Abstract
Vitamin D3 (VD3) is a steroid hormone that regulates bone health and numerous aspects of immune function and may play a role in respiratory health. We hypothesized that T helper type 2 (Th2) disorders, chronic rhinosinusitis with nasal polyps (CRSwNP) and allergic fungal rhinosinusitis (AFRS) would have VD3 deficiencies, resulting in increased mature dendritic cells (DCs) and bone erosion. We conducted a retrospective study examining VD3 levels in patients with AFRS (n = 14), CRSwNP (n = 9), chronic rhinosinusitis without nasal polyps (CRSsNP) (n = 20) and cerebrospinal fluid leak repair (non-diseased controls) (n = 14) at time of surgery. Circulating immune cell levels were determined by immunostaining and flow cytometric analysis. Plasma VD3 and immune regulatory factors (granulocyte–macrophage colony-stimulating factor and prostaglandin E2) were measured by enzyme-linked immunosorbent assay. It was observed that CRSwNP and AFRS demonstrated increased circulating DCs, while chronic rhinosinusitis without nasal polyps displayed increased circulating macrophages. CRSwNP and AFRS were to found to have insufficient levels of VD3 which correlated inversely with circulating numbers of mature DCs, DC regulatory factors and bone erosion. CRSsNP displayed no change in circulating DC numbers or VD3 status compared to control, but did display increased numbers of circulating macrophages that was independent of VD3 status. Lastly, VD3 deficiency was associated with more severe bone erosion. Taken together, these results suggest support a role for VD3 as a key player in the immunopathology of CRSwNP and AFRS.
Keywords: allergic fungal rhinosinusitis, bone erosion, chronic rhinosinusitis, dendritic cells, vitamin D
Introduction
While the exact cause of the persistent symptomatic inflammation associated with chronic rhinosinusitis (CRS) is unknown, it is thought to be the result of numerous interactions between environmental factors and the host immune system. CRS can be subdivided into two categories: CRS without nasal polyps (CRSsNP), which displays elevated levels of T helper type 1 (Th1) and Th2 cytokines, and CRS with nasal polyps (CRSwNP) which is heavily Th2 skewed [1]. Elevated levels of Th2 cytokines contribute to the symptoms of CRS by stimulating mucus production and recruitment of eosinophils [2].
Dispersed throughout the nasal and sinus mucosa are antigen-presenting cells (APC), among which are dendritic cells (DCs) and macrophages, that play a critical role in regulating Th1/Th2 skewing. Additionally, monocytes are recruited from the peripheral blood to the site of exposure, where they are differentiated into DCs or macrophages under the influence of mediators such as granulocyte–macrophage colony-stimulating factor (GM-CSF) [3]. Once matured, DCs direct naive T cells towards either a Th1 or Th2 phenotype, based on the type of stimulus inducing maturation and cues from the external environment. For example, DCs matured in the presence of prostaglandin E2 (PGE2) promote Th2 responses [4]. Furthermore, DC expression of CD86+ has been shown to be elevated in Th2-skewed respiratory diseases such as asthma and allergic rhinitis [5,6]. Macrophages represent another class of APC that regulate inflammation. In response to cytokines and microbial products, macrophages produce proinflammatory and anti-inflammatory mediators [7,8]. Elevated numbers of macrophages are observed in asthma [9], yet it is unclear if they are elevated systemically in sinusitis. Like DCs, their ability to regulate downstream immune responses suggests that they may contribute to the inflammatory response in sinusitis.
Vitamin D3 (VD3) is an immunomodulatory steroid hormone that regulates DC, monocyte, macrophage and T cell functions. VD3 plays an important role as an immune regulator through its ability to block monocyte to DC differentiation and maturation, thereby diminishing DCs ability to stimulate T cell Th1/Th2 differentiation [10]. Several studies have also shown that exposure of DCs to VD3 re-programs them to support a tolerogenic phenotype [11–13]. In macrophages, VD3 has been shown to exert an opposite effect, promoting monocyte to macrophage differentiation and proliferation [14]. Therefore, VD3 may play an important role in inflammatory diseases such as CRS.
Increasing evidence suggests that VD3 plays an important role in respiratory health. For example, in a study of 6–14-year-old children with asthma, 28% were determined to have severe VD3 deficiencies. Furthermore, increased VD3 levels were associated with reduced likelihood for being hospitalized and reduced use of anti-inflammatory medications [15]. In steroid-resistant asthmatics it has been shown that VD3 administration can down-regulate Th2 skewing [16]. Data from the Third National Health and Nutrition Examination Survey (NHANES III) showed that VD3 levels are associated inversely with the occurrence of upper respiratory tract infections, and this association was even stronger in those with asthma [17].
In the upper airway, two reports have examined the role of VD3 in allergic rhinitis. Using data from the NHANES III, Wjst and Hypponen found that the prevalence of allergic rhinitis increased across quartile groups of VD3 serum levels [18]. Pinto et al. observed that African Americans with allergic rhinitis have lower VD3 levels than race- and age-matched controls, suggesting that VD3 has a potential role in upper respiratory disease in African Americans [19]. Previous studies by our group have reported that some forms of Th2 skewed CRS, such as allergic fungal rhinosinusitis (AFRS), occur more commonly in African Americans, who are more susceptible to VD3 deficiency, raising the possibility of a role in AFRS. Additionally, African Americans with AFRS demonstrate more bone erosion than Caucasians, further supporting a potential role of VD3[20,21]. Therefore, in these studies we examined if VD3 deficiency may contribute to immune dysfunction and bone erosion in CRS.
Methods
Clinical evaluation
Studies were conducted retrospectively at the Medical University of South Carolina with Institutional Review Board approval. The Medical University of South Carolina Institutional Review Board granted approval prior to initiation of the study and informed written consent was obtained from all participants. Patients were divided among four diagnostic groups: AFRS, CRSwNP, CRSsNP and control. AFRS patients met the classic Bent and Kuhn criteria, with immunoglobulin (Ig)E hypersensitivity to fungi demonstrated by either skin testing or elevated serum IgE [22]. CRSsNP patients were diagnosed through clinical and radiographic examinations that revealed inflammatory sinus disease without frank nasal polyposis and no subjective history of atopy. Control patients were undergoing repair of spontaneous cerebrospinal fluid leak and had no history of sinusitis and no radiographic or endoscopic evidence of inflammatory sinus disease at time of surgery. Patients who had taken oral steroids or immunotherapy within 30 days of surgery were excluded from the study.
Determination of VD3 deficiency
Levels of 25-dihydroxy VD3 were measured by enzyme-linked immunosorbent assay (ELISA) (Alpco Immunoassays, Salem, NH, USA) according to the manufacturer's instructions. VD3 insufficiency was defined as <32 ng/ml and deficiency as ≤20 ng/ml [23–25]. Samples analysed in these studies were collected from mid-March to late August 2009 and March to May 2010 at latitude 32°N (spring/summer) to minimize the impact of seasonal variation in VD3 levels.
Analysis of circulating immune cells and immune regulatory products
Peripheral blood was collected at time of sinus surgery and used as the source of plasma and peripheral blood mononuclear cells (PBMCs). Circulating levels of DCs and monocytes were determined by immunostaining followed by flow cytometric analysis. Prior to staining, PBMCs were incubated in phosphate-buffered saline (PBS) with 1% bovine serum albumin (BSA) to block non-specific binding. DCs were identified by positive staining for CD209 (DC-SIGN), CD1a and CD1c. CD209 is expressed in a small number of circulating DCs [26]; it has been shown to up be up-regulated in the sinuses of patients with CRS and has been shown to support Th2 skewing [27–29]. CD86 was examined to identify macrophages and DCs and for its role in initiation of Th2 responses [30,31]. CD14 was used to identify monocytes. Expression of the co-stimulatory molecule CD86 was also examined on DCs and macrophages. Macrophages were identified by staining for CD68, after treatment with Cytofix/Perm. CD209, CD1c and CD1a+ cells were confirmed as DCs by staining lineage cocktail 1 (CD3, CD14, CD16, CD19, CD20 and CD56) and CD68-negative. Live cell gating was determined by propidium iodide staining and marker channels were set using the isotype controls specific to each antibody. Analysis was performed on a BD fluorescence activated cell sorter (FACS) FACSCantos using FACS Diva software. All reagents for immunostaining were from BD Biosciences (San Diego, CA, USA).
Plasma levels of GM-CSF (BD Biosciences) and PGE2 (R&D Systems, Minneapolis, MN, USA) were measured by ELISA and performed according to the manufacturers' instructions.
Bone erosion scoring
Degree of bone erosion was analysed by two graders using a previously published staging system [32]. A computed tomography (CT) bone remodelling score was assigned by both graders and then averaged to yield a mean CT bone erosion score for each patient. Graders were blinded to age, race, gender and VD3 status of the patients.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism version 5.02 software (La Jolla, CA, USA). Values were first determined to follow a normal distribution using a D'Agostino and Pearson omnibus normality test. A one-way analysis of variance (anova) with post-hoc unpaired Student's t-test was then used to determine statistically significant differences between patient cohorts and indicated parameters. A Pearson's correlation analysis was used to determine if there was a correlation between VD3 levels and the aforementioned immune parameters. Two-way anova was conducted to determine if differences observed in VD3 levels were influenced by age, gender, body mass index (BMI) or race.
Within the subset of patients whose mean CT bone remodelling score was greater than 0, an unpaired t-test was used to determine statistical significance those with adequate VD3 (greater than or equal to 32 ng/ml) or insufficient VD3 levels (<32 ng/ml) on the CT bone remodelling score. An unpaired Student's t-test was used to determine differences in bone erosion scores between VD3-deficient and -insufficient patients. A Pearson's correlation analysis was used to determine if there was a correlation between VD3 levels and bone erosion severity.
Results
CRSwNP and AFRS patients have elevated numbers of circulating DCs and DC regulatory products, while CRSsNP displays elevated numbers of circulating macrophages
In these retrospective studies, we examined PBMCs from patients with CRSsNP, CRSwNP or AFRS to determine if there were differences in circulating numbers of APCs and monocytes compared to controls. First, expression of CD86 was assessed due to its role in Th2 initiation [5,6]. Compared to controls, we found elevated numbers of CD86+ PBMCs in CRSsNP (P = 0·007), CRSwNP (P < 0·0001) and AFRS (P < 0·0001) (Fig. 1a). There was no statistically significant difference between CRSsNP and CRSwNP (P = 0·368) or AFRS (P = 0·190).
Fig. 1.
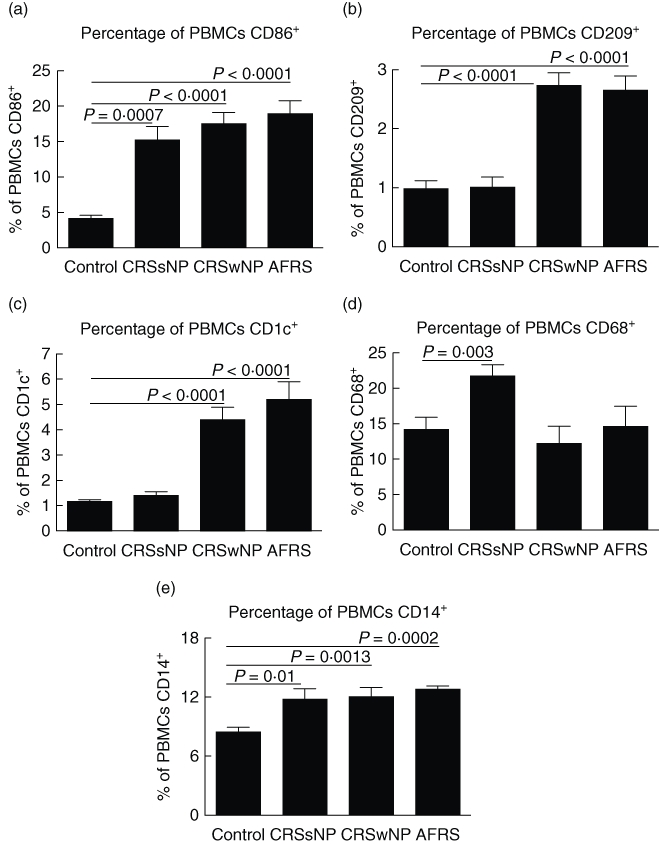
Circulating levels of antigen-presenting cells (APCs) and monocytes are altered in chronic rhinosinusitis (CRS). Immunostaining and flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) in patients with CRS for (a) CD86+ dendritic cells (DCs) and macrophages, (b) CD209+ DCs, (c) CD1c+ DCs, (d) CD68+ macrophages and (e) CD14+ monocytes. Statistics shown represent Student's t-test between indicated patient groups.
Next, staining for CD209 and CD68 was conducted to identify circulating DCs and macrophages, respectively, more definitively. Only CRSwNP and AFRS displayed elevated levels of CD209+ DCs (Fig. 1b) compared to control (P < 0·0001 for each group). CRSwNP and AFRS circulating DC numbers were also elevated compared to CRSsNP (P = 0·0001 and P = 0·0014, respectively). Similar to the CD209 results, circulating numbers of CD1c+ DCs (Fig. 1c) were elevated in CRSwNP and AFRS versus control (P < 0·0001 for both analyses). No differences were observed between control and CRSsNP levels of CD1c+ DCs (P = 0·15). Unlike changes in DC numbers, only CRSsNP had increased numbers of circulating CD68+ macrophages (Fig. 1d) compared to control (P = 0·003), CRSwNP (P = 0·004) and AFRS (P = 0·03). Lastly, we measured circulating monocyte levels (Fig. 1e). Compared to control there were elevated numbers of CD14+ cells in CRSsNP (P = 0·01), CRSwNP (P = 0·0013) and AFRS (P = 0·0002). There was no significant difference in levels between the three sinusitis subclasses. Taken together, these results demonstrate that all three sinusitis subclasses have increased circulating monocytes. However, only CRSwNP and AFRS have increased numbers of circulating DCs, while only CRSsNP has increased circulating macrophages. These differences in immune cell composition may help to account for differences in Th1/Th2 skewing observed in the various sinusitis subclasses.
CRSwNP and AFRS, but not CRSsNP, have insufficient circulating levels of vitamin D3
After observing increased numbers of circulating DCs in CRSwNP and AFRS, we next determined if these patients were VD3-deficient, as VD3 has been shown to block monocyte to DC differentiation and DC maturation. Mean plasma 25-OH VD3 levels for controls (51 ± 4 ng/ml) and CRSsNP (45 ± 2 ng/ml) were well above the recommended minimum level of 32 ng/ml (Fig. 2). Mean 25-OH VD3 levels for CRSwNP (18 ± 4 ng/ml) and AFRS (21 ± 5 ng/ml) were significantly lower when compared to either control or CRSsNP (P ≤ 0·0001 for all comparisons).
Fig. 2.
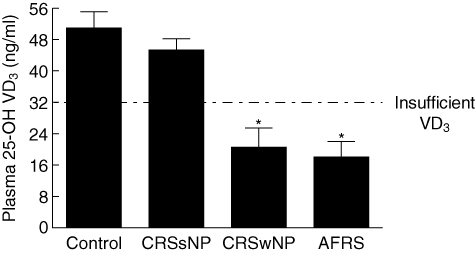
Chronic rhinosinusitis with nasal polyps (CRSwNP) and allergic fungal rhinosinusitis (AFRS) have insufficient levels of circulating 25-OH vitamin D3 (VD3). Compared to control and CRSwNP, AFRS and CRSwNP have significantly lower plasma VD3 levels with means below the recommended level of 32 ng/ml. (*) P ≤ 0·0001 versus control or chronic rhinosinusitis without nasal polyps (CRSsNP).
Two-way anova analysis was used to determine if differences in VD3 were influenced by gender, race or BMI, all of which are known to effect VD3 levels (summarized in Table 1). It was determined that gender (P = 0·58), race (P = 0·12) and BMI (P = 0·18) did not influence significantly the differences in VD3 observed among the various patient cohorts. Post-hoc t-test analysis identified that overweight patients with AFRS have significantly lower VD3 than AFRS patients, whose BMI was in the healthy range (P = 0·03), suggesting that weight can contribute further to VD3 insufficiency associated with AFRS. These results demonstrate that CRSwNP and AFRS are VD3-insufficient compared to control. Conversely, CRSsNP was found to be VD3-sufficient, implicating VD3 in the pathophysiology of the different subtypes of chronic sinusitis.
Table 1.
| Control (n = 14) | CRSsNP (n = 20) | CRSwNP (n = 9) | AFRS (n = 14) | |
|---|---|---|---|---|
| Mean ± s.d. | Mean ± s.d. | Mean ± s.d. | Mean ± s.d. | |
| Male | 53·3 ± 14 | 50·2 ± 30 | 18·0 ± 17 | 26·4 ± 24 |
| Female | 45·3 ± 15 | 58·3 ± 28 | 15·8 ± 7 | 15·5 ± 9 |
| P-value | 0·33 | 0·15 | 0·79 | 0·32 |
| Caucasian | 56·0 ± 14 | 43·8 ± 13 | 21·3 ± 17 | 31·2 ± 22 |
| African American | 41·2 ± 15 | 59·0 ± 4 | 8·5 ± 9 | 13·0 ± 7 |
| P-value | 0·09 | 0·11 | 0·35 | 0·07 |
| Healthy | 53·0 ± 15 | 39·7 ± 14 | 21·7 ± 5 | 32·0 ± 2 |
| Overweight | 50·0 ± 18 | 45·3 ± 13 | 18·5 ± 10 | 8·9 ± 6·8 |
| P-value | 0·78 | 0·44 | 0·64 | 0·03* |
Statistically significant.
For stratification by race, data from one Hispanic individual was excluded.
Using the classification system defined by the US National Institute of Health, individuals were stratified by BMI as healthy (≥18·5 to 24·9) or overweight (≥25). CRSsNP, chronic rhinosinusitis without nasal polyps; CRSwNP, chronic rhinosinusitis with nasal polyps; AFRS, allergic fungal rhinosinusitis; s.d., standard deviation.
Vitamin D3 levels correlate inversely with numbers of circulating mature DCs and DC regulatory factors
After determining that CRSwNP and AFRS have lower VD3 levels, we next determined if there was an association between VD3 and elevated numbers of circulating DCs. First, we examined the impact VD3 on circulating CD86+ and CD209+ PBMCs. VD3-insufficient patients had double the number of circulating CD86+ cells than those with healthy VD3 levels (P = 0·01) (Fig. 3a). Those who were VD3-deficient had nearly four times as many CD86+ cells as control (P < 0·0001) and twice as many as those who were insufficient (P = 0·01). CD209+ DCs (Fig. 3b) followed a similar trend, with increased numbers of cells in those whose VD3 levels were healthy compared to insufficient (P = 0·008) or deficient (P < 0·0001). Patients who were deficient also had significantly more CD209+ DCs than those who were insufficient (P = 0·003). Furthermore, those who were VD3-insufficient or -deficient also had significantly higher circulating levels of CD1c+ DCs compared to healthy controls (P = 0·0003 and P < 0·0001, respectively). As shown in Fig. 3d, a strong inverse correlation exists between circulating CD86+ DCs and VD3 status (R2 = 0·8501, P < 0·0001). VD3 also correlated inversely with PBMC expression of CD209+ (Fig. 3e) (R2 = 0·7977, P < 0·0001), CD1c (Fig. 3f) (R2 = 0·8404, P < 0·0001) and CD1a (R2 = 0·9197, P < 0·0001, data not shown). Of the nine CRSwNP patients with CD209+ measurement, five had negative allergy testing, three had positive allergy testing and one was untested. Further evaluation determined that there were no significant differences between circulating CD209+ DCs levels in atopic versus non-atopic CRSwNP individuals (data not shown, P = 0·88). This would suggest that while atopic status may contribute to elevated numbers of DCs, such as in AFRS, there are mechanisms such as VD3 deficiency that result in an altered immune profile independent of atopy.
Fig. 3.
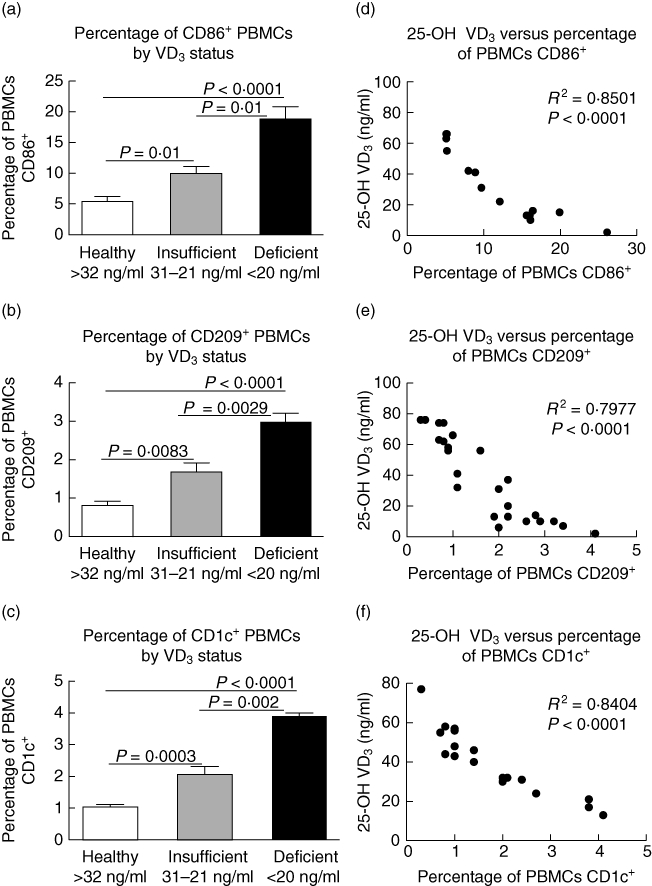
25-OH vitamin D3 (VD3) levels correlate inversely with levels of circulating CD86+, CD209+ and CD1c+ cells. Clinical stratification based upon VD3 levels and corresponding levels of (a) CD86+, (b) CD209+ cells and (c) CD1c+. Pearson's correlation analysis demonstrating (d) 25-OH VD3versus percentage of peripheral blood mononuclear cells (PBMCs) CD86+, (e) 25-OH VD3versus percentage of PBMC CD209+ and (f) 25-OH VD3versus percentage of PBMC CD1c+.
While the CRSsNP cohort was overall VD3-sufficient, a correlation analysis was conducted between VD3 and CD68+. As expected, there was no association between VD3 and circulating numbers of CD68+ cells (data not shown; R2 = 0·08, P = 0·72). Similarly, there was no correlation between VD3 plasma levels and circulation CD14+ monocyte levels among any of the cohorts (data not shown; R2 = 0·015, P = 0·71).
Next we assessed plasma levels of macrophage and DC regulatory products, GM-CSF and PGE2. Figure 4a,b demonstrates that compared to control, GM-CSF and PGE2 were increased in CRSsNP (P = 0·02 and P = 0·0011, respectively), CRSwNP (P < 0·0001 and P = 0·0004, respectively) and AFRS (P = 0·0067 and P = 0·0057, respectively). Levels of GM-CSF were also significantly higher in CRSwNP and AFRS compared to CRSsNP (P = 0·03 and P = 0·01, respectively) and levels of PGE2 were significantly higher in AFRS compared to CRSsNP (P = 0·005). There was no statistically significant difference between CRSsNP and CRSwNP plasma PGE2 levels (P = 0·08). Similar to the DCs/VD3 correlation, VD3 correlated inversely with GM-CSF (R2 = 0·7039, P = 0·0012) (Fig. 4c) and PGE2 (Fig. 4d) (R2 = 0·7401, P = 0·0081). These results demonstrate that VD3 deficiency is associated with elevated levels of circulating DCs and DC regulatory products in CRSwNP and AFRS.
Fig. 4.
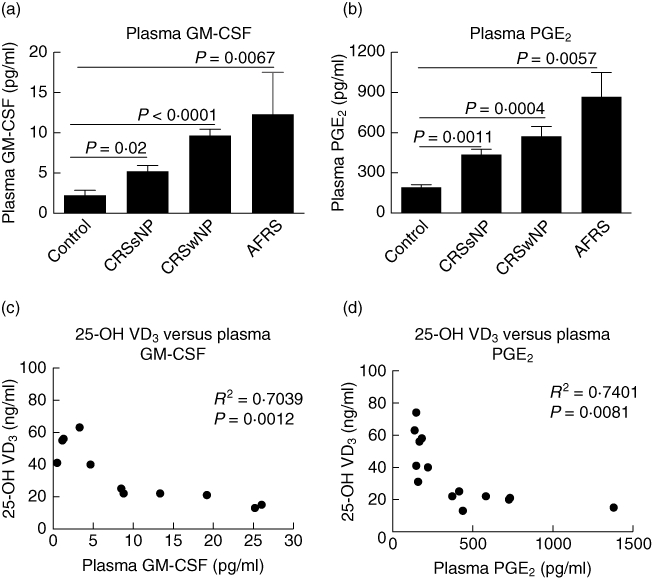
Circulating levels of dendritic cell (DC) regulatory factors are up-regulated in chronic rhinosinusitis (CRS) correlated inversely with 25-OH vitamin D3 (VD3). Plasma levels of (a) granulocyte–macrophage colony-stimulating factor (GM-CSF) and (b) prostaglandin E2 (PGE2) as measured by enxyme-linked immunosorbent assay (ELISA). Statistics shown represent Student's t-test between indicated patient groups. Pearson's correlation analysis of plasma levels for GM-CSF (c) and PGE2 (d) as determined by ELISA versus plasma 25-OH VD3.
VD3 deficiency is associated with increased bone erosion in CRS
VD3 has long been known as a regulator of bone health due to its ability to stimulate calcium absorption. Therefore we measured the severity of bone erosion on preoperative CT scans in patients with varying levels of VD3. As shown in Fig. 5a, the average CT bone remodelling score in patients with insufficient levels (<32 ng/ml) of serum VD3 was significantly greater than in patients with adequate (≥32 ng/ml) VD3 (P = 0·016) levels. Furthermore, as in our immune studies, a strong inverse relationship was identified between reduced plasma levels of VD3 and an increase in the CT bone remodelling score (R2 = 0·553 and P = 0·009) (Fig. 5b). Figure 5c is a representative CT scan from an AFRS patient with a bone erosion score of 22 and VD3 level of 11 ng/ml. These results support the role of VD3 in the exacerbation of CRS-associated bone erosion.
Fig. 5.
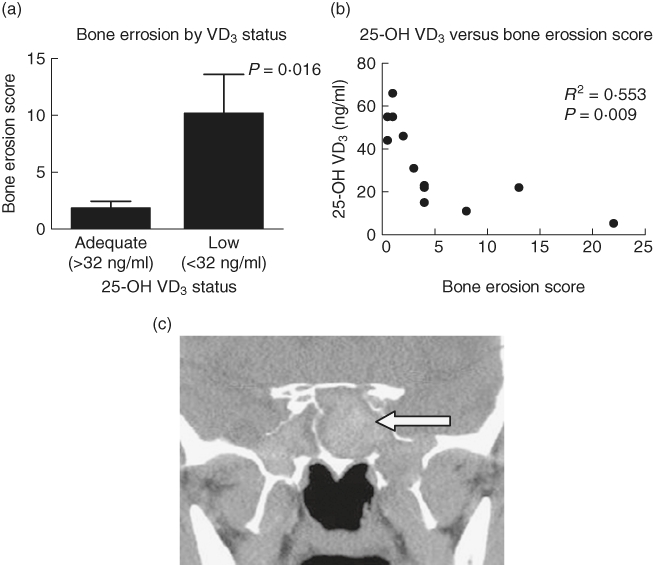
Vitamin D3 (VD3) deficiency is associated with increased bone erosion. (a) Patients with sufficient VD3 had significantly less bone erosion than those with insufficient VD3. (b) Pearson's correlation examining 25-OH VD3versus bone erosion score. (c) Representative coronal computed tomography (CT) from allergic fungal rhinosinusitis (AFRS) patient demonstrates bone erosion bilaterally along the lateral and inferior walls of the sphenoid sinus (arrow).
Discussion
In these retrospective studies we investigated circulating levels of APCs in chronic rhinosinusitis. Patients with CRSwNP and AFRS displayed elevated numbers of circulating DCs, while CRSsNP had increased numbers of macrophages. In other respiratory diseases, such as asthma, DC numbers are elevated and make a significant contribution to disease pathogenesis, including the initiation of Th2 skewing [5,6,31].
Investigation into the potential mechanism driving elevated numbers of DCs led us to examine VD3. Both CRSwNP and AFRS patients were identified as being VD3-insufficient (<32 ng/ml) compared to control and CRSsNP. Furthermore, a strong association between VD3 deficiency and increased levels of circulating DCs in CRSwNP and AFRS was identified. Atopic status was examined as additional mechanism accounting for elevated numbers of DCs, although it was determined that there was no difference in circulating DC numbers between atopic and non-atopic CRSwNP individuals. It is hypothesized that lack of VD3 allows the elevated numbers of monocytes in CRSwNP and AFRS to proceed systemically to DC differentiation and maturation more freely. While a large body of literature supports VD3 as promoting Th1 or Th2 skewing in various disease states [33], ultimately all these demonstrate a failure of DCs to be kept in a tolerogenic state. In studies by Penna et al. it was shown that the 1,25 VD3 promoted myeloid DCs to promote a tolerogenic state [34]. The lack of the 1,25 VD3 precursor, 25-OH VD3, observed in CRSwNP and AFRS may therefore allow DCs to mature with other environmental or host signals driving DCs to promote Th2 inflammation.
VD3 did not correlate with all the changes in immune parameters observed in these studies. No correlation was observed between VD3 and CD14+ monocytes, suggesting that the presence of DC and macrophage precursors is not dependent upon VD3. Additionally, elevations in CD68+ macrophages did not correlate with VD3. This was not entirely unexpected, because in contrast to its inhibitory effects upon DC maturation, VD3 promotes monocyte to macrophage differentiation. Thus, patients with CRSsNP who had normal VD3 levels had higher macrophage levels than CRSwNP and AFRS patients who were VD3-insufficient.
Our studies also identified that plasma levels of PGE2 and GM-CSF were up-regulated in CRSsNP and to an even greater extent in CRSwNP and AFRS. Moreover, both of these factors were found to correlate inversely with VD3 in CRSwNP and AFRS. These results are consistent with reports in asthma showing elevated PGE2[35]. One cellular source of PGE2 identified previously in asthmatics is macrophages [36], which may account for the slight increase in CRSsNP, as these patients possess elevated numbers of circulating macrophages. As with PGE2, GM-CSF has also been identified as being elevated in asthma [37] and has been shown to be a contributor to airway inflammation and hyperresponsiveness [38]. While our studies are the first to identify GM-CSF as being elevated systemically, previous studies have shown GM-CSF up-regulation locally in allergic and non-allergic polyp tissue compared to turbinate [39]. However, the role of both of these factors in CRSsNP and CRSwNP remains to be identified.
In addition to examination of immune parameters, the impact of VD3 on bone erosion in CRS was investigated. Patients with more severe forms of CRS that present with bone erosion into the orbit and/or skull base demonstrated more severe VD3 deficiencies. These results echo similar findings in other diseases, such as rheumatoid arthritis, that report a relationship between VD3 receptor polymorphisms and accelerated bone loss [40]. It is unclear if VD3 deficiencies lead to systemic abnormalities of bone metabolism or if they even play a major role in localized bone loss within the sinonasal cavity.
VD3 targets many of the same DC regulatory pathways as corticosteroids, such as prednisone, one of the most commonly prescribed treatments for CRS. Based on this, it could be suggested that supplementation with VD3 in CRSwNP and AFRS may be analogous to replacing one's natural prednisone. Based on the results of the above-mentioned studies and the results presented here, there is increasing evidence to support a role for VD3 as a key player in the immunopathology of CRSwNP and AFRS.
Acknowledgments
The authors would like to thank Helen Accerbi RN for her technical assistance with these studies. These studies were supported by grants to R.J.S. and J.K.M. from the Flight Attendant Medical Research Institute.
Disclosure
None of the authors listed have any potential conflicts to disclose related to the research presented herein.
References
- 1.Schleimer RP, Kato A, Peters A, et al. Epithelium, inflammation, and immunity in the upper airways of humans: studies in chronic rhinosinusitis. Proc Am Thorac Soc. 2009;6:288–94. doi: 10.1513/pats.200808-088RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol. 2008;8:193–204. doi: 10.1038/nri2275. [DOI] [PubMed] [Google Scholar]
- 3.Rate A, Upham JW, Bosco A, McKenna KL, Holt PG. Airway epithelial cells regulate the functional phenotype of locally differentiating dendritic cells: implications for the pathogenesis of infectious and allergic airway disease. J Immunol. 2009;182:72–83. doi: 10.4049/jimmunol.182.1.72. [DOI] [PubMed] [Google Scholar]
- 4.Kalinski P, Hilkens CM, Snijders A, Snijdewint FG, Kapsenberg ML. Dendritic cells, obtained from peripheral blood precursors in the presence of PGE2, promote Th2 responses. Adv Exp Med Biol. 1997;417:363–7. doi: 10.1007/978-1-4757-9966-8_59. [DOI] [PubMed] [Google Scholar]
- 5.van Rijt LS, Lambrecht BN. Dendritic cells in asthma: a function beyond sensitization. Clin Exp Allergy. 2005;35:1125–34. doi: 10.1111/j.1365-2222.2005.02321.x. [DOI] [PubMed] [Google Scholar]
- 6.Hattori H, Okano M, Yoshino T, et al. Expression of costimulatory CD80/CD86-CD28/CD152 molecules in nasal mucosa of patients with perennial allergic rhinitis. Clin Exp Allergy. 2001;31:1242–9. doi: 10.1046/j.1365-2222.2001.01021.x. [DOI] [PubMed] [Google Scholar]
- 7.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 8.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–92. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 10.Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34. doi: 10.1038/nri1256. [DOI] [PubMed] [Google Scholar]
- 11.May E, Asadullah K, Zugel U. Immunoregulation through 1,25-dihydroxyvitamin D3 and its analogs. Curr Drug Targets Inflamm Allergy. 2004;3:377–93. doi: 10.2174/1568010042634596. [DOI] [PubMed] [Google Scholar]
- 12.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–11. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 13.Szeles L, Keresztes G, Torocsik D, et al. 1,25-Dihydroxyvitamin D3 is an autonomous regulator of the transcriptional changes leading to a tolerogenic dendritic cell phenotype. J Immunol. 2009;182:2074–83. doi: 10.4049/jimmunol.0803345. [DOI] [PubMed] [Google Scholar]
- 14.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann NY Acad Sci. 2007;1117:94–105. doi: 10.1196/annals.1402.036. [DOI] [PubMed] [Google Scholar]
- 15.Brehm JM, Celedon JC, Soto-Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179:765–71. doi: 10.1164/rccm.200808-1361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Litonjua AA, Weiss ST. Is vitamin D deficiency to blame for the asthma epidemic? J Allergy Clin Immunol. 2007;120:1031–5. doi: 10.1016/j.jaci.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Ginde AA, Mansbach JM, Camargo CA., Jr Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2009;169:384–90. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62:1085–6. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 19.Pinto JM, Schneider J, Perez R, DeTineo M, Baroody FM, Naclerio RM. Serum 25-hydroxyvitamin D levels are lower in urban African American subjects with chronic rhinosinusitis. J Allergy Clin Immunol. 2008;122:415–7. doi: 10.1016/j.jaci.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 20.Wise SK, Rogers GA, Ghegan MD, Harvey RJ, Delgaudio JM, Schlosser RJ. Radiologic staging system for allergic fungal rhinosinusitis (AFRS) Otolaryngol Head Neck Surg. 2009;140:735–40. doi: 10.1016/j.otohns.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 21.Ghegan MD, Lee FS, Schlosser RJ. Incidence of skull base and orbital erosion in allergic fungal rhinosinusitis (AFRS) and non-AFRS. Otolaryngol Head Neck Surg. 2006;134:592–5. doi: 10.1016/j.otohns.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 22.Bent JP, 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111:580–8. doi: 10.1177/019459989411100508. [DOI] [PubMed] [Google Scholar]
- 23.Binkley N, Ramamurthy R, Krueger D. Low vitamin D status: definition, prevalence, consequences, and correction. Endocrinol Metab Clin North Am. 2010;39:287–301. doi: 10.1016/j.ecl.2010.02.008. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmed W, Khan N, Glueck CJ, et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis–myalgia in statin-treated patients. Transl Res. 2009;153:11–6. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Heaney RP. Vitamin D in health and disease. Clin J Am Soc Nephrol. 2008;3:1535–41. doi: 10.2215/CJN.01160308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soilleux EJ, Morris LS, Leslie G, et al. Constitutive and induced expression of DC-SIGN on dendritic cell and macrophage subpopulations in situ and in vitro. J Leukoc Biol. 2002;71:445–57. [PubMed] [Google Scholar]
- 27.Rampey AM, Lathers DM, Woodworth BA, Schlosser RJ. Immunolocalization of dendritic cells and pattern recognition receptors in chronic rhinosinusitis. Am J Rhinol. 2007;21:117–21. doi: 10.2500/ajr.2007.21.2998. [DOI] [PubMed] [Google Scholar]
- 28.Zhou T, Chen Y, Hao L, Zhang Y. DC-SIGN and immunoregulation. Cell Mol Immunol. 2006;3:279–83. [PubMed] [Google Scholar]
- 29.Wills-Karp M. Allergen-specific pattern recognition receptor pathways. Curr Opin Immunol. 2010 doi: 10.1016/j.coi.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–83. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- 31.Okano M, Azuma M, Yoshino T, et al. Differential role of CD80 and CD86 molecules in the induction and the effector phases of allergic rhinitis in mice. Am J Respir Crit Care Med. 2001;164:1501–7. doi: 10.1164/ajrccm.164.8.2011072. [DOI] [PubMed] [Google Scholar]
- 32.Wise SK, Rogers GA, Ghegan MD, Harvey RJ, DelGaudio JM, Schlosser RJ. Radiologic staging system for allergic fungal rhinosinusitis (AFRS) Otolaryngol Head Neck Surg. 2009;140:735–40. doi: 10.1016/j.otohns.2008.12.053. [DOI] [PubMed] [Google Scholar]
- 33.Wagner CL, Taylor SN, Hollis BW. Does vitamin D make the world go ‘round’? Breastfeed Med. 2008;3:239–50. doi: 10.1089/bfm.2008.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penna G, Amuchastegui S, Giarratana N, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–53. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal S, Moodley YP, Thompson PJ, Misso NL. Prostaglandin E2 and cysteinyl leukotriene concentrations in sputum: association with asthma severity and eosinophilic inflammation. Clin Exp Allergy. 2008;40:85–93. doi: 10.1111/j.1365-2222.2009.03386.x. [DOI] [PubMed] [Google Scholar]
- 36.Profita M, Sala A, Bonanno A, et al. Increased prostaglandin E2 concentrations and cyclooxygenase-2 expression in asthmatic subjects with sputum eosinophilia. J Allergy Clin Immunol. 2003;112:709–16. doi: 10.1016/s0091-6749(03)01889-x. [DOI] [PubMed] [Google Scholar]
- 37.Brown PH, Crompton GK, Greening AP. Proinflammatory cytokines in acute asthma. Lancet. 1991;338:590–3. doi: 10.1016/0140-6736(91)90605-o. [DOI] [PubMed] [Google Scholar]
- 38.Yamashita N, Tashimo H, Ishida H, et al. Attenuation of airway hyperresponsiveness in a murine asthma model by neutralization of granulocyte–macrophage colony-stimulating factor (GM-CSF) Cell Immunol. 2002;219:92–7. doi: 10.1016/s0008-8749(02)00565-8. [DOI] [PubMed] [Google Scholar]
- 39.Hamilos DL, Leung DY, Huston DP, Kamil A, Wood R, Hamid Q. GM-CSF, IL-5 and RANTES immunoreactivity and mRNA expression in chronic hyperplastic sinusitis with nasal polyposis (NP) Clin Exp Allergy. 1998;28:1145–52. doi: 10.1046/j.1365-2222.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 40.Gough A, Sambrook P, Devlin J, et al. Effect of vitamin D receptor gene alleles on bone loss in early rheumatoid arthritis. J Rheumatol. 1998;25:864–8. [PubMed] [Google Scholar]


