor, which consists of the IL-7 receptor alpha chain and a unique TSLP receptor chain (TSLPR) [12]. Studies of TSLP signalling show that phosphorylation of signal transducer and activator of transcription 5 (STAT5) induces and mediates multiple biological functions following TLSP ligation by its heterodimeric receptor [13,14]. It is accepted that TSLP represents a key switch for allergic inflammation; therefore, blocking TSLP signalling is an attractive intervention for the treatment of allergic diseases. However, TSLP is associated with the development and function of immunological organs; therefore, tslp over-expression or tslpr knock-out could result in systemic side effects. Indeed, previous studies have shown that disruption of TSLP signalling is associated with some immunological disorders [15,16]. Soluble receptor-based therapy has been used to neutralize the function of soluble cytokines in vivo. The intrapulmonary application of antagonizing agents that target molecules on effector cells is effective in eliminating the cardinal features of asthma. In fact, this approach is more effective than other approaches, such as the intraperitoneal administration of alpha4 integrin (CD49d) antagonists [e.g. CD49d monoclonal antibodies (mAb)] that act on circulating lymphocytes to inhibit airway responses [17]. In the present study a soluble TSLPR, TSLPR-Ig, was prepared by fusing the extracellular domain of murine TSLPR with a murine IgG2a Fc tail. We administered TSLPR-Ig locally into the airways of asthmatic mice to investigate the effects of blocking TSLP function on airway inflammation. The results suggest that TSLP-Ig acts, at least in part, by altering the function of DCs, which are critical in Th2-mediated allergic disorders.
Materials and methods
Mice
Female Balb/C mice were purchased from the animal centre of Jingling Hospital and maintained in a pathogen-free authorized facility where the temperature was maintained at 20–22°C and the humidity was kept at 50–60%. The dark/light cycle was 12 h. All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Ovalbumin (OVA) sensitization and airway challenge
Mice were divided randomly into the four groups (n = 6 per group) as follows: (i) mice sensitized with phosphate-buffered saline (PBS) and challenged with OVA (PBS/OVA; control group); (ii) mice sensitized and challenged with OVA (OVA/OVA; model group); (iii) mice pretreated with control immunoglobulin (Ig)G (AB-108-C; R&D Systems, Minneapolis, MN, USA) and sensitized and challenged with OVA (OVA/IgG/OVA; processing control group); and (iv) mice pretreated with TSLPR-Ig and sensitized and challenged with OVA (OVA/TSLPR-Ig/OVA; treatment group). A murine allergic asthma model was established as described previously [5]. In brief, mice were sensitized by intraperitoneal injection of 20 µg OVA (grade V; Sigma Chemical Co., St Louis, MO, USA) emulsified in 2 mg aluminium hydroxide in a total volume of 200 µl PBS on days 0 and 14. Non-sensitized control mice were given PBS plus aluminium hydroxide without OVA. On days 28, 29 and 30, anaesthetized mice were challenged by exposure to an ultrasonically nebulized aerosol containing 1% (w/v) OVA in PBS for 30 min. Twenty-four hours after the final challenge the mice were killed, and airway inflammation was characterized by histological examination. For the TSLPR blocking experiment, anaesthetized mice received an intratracheal (i.t.) injection of 20 µg of TSLPR-Ig or control IgG (AB-108-C; R&D Systems) 30 min before each OVA sensitization. The dose of TSLPR-Ig was predetermined by staining analysis of airway inflammation in mice receiving 10–40 µg TSLPR-Ig.
Establishment of the expression construct for soluble murine TSLPR (TSLPR-Ig)
The extracellular domain of murine TSLPR (mTSLPR) was amplified from a mouse thymus cDNA library using the primers 5′-CGG AAT TCA GTG CTG AGA TGG CAT GGG-3′ and 5′-GAA GAT CTA GGG GCG GGG CCA GGG CCG-3′, and then subcloned into the region upstream of a murine IgG2a Fc tail using the EcoR I/Bgl II cutting sites within the pcDNA-Ig vector established within our laboratory by cloning the murine IgG2a Fc tail into the pcDNA3 vector. The recombinant plasmid, pTSLPR-Ig, was confirmed by restriction enzyme digestion and DNA sequencing (Shenggong Co., Shanghai, China).
Expression and purification of recombinant soluble TSLPR-Ig
COS-7 cells were seeded in 100 mm culture flasks (2 × 106 cells/flask) in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS) (Gibco, Grand Island, NY, USA) and 1% penicillin/streptomycin. The expression plasmid, pTSLPR-Ig, was transfected into COS-7 cells using Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. After 72 h, the supernatant was collected and ectopic soluble TSLPR-Ig expression in the confirmed by Western blotting using anti-mIgG antibodies (Zhongshan Co., Beijing, China) and enhanced chemiluminescence (ECL) (Pierce, Rockford, IL, USA). After the first round of culture and supernatant collection, the transfected cells were supplemented with fresh serum-free medium (Thermo Scientific, Rockford, IL, USA) for one more round of supernatant collection. Soluble TSLPR-Ig was purified from the culture supernatants by standard protein A Sepharose affinity chromatography (Amersham Biosciences, Piscataway, NJ, USA) and visualized by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
Analysis of co-stimulatory molecule expression on DCs in vitro
Mouse bone marrow-derived DCs (BMDCs) were generated as described previously [18]. After 8 days of culture with 10 ng/ml granulocyte–macrophage colony-stimulating factor (GM-CSF) and 1 ng/ml IL-4, DCs were purified using anti-CD11c-coated microbeads (Miltenyi-Biotec, Auburn, CA, USA) and treated with medium, TSLP (100 ng/ml; R&D Systems), TSLP (100 ng/ml) + control IgG (200 ng/ml, AB-108-C; R&D Systems) or TSLP (100 ng/ml) + TSLPR-Ig (200 ng/ml) for 24 h. The dose of TSLPR-Ig was predetermined by flow cytometry analysis of co-stimulatory molecule expression by TSLP-DCs treated with 100–400 ng/ml TSLPR-Ig. The following day, DCs were collected, washed and stained with Allophycocyanin (APC)-CD11c (eBioscience Inc., San Diego, CA, USA) and phycoerythrin (PE)-CD40, CD80 or CD86 mAbs (eBioscience Inc.). At least 10 000 cells were analysed using a fluorescence activated cell sorter (FACS) FACScan (Becton Dickinson, San Jose, CA, USA) and CellQuest software (Becton Dickinson). DC (CD11c+) expression of the co-stimulatory molecules CD40, CD80 and CD86 was detected by flow cytometry.
Cellular composition of the bronchoalveolar lavage fluid (BALF)
To investigate cellular infiltration of the airways, mice were killed 24 h after allergen challenge using an overdose of ketamine. The BALF was then collected from each animal via cannulation of the exposed trachea and gentle flushing of the lungs three times with 0·5 ml of PBS (total 1·5 ml). BALF cells were coated onto glass slides using a Cytospin, fixed in methanol, and stained with Diff/Quick using standard procedures (Merck, Darmstadt, Germany). Differential cell counts were performed in duplicate on coded slides for 200 cells from each sample. BALF was stored at −70°C and then used to determine the levels of IL-4, IL-5 and IFN-γ using specific enzyme-linked immunosorbent assays ( ELISAs) according to the manufacturer's instructions (ELISA kits; eBioscience).
Histological analysis
Immediately after the collection of BALF the excised lungs were inflated under constant pressure, fixed overnight with neutral buffered 4% formalin and the lung tissues embedded in paraffin. For histological examination, 4-mm slices of the fixed, embedded tissues were cut on a Leica rotary microtome (Leica Microsystems Nussloch GmbH, Nussloch, Germany), placed onto glass slides, deparaffinized and stained with haematoxylin 2, eosin-Y (Richard-Allan Scientific, Kalamazoo, MI, USA). The bronchoalveolar changes were observed under a light microscope.
Determination of OVA-specific IgE levels in serum
The concentration of OVA-specific IgE was determined using ELISAs as follows: 96-well plates were coated overnight with 100 µl of OVA (10 µg/ml in 0·1 mol/l carbonate buffer, pH 9·6) at 4°C. The antigen-coated plates were then washed five times with 0·5% Tween-20 in PBS (PBST). Mouse serum was added to the antigen-coated wells and the plates were incubated overnight at 4°C with a peroxidase-conjugated anti-mouse IgE antibody (Biotechnology Associates, Birmingham, AL, USA) followed by washing five times with PBST. Finally, citric acid-phosphate buffer (pH 5·0) containing 0·5 mg/ml of O-phenylenediamine (Sigma Chemical Co.) was added to the wells. Colour was allowed to develop at 37°C and the reaction was stopped with 2·5 mol/l sulphuric acid. Absorbance values were measured at 450 nm.
Evaluation of co-stimulatory molecule expression on pulmonary DCs
Lungs were removed aseptically and cut into small fragments. The pieces were digested enzymatically with collagenase and DNase I, as described previously [19]. Briefly, lung tissue was cut into small pieces of about 2–3 mm in size and washed extensively in RPMI-1640 culture medium. Each tissue sample was incubated for 1 h in RPMI-1640 containing 0·1% collagenase (Type IV; Sigma) and 0·002% DNase (Sigma). After incubation, the tissues were ground gently in a tissue grinder and the cells were collected. The cells were washed three times and suspended in cold PBS. Suspended cells (95% viable) were stained with APC-CD11c (eBioscience Inc.) and PE-CD40, CD80 or CD86 mAbs (eBioscience Inc.). At least 100 000 cells were analysed using a FACScan (Becton Dickinson) and CellQuest software (Becton Dickinson). The expression of CD40, CD80 and CD86 on pulmonary DCs (CD11c+) was detected by flow cytometry.
Th2 priming induced by i.t. injection of bone marrow-derived DCs (BMDCs)
BMDCs (cultured for 8 days) were purified and pulsed (or not) with OVA overnight. For the in vivo experiments, mice were divided randomly into three groups (n = 6 per group) as follows: (i) mice received an i.t. injection of PBS 30 min before i.t. injection of 2 × 106 non-pulsed DCs (PBS/DCs); (ii) mice received an i.t. injection of control IgG 30 min before i.t. injection of 2 × 106 OVA-pulsed DCs (IgG/OVA-DCs); and (iii) mice received an i.t. injection of TSLPR-Ig 30 min before i.t. injection of 2 × 106 OVA-pulsed DCs (TSLPR-Ig/OVA-DCs). The blocking dose of TSLPR-Ig, or control IgG, was 20 µg. On days 10–12, mice were challenged with an OVA aerosol for 30 min once per day and killed 24 h after the final challenge [20].
Statistical analysis
Experimental differences were tested for statistical significance using analysis of variance (anova) and Student's t-test. P-values of <0·05 or <0·01 was considered to be significant.
Results
Expression and purification of mouse soluble TSLPR-Ig
The cDNA encoding the extracellular domain of murine TSLPR was obtained by polymerase chain reaction (PCR) amplification of a mouse thymus cDNA library, to yield a 723 base pairs (bp) DNA fragment. This fragment was digested with EcoRI/BglII and cloned into the pcDNA-Ig vector. The soluble TSLPR-Ig expression construct (pTSLPR-Ig) was then validated by sequencing. We first tested TSLPR-Ig secretion into the culture medium of transfected COS-7 cells. Western blot analysis of culture medium from pTSLPR-Ig-transfected cells identified a 51·1 kD protein, which is in agreement with the combined molecular weight of the extracellular domain of murine TSLPR and the murine IgG2a Fc (Fig. 1a). In contrast, Western blotting failed to detect any product in cells transfected with pcDNA-Ig. We then purified TSLPR-Ig from the culture medium using protein A Sepharose CL-4B affinity chromatography, and the purified TSLPR-Ig resolved as a unique band with a molecular weight of 51·1 kD on SDS-PAGE gels after Coomassie Blue staining (Fig. 1b).
Fig. 1.
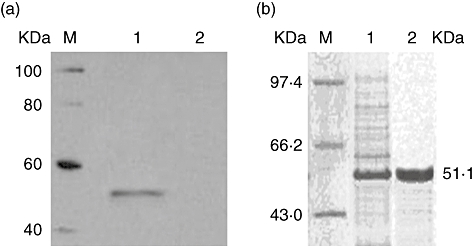
Expression and purification of the thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) fusion protein. (a) Western blot analysis of TSLPR-Ig in the culture medium of transfected COS-7 cells. Lane 1: pTSLPR-Ig transfected COS-7 cells; lane 2: pcDNA-Ig transfected COS-7 cells. M: protein markers. The membrane was then probed with an anti-murine IgG antibody. (b) Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis of purified TSLPR-Ig by Coomassie Blue staining. Soluble TSLPR-Ig was purified from the culture supernatants by protein A Sepharose CL-4B affinity chromatography. Lane 1: total proteins in the culture supernatants from transfected COS-7 cells; lane 2: the purified TSLPR-Ig. M: protein marker.
TSLPR-Ig inhibits co-stimulatory molecule expression by TSLP-DCs
To examine whether TSLPR-Ig influences co-stimulatory molecule expression by TSLP-DCs in vitro, 8-day cultured DCs were enriched and treated with medium, TSLP, TSLP + TSLPR-Ig or TSLP + control IgG for 24 h. The expression of co-stimulatory molecules by DCs was assessed using flow cytometry. Table 1 shows that DCs cultured with TSLP for 24 h in the presence and absence of control IgG up-regulated CD40, CD80 and CD86 expression to a larger extent than DCs cultured in medium alone (P < 0·01). TSLPR-Ig effectively inhibited the TSLP-induced increase in CD40, CD80 and CD86 expression (P < 0·01 versus TSLP group or TSLP + control IgG group). These results indicate that TSLPR-Ig interferes with co-stimulatory molecule expression by TSLP-treated DCs in vitro.
Table 1.
Thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) suppresses co-stimulatory molecule expression (%) by TSLP-dendritic cells (DCs)
| Groups | CD40 | CD80 | CD86 |
|---|---|---|---|
| Medium | 4·46 ± 1·22 | 10·42 ± 1·53 | 4·89 ± 1·74 |
| TSLP | 20·47 ± 2·23*,** | 22·05 ± 3·41*,** | 21·16 ± 3·28*,** |
| TSLP + TSLPR-Ig | 5·15 ± 1·45 | 11·57 ± 1·78 | 5·49 ± 1·85 |
| TSLP + control IgG | 21·64 ± 3·56*,** | 21·51 ± 3·42*,** | 22·66 ± 3·94*,** |
P < 0·01 compared with the TSLP + TSLPR-Ig group.
P < 0·01 compared with the medium group.
TSLPR-Ig decreases the number of inflammatory cells in the BALF and reduces tissue pathology
Increasing evidence shows that TSLP expression is associated closely with asthmatic inflammation in mice. Therefore, we investigated whether blockade of TSLP signalling decreased the levels of Th2 cytokine production and eosinophilic inflammation. To evaluate the effects of TSLP blocking on allergen-primed airway inflammation, we administered the TSLPR-Ig fusion protein (or control IgG) locally before OVA sensitization. As expected, OVA inhalation by OVA-sensitized mice increased the total cell number significantly, and that of eosinophils and lymphocytes in the BALF compared with that in PBS-sensitized mice (P < 0·05 or < 0·01). However, administration of TSLPR-Ig before OVA sensitization resulted in a decrease in the total number of BALF cells, eosinophils and lymphocytes compared with that in IgG-treated mice (P < 0·05 or < 0·01, Fig. 2a). Furthermore, local blockade of TSLP signalling by TSLPR-Ig led to a marked reduction in IL-4 and IL-5 levels (P < 0·01 versus OVA/OVA group or OVA/IgG/OVA group), but an increase in the level of the Th1-associated cytokine, IFN-γ (P < 0·05 versus OVA/OVA group or OVA/IgG/OVA group, Fig. 2b).
Fig. 2.
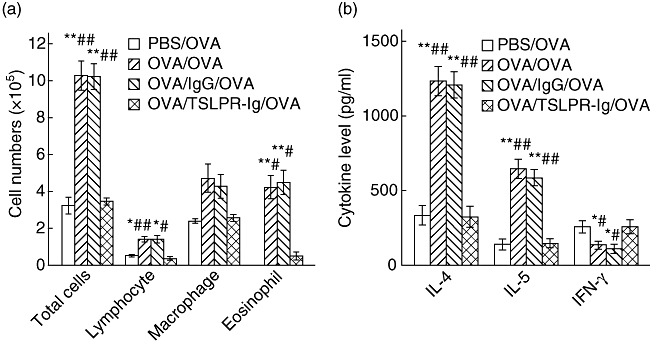
Thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) pretreatment suppressed allergic responses. Ovalbumin (OVA)-sensitized and -challenged mice received an intratracheal (i.t.) injection of TSLPR-Ig or control IgG 30 min before each OVA sensitization. (a) Total and differential cells counts and (b) cytokine profiles in the bronchoalveolar lavage fluid (BALF) were analysed 24 h after final OVA exposure. Data represent mean values ± standard error of the mean from six mice per group. *P < 0·05 compared with the phosphate-buffered saline (PBS)/OVA group. **P < 0·01 compared with the PBS/OVA group. #P < 0·05 compared with the OVA/TSLPR-Ig/OVA group. ##P < 0·01 compared with the OVA/TSLPR-Ig/OVA group.
We also compared changes in lung pathology in the different groups of mice. As shown in Fig. 3, OVA-challenged mice showed inflammatory cell infiltration of the peribronchiolar and perivascular connective tissues, together with the hypersecretion of mucus by epithelial cells. In contrast, TSLPR-Ig administration markedly inhibited both OVA-induced, eosinophil-rich leucocyte infiltration and mucus hypersecretion into the airways. All the results indicated that local blockade of TSLP signalling with TSLPR-Ig prior to sensitization reduced significantly the level of inflammatory cell infiltration and the severity of allergic airway responses.
Fig. 3.
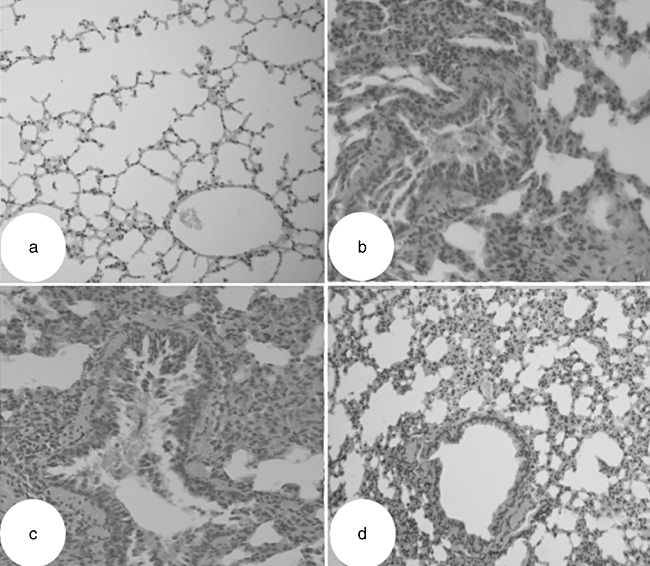
Thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) pretreatment inhibited lung inflammation. Ovalbumin (OVA)-sensitized and -challenged mice received an intratracheal (i.t.) injection of TSLPR-Ig or control IgG 30 min before each OVA sensitization, and histological examination of the lung tissues (haematoxylin and eosin, ×200 original) was performed 24 h after the final OVA exposure. (a) Phosphate-buffered saline (PBS)/OVA group. (b) OVA/OVA group. (c) OVA/IgG/OVA group. (d) OVA/TSLPR-Ig/OVA group.
Inhibition of allergen-specific IgE synthesis by TSLPR-Ig
To assess the potential utility of TSLPR-Ig administration in modulating the immune response, we measured changes in serum IgE antibodies by ELISA. OVA-specific IgE levels 24 h after the final allergen challenge are shown in Fig. 4. The levels of OVA-specific IgE were increased markedly in OVA-sensitized and -challenged mice (P < 0·01 versus PBS/OVA group), while mice pretreated with TSLPR-Ig had significantly lower levels of OVA-specific IgE compared with mice pretreated with IgG (P < 0·01). This suggested that TSLPR-Ig administration was more effective in suppressing IgE responses.
Fig. 4.
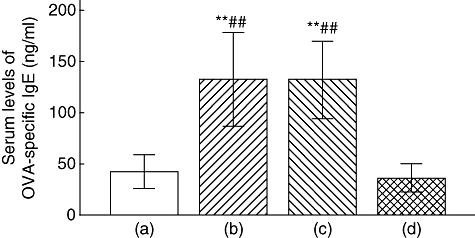
Thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) pretreatment suppressed ovalbumin (OVA)-specific IgE synthesis. Serum levels of OVA-specific IgE in mice from the phosphate-buffered saline (PBS)/OVA group (a), the OVA/OVA group (b), the OVA/IgG/OVA group (c) and the OVA/TSLPR-Ig/OVA group (d), were assessed by enzyme-linked immunosorbent assay. **P < 0·01 compared with group (a). ##P < 0·01 compared with group (d).
TSLPR-Ig affects the expression of co-stimulatory molecules by pulmonary DCs
As pulmonary DCs play a central role in initiating allergen-specific Th2 cell differentiation and sensitization, and TSLP is thought to be a powerful activator of DCs, we hypothesized that the anti-asthmatic effects of TSLPR-Ig might be related to functional changes in pulmonary DCs. Therefore, we investigated the expression of co-stimulatory molecules, which are a prerequisite for DC-induced Th2 cell differentiation. As shown in Fig. 5, there was a marked reduction in CD40, CD80 or CD86 expression by pulmonary DCs in the TSLPR-Ig-treated group compared with the IgG-treated group (P < 0·05 or < 0·01), suggesting that TSLPR-Ig treatment before sensitization inhibited the maturation of pulmonary DCs substantially.
Fig. 5.
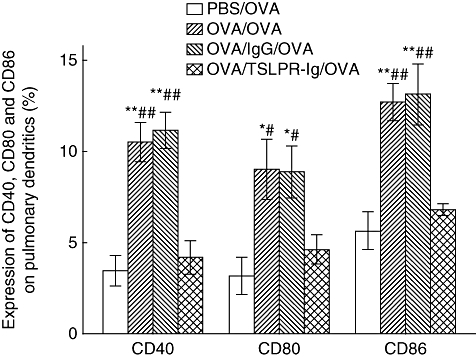
Thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) pretreatment inhibited co-stimulatory molecule expression by pulmonary dendritic cells (DCs). Ovalbumin (OVA)-sensitized and challenged mice received an intratracheal (i.t.) injection of TSLPR-Ig or control IgG 30 min before each OVA sensitization, and the expression of CD40, CD80 and CD86 on pulmonary DCs was analysed by flow cytometry. Data represent mean values ± standard error of the mean from six mice per group. *P < 0·05 compared with the phosphate-buffered saline (PBS)/OVA group. **P < 0·01 compared with the PBS/OVA group. #P < 0·05 compared with the OVA/TSLPR-Ig/OVA group. ##P < 0·01 compared with the OVA/TSLPR-Ig/OVA group.
TSLPR-Ig abrogates the ability of mDCs to induce Th2 development in vivo
To clarify the impact of TSLPR-Ig on the potential of DCs to promote Th2 skewing in vivo, we transferred OVA-pulsed DCs (OVA-DCs) adoptively into mice airways following intratracheal administration of control IgG or TSLPR-Ig. As expected, mice given control IgG and OVA-DCs showed marked asthmatic inflammation characterized by the infiltration of lymphocytes and eosinophils into the BALF compared with mice given PBS and non-pulsed DCs (P < 0·05 or <0·01). However, TSLPR-Ig pretreatment before transferring OVA-DCs reduced significantly the number of total cells, macrophages and eosinophils in the BALF (P < 0·05 or <0·01 versus IgG/OVA-DCs group Fig. 6a), ameliorated the disequilibrium of Th1/Th2 associated cytokines, as shown by the decreased IL-4 and IL-5 levels (P < 0·01 versus IgG/OVA-DCs group) and maintained the high levels of IFN-γ in the BALF (P > 0·05 versus IgG/OVA-DCs group, Fig. 6b). The above data confirmed that TSLPR-Ig pretreatment inhibited the capability of OVA-DCs to induce Th2 skewing in asthmatic mice.
Fig. 6.
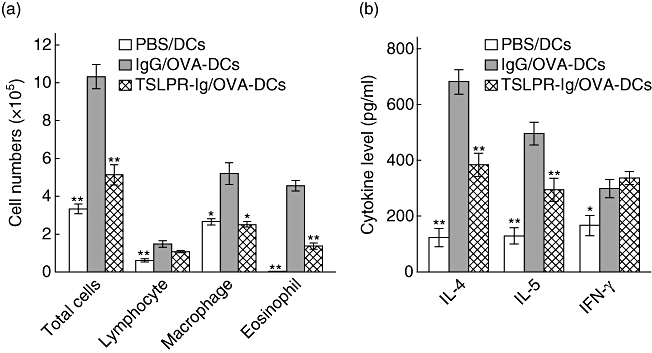
Thymic stromal lymphopoietin receptor-immunoglobulin (TSLPR-Ig) pretreatment impaired the ability of dendritic cells (DCs) to prime T helper type 2 (Th2) responses in vivo. Mice received an intratracheal (i.t.) injection of phosphate-buffered saline (PBS), control IgG or TSLPR-Ig 30 min before i.t. injection of 2 × 106 non-pulsed DCs, or OVA-pulsed DCs (OVA-DCs). They were then exposed to OVA aerosols on days 10–12. (a) Total and differential cells counts and (b) cytokine profiles in the bronchoalveolar lavage fluid (BALF) were assessed 24 h after the final OVA exposure. Data are expressed as mean ± standard error of the mean. *P < 0·05 compared with the IgG/OVA-DCs group. **P < 0·01 compared with the IgG/OVA-DCs group.
Discussion
The mechanisms involved in the pathological changes seen in asthma are complicated, with various inflammatory cells, including eosinophils, macrophages, mast cells, neutrophils and Th2 cells, contributing to the pathogenesis. Also, emerging data have suggested that other T cell subsets, including Th1 cells, Th9 cells, Th17 cells, NK T cells, regulatory T cells (Tregs), CD8+ T cells and γδ T cells play a potential role in regulating the asthmatic response [21–27]. However, pulmonary DCs are involved in both innate and adaptive immune responses, and play a role as upstream initiators of the allergic inflammatory response. Therefore, they are the focus of investigations into the pathogenesis of asthma and are potential therapeutic targets. Many experiments indicate that inhaled external agents, such as diesel exhaust particles, induce a skewed Th2 response by facilitating the maturation and migration of airway DCs to the local lymph nodes and the production of Th2-type proinflammatory cytokines and Th2-attracting chemokines [28]. Also endogenous factors, such as extracellular ATP, induce Th2 responses by recruiting and activating DCs [29]. Of these factors, TSLP is thought to play a critical role in the pathophysiology of asthma.
TSLP is an interleukin (IL)-7-like cytokine produced by epithelial cells following allergen contact, which activates dendritic cells. These TSLP-DCs exert a profound influence on CD4+ T cells to produce a Th2 cytokine profile and initiate the allergic cascade. Accumulating evidence shows that TSLP is expressed highly by keratinocytes in the skin lesions seen in atopic dermatitis and by bronchial epithelial cells in asthma [30,31]. Also, recent evidence shows that TSLP aggravates allergic inflammation during the effector stages, and the synergy between TSLP, IL-1 and tumour necrosis factor (TNF)-α enhancing the production of Th2 cytokines by mast cells [32]. These findings suggest that TSLP represents a master switch for allergic inflammation at the epithelial cell/dendritic cell interface and could be a therapeutic target in allergic inflammation. In this study, we examined the effect of blocking TSLP signalling in DCs in vitro using TSLPR-Ig, and evaluated the effect of TSLPR-Ig on co-stimulatory molecule expression by TSLP-DCs. We found that TSLPR-Ig reduced significantly the expression of CD40, CD80 and CD86 by TSLP-DCs. We also evaluated the effect of TSLPR-Ig on airway inflammation in an asthmatic mouse model. The results showed that TSLPR-Ig pretreated mice had low levels of OVA-specific IgE in the serum, little infiltration of the BALF by eosinophils and lymphocytes and decreased production of Th2 cytokines. This finding confirmed the critical role played by TSLP/TSLPR signalling in Th2 skewing during local airway inflammation without the need for tslp or tslpr knock-out mice, and suggested a therapeutic effect of blocking TSLP/TSLPR signalling in allergic airway inflammation. To explore further the mechanisms underlying the anti-asthmatic effects of TSLPR-Ig we focused on pulmonary DCs, which play a major role in the pathogenesis of asthma, and also express TSLPR preferentially [32]. As the level of maturation is the hallmark of DC activity, and is critical for priming naive T cell responses, we assessed the maturation status of pulmonary DCs upon encountering a soluble allergen. The results showed that pulmonary DCs from mice injected with TSLPR-Ig showed reduced expression of CD40, CD80 and CD86 compared with DCs treated with control IgG.
Furthermore, our study demonstrated that TSLPR-Ig inhibited the asthmatic response in the airways of mice, at least in part, by regulating DC function. As TSLPR is expressed on DCs, CD4+ T cells and mast cells [33], TSLPR-Ig may also have direct inhibitory effects on other effector cells during allergic inflammation. To prove that TSLPR-Ig inhibits Th2 polarization predominantly by blocking TSLPR on DCs, we investigated the effects of TSLPR-Ig treatment prior to adoptive transfer of OVA-pulsed DCs on airway inflammation in naive mice. Consistent with previous reports, OVA-DCs in the airways induced a strong Th2 skewing response characterized by eosinophilic inflammation of the airways and the massive release of Th2 cytokines such as IL-4 and IL-5. However, pretreatment with TSLPR-Ig reduced significantly the degree of eosinophil and macrophage infiltration, as well as the release of Th2-type cytokines. These results strongly support the important role of TSLP/TSLPR signalling in DC-primed allergic disease, and demonstrate the potential of TSLPR-Ig for controlling airway inflammation in asthmatic mice mainly by blocking TSLP/TSLPR signalling in DCs.
In conclusion, local administration of TSLPR-Ig into mouse airways before sensitization resulted in inhibition of the Th2-mediated features of asthma via regulation of pulmonary DC function. These results increase our understanding of the biological function of TSLP/TSLPR signalling and support the important role of TSLP as an initiator and activator of Th2 skewing in atopic diseases. It will be interesting to determine whether the administration of TSLPR-Ig at the allergen-challenge phase has the same therapeutic effects. These findings highlight the pathological significance of TSLP in asthma and open up the possibility of TSLP-orientated therapies.
Acknowledgments
This work was supported by grants from China Postdoctoral Science Foundation (no. 20070411050) and Jiangsu Planned Projects for Postdoctoral Research Funds (no. 0701023B).
Disclosure
None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Komai M, Tanaka H, Masuda T, et al. Role of Th2 responses in the development of allergen-induced airway remodelling in a murine model of allergic asthma. Br J Pharmacol. 2003;138:912–20. doi: 10.1038/sj.bjp.0705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazzarella G, Bianco A, Catena E, De Palma R, Abbate GF. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55(Suppl. 61):6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]
- 3.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–89. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol. 2004;16:702–8. doi: 10.1016/j.coi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–7. [PubMed] [Google Scholar]
- 6.Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr Pharm Des. 2006;12:4247–54. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- 7.Idzko M, Hammad H, van Nimwegen M, et al. Inhaled iloprost suppresses the cardinal features of asthma via inhibition of airway dendritic cell function. J Clin Invest. 2007;117:464–72. doi: 10.1172/JCI28949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol. 2009;182:1641–7. doi: 10.4049/jimmunol.182.3.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, Wang YH, Duramad O, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He R, Geha RS. Thymic stromal lymphopoietin. Ann NY Acad Sci. 2010;1183:13–24. doi: 10.1111/j.1749-6632.2009.05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–39. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandey A, Ozaki K, Baumann H, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 13.Isaksen DE, Baumann H, Trobridge PA, Farr AG, Levin SD, Ziegler SF. Requirement for stat5 in thymic stromal lymphopoietin-mediated signal transduction. J Immunol. 1999;163:5971–7. [PubMed] [Google Scholar]
- 14.Rochman Y, Kashyap M, Robinson GW, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci USA. 2010;107:19455–60. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborn MJ, Ryan PL, Kirchhof N, Panoskaltsis-Mortari A, Mortari F, Tudor KS. Overexpression of murine TSLP impairs lymphopoiesis and myelopoiesis. Blood. 2004;103:843–51. doi: 10.1182/blood-2003-05-1557. [DOI] [PubMed] [Google Scholar]
- 16.Al-Shami A, Spolski R, Kelly J, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–68. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson WR, Jr, Chi EY, Albert RK, et al. Blockade of CD49d (alpha4 integrin) on intrapulmonary but not circulating leukocytes inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J Clin Invest. 1997;100:3083–92. doi: 10.1172/JCI119863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahrens B, Gruber C, Rha RD, et al. BCG priming of dendritic cells enhances T regulatory and Th1 function and suppresses allergen-induced Th2 function in vitro and in vivo. Int Arch Allergy Immunol. 2009;150:210–20. doi: 10.1159/000222673. [DOI] [PubMed] [Google Scholar]
- 19.Sung S, Rose CE, Fu SM. Intratracheal priming with ovalbumin- and ovalbumin 323–339 peptide-pulsed dendritic cells induces airway hyperresponsiveness, lung eosinophilia, goblet cell hyperplasia, and inflammation. J Immunol. 2001;166:1261–71. doi: 10.4049/jimmunol.166.2.1261. [DOI] [PubMed] [Google Scholar]
- 20.Lambrecht BN, De Veerman M, Coyle AJ, Gutierrez-Ramos JC, Thielemans K, Pauwels RA. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J Clin Invest. 2000;106:551–9. doi: 10.1172/JCI8107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang TJ, MacAry PA, Eynott P, et al. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol. 2001;166:207–17. doi: 10.4049/jimmunol.166.1.207. [DOI] [PubMed] [Google Scholar]
- 22.Soroosh P, Doherty TA. Th9 and allergic disease. Immunology. 2009;127:450–8. doi: 10.1111/j.1365-2567.2009.03114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–87. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 24.Akbari O, Stock P, Meyer E, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 25.Kearley J, Barker JE, Robinson DS, Lloyd CM. Resolution of airway inflammation and hyperreactivity after in vivo transfer of CD4+CD25+ regulatory T cells is interleukin 10 dependent. J Exp Med. 2005;202:1539–47. doi: 10.1084/jem.20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamelmann E, Oshiba A, Paluh J, et al. Requirement for CD8+ T cells in the development of airway hyperresponsiveness in a marine model of airway sensitization. J Exp Med. 1996;183:1719–29. doi: 10.1084/jem.183.4.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahn M, Kanehiro A, Takeda K, et al. Negative regulation of airway responsiveness that is dependent on gammadelta T cells and independent of alphabeta T cells. Nat Med. 1999;5:1150–6. doi: 10.1038/13476. [DOI] [PubMed] [Google Scholar]
- 28.Provoost S, Maes T, Willart MA, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol. 2010;184:426–32. doi: 10.4049/jimmunol.0902564. [DOI] [PubMed] [Google Scholar]
- 29.Idzko M, Hammad H, van Nimwegen M, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–19. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 30.Oyoshi MK, He R, Kumar L, Yoon J, Geha RS. Cellular and molecular mechanisms in atopic dermatitis. Adv Immunol. 2009;102:135–226. doi: 10.1016/S0065-2776(09)01203-6. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z, Hener P, Frossard N, et al. Thymic stromal lymphopoietin overproduced by keratinocytes in mouse skin aggravates experimental asthma. Proc Natl Acad Sci USA. 2009;106:1536–41. doi: 10.1073/pnas.0812668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allakhverdi Z, Comeau MR, Jessup HK, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–8. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol. 2007;178:1396–404. doi: 10.4049/jimmunol.178.3.1396. [DOI] [PubMed] [Google Scholar]


