Abstract
Familial Mediterranean fever (FMF) is an autoinflammatory disease characterized by recurrent episodes of fever and serosal or synovial inflammation. We examined the utility of CD64 (FcγRI) expression in polymorphonuclear neutrophils (PMNs) as clinical and biological parameters in patients with FMF. We studied 12 Japanese FMF patients (mean age; 22·8 ± 15·5 years, male/female: 2/10), along with rheumatoid arthritis patients (RA, n = 38 male/female: 6/32, mean age; 52·2 ± 15·3 years), systemic lupus erythematosus (SLE, n = 15 male/female: 0/15, mean age; 38·5 ± 15·9 years) and 12 healthy subjects (male/female: 3/9, mean age; 37·9 ± 17·2 years). CD64 expression on PMNs was determined using flow cytometry. The quantitative expression of CD64 in patients with FMF (2439·6 ± 2215·8 molecules per PMN) was significantly higher than in healthy subjects (547·8 ± 229·5, P = 0·003) or in patients with RA (606·5 ± 228·2, P < 0·0001) and SLE (681·3 ± 281·1, P = 0·004). The increased CD64 expression on PMNs isolated from untreated FMF patients was down-regulated by colchicine treatment. NACHT-LRR-PYD-containing protein 3 (NLRP3) activation using MurNAc-L-Ala-D-isoGln (MDP) resulted in increased CD64 expression on PMNs from healthy subjects. Our results suggest that quantitative measurement of CD64 expression on PMNs can be a valuable tool to discriminate between FMF and autoimmune diseases.
Keywords: autoinflammatory disease, CD64, familial Mediterranean fever, polymorphonuclear neutrophil
Introduction
Autoinflammatory diseases are a group of disorders characterized by unprovoked inflammation in the absence of high-titre autoantibodies or antigen-specific T cells [1]. Familial Mediterranean fever (FMF) is a most prevalent genetic autoinflammatory disease [2]. It is characterized by self-limiting fever and polyserositis. During the attack, neutrophilia and an acute-phase response occur, and histologically there is massive sterile influx of leucocytes to the affected site [3]. FMF is caused by mutations in MEFV, which encodes pyrin [4,5]. Pyrin is expressed predominantly in neutrophils and is considered to be involved in the regulation of caspase-1 activation and consequently interleukin (IL)-1 beta production [6].
CD64 (FCγRI), one of the FC receptors for immunoglobulin (Ig)G, plays a role in antibody-dependent cytotoxicity, clearance of immune complexes and phagocytosis of targets opsonized with IgG [7]. CD64 is expressed constitutively by monocyte/macrophages, and is up-regulated in activated neutrophils in response to microbial wall components and cytokines such as granulocyte colony-stimulating factor (G-CSF) [8]. Quantitative flow cytometric techniques have been established to measure CD64 expression in myeloid cells. Matsui et al. demonstrated that neutrophil expression of CD64 is a highly sensitive and specific marker for detecting inflection in rheumatoid arthritis (RA) and can distinguish infection from an RA flare [9]. Myeloid CD64 expression has been thought to be suitable as a diagnostic indicator of active acute inflammatory responses, such as infection.
Although some information is available regarding immune activation in FMF, there is little information concerning neutrophil activation marker expression in peripheral blood. More recently, S100A12, a product of activated neutrophils, has been reported to be a sensitive biomarker for FMF [10]. It is likely that polymorphonuclear neutrophil (PMN) expression of CD64 can have great clinical utility in the differential diagnosis of FMF, as the hallmark of FMF is PMN activation. In this study, we investigated whether CD64 expression is up-regulated in circulating PMNs in patients with FMF.
Materials and methods
Patients
FMF patients were followed at the departments of paediatrics or rheumatology at Shinshu University Hospital, Nagasaki Medical Center and Nagasaki University Hospital. Patients with RA (n = 38) and systemic lupus erythematosus (SLE, n = 15) were followed at the department of rheumatology at Nagasaki Medical Center. Mean values of white blood cells (WBC) were 6903 ± 2280 (/µl) in RA and 6725 ± 2762 (/µl) in SLE; C-reactive protein (CRP) values were 1·40 ± 1·14 (mg/dl) in RA and 0·30 ± 0·01 (mg/dl) in SLE, and erythrocyte sedimentation rate (ESR) were 39·6 ± 31·7 (mm/h) in RA and 17·6 ± 11·9 (mm/h) in SLE. RA patients having interstitial pneumonia or vasculitis were excluded from this study. Informed consent was obtained from each patient.
Reagents
Polymorphprep™ was obtained from NycoMed (Axis-Shield PoC AS, Oslo, Norway). MurNAc-L-Ala-D-isoGln (MDP) and MDP-negative control were purchased from Invivogen (San Diego, CA, USA).
Flow cytometric analysis
The expression of CD64 was measured by flow cytometry using a Coulter Epics XL flow cytometer (Beckman Coulter, Brea, CA, USA) using Expo32 ADC analysis software (Beckman Coulter). After delineating the leucocytes by staining with the pan-leucocyte marker CD45, the different cell types were gated. CD64 expression was determined as the percentage of positive cells in the PMN gate. To determine the number of CD64 molecules per cell, a phycoerythrin (PE) fluorescence kit (Quantibrite PE; BD Biosciences, San Jose, CA, USA) was used according to the manufacturer's instructions, which allows conversion of fluorescence reading to CD64 molecules per cell. PE-conjugated anti-human CD64 antibody (clone 10·1) was purchased from BD Biosciences.
PMNs isolation
Venous peripheral blood was collected from healthy volunteers. All participating subjects had given their informed consent. The blood was layered onto a Polymorphprep™ cushion and cells were isolated according to the manufacturer's protocol. Briefly, PMNs were isolated on the basis of density, washed once in 0·5 N RPMI-1640 to restore osmolarity, and then washed once more in RPMI-1640. The PMNs were subsequently diluted in complete medium consisting of RPMI-1640 supplemented with 0·3 g/l l-glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin.
Statistical analysis
The SigmaStat statistical program (SPSS Science, Chicago, IL, USA) was used for statistical analysis. CD64 values on PMN were reported as median and interquartile range (IQR). Comparisons of the expression levels of CD64 on PMN were made using the Mann–Whitney U-test for evaluation of differences between groups. Student's t-test was used for matched paired samples. P < 0·05 was considered significant.
Results
Patients
Between January 2010 and 31 July 2010, blood samples were obtained from nine FMF patients who attended the out-patient clinics at Shinshu University Hospital, Nagasaki Medical Center and Nagasaki University Hospital. The patient characteristics are listed in Table 1. All patients fulfilled the clinical criteria of FMF [11]. Of these nine patients, seven patients had been treated with daily colchicine and the remaining two untreated patients had been treated by colchicine after the diagnosis was established.
Table 1.
Patient characteristics and CD64 expression levels in familial Mediterranean fever (FMF) patients
| Patient no. | Gender | Age | Age of onset | Dose of colchicine (mg/day) | Mutations | CD64 expression* | WBC (/µl) | CPR (mg/dl) | ESR (mm/h) |
|---|---|---|---|---|---|---|---|---|---|
| Untreated FMF | |||||||||
| 1 | F | 46 | 23 | (−) | E84K/– | 9166 | 4 900 | 6·54 | 38 |
| 2 | M | 46 | 21 | (−) | E148Q–L110P/– | 1817 | 7 500 | 3·78 | 73 |
| Treated FMF (on attack) | |||||||||
| 3 | F | 7 | 6 | 0·5 | (−) | 1411 | 11 000 | 3·0 | n.a. |
| 4 | M | 24 | 12 | 0·75 | M694I/L110P | 2234 | 9 890 | 7·63 | n.a. |
| 5 | F | 10 | 7 | 0·5 | M694I/E148Q | 2456 | 5 900 | 19 | 70 |
| 6 | F | 12 | 4 | 0·5 | (−) | 2079 | 18 200 | 11·6 | n.a. |
| Treated FMF (no attack) | |||||||||
| 7 | F | 7 | 7 | 0·5 | E148Q/– | 2346 | 7 390 | 0·2 | n.a. |
| 8 | F | 42 | 20 | 1·0 | M694I/M694I | 2051 | 5 600 | Negative | 13 |
| 9 | F | 32 | 29 | 1·0 | R202Q–E148Q/– | 1658 | 4 900 | Negative | 4 |
| 10 | F | 19 | 18 | 0·5 | M694I/E148Q–L110P | 722 | n.a. | n.a. | n.a. |
| 11 | F | 23 | 6 | 1·0 | M694I/E148Q–L110P | 611 | 7 500 | Negative | n.a. |
| 12 | F | 5 | 3 | 0·125 | E148Q/– | 2724 | 8 100 | Negative | n.a. |
Expression of CD64 molecules per neutrophil. WBC, white blood cell; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; n.a., not available.
CD64 expression on PMNs from FMF patients
CD64 expression on PMNs in patients with RA (median 567·0, IQR 393–786) or SLE (median 742·0, IQR 480–876) did not differ significantly from that of healthy subjects (median 528·5, IQR 410·5–742). However, CD64 expression in FMF patients in FMF patients (median 2051, IQR 1411–2346) differed significantly from those of the RA, SLE and healthy subjects (Fig. 1). CD64 expression on PMN were elevated in all FMF patients who were not treated, or during the phase of attack, except for two patients in remission state under colchicine treatment (Table 1). A representative histogram of CD64 expression on PMNs and monocytes from blood samples of a FMF patient (case 8) and a healthy subject is shown in Fig. 2. CD64 expression on PMNs was elevated markedly in FMF patients compared to those in healthy subjects. CD64 expression on monocytes was also elevated marginally in FMF patients. To determine whether CD64 expression could be modulated by colchicine treatment, we investigated the CD64 expression on PMNs in newly diagnosed FMF patients, before and after colchicine treatment. CD64 expression was elevated in FMF patients without treatment. This increased CD64 expression was down-regulated by colchicine (1·0 mg/day) treatment (Fig. 3a and b).
Fig. 1.
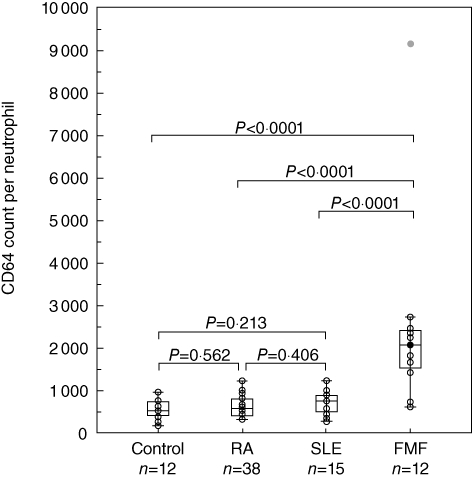
Expression of CD64 molecules per neutrophil in familial Mediterranean fever (FMF) patients. The line inside the box indicates the median value, box shows the 25th and 75th percentiles.
Fig. 2.
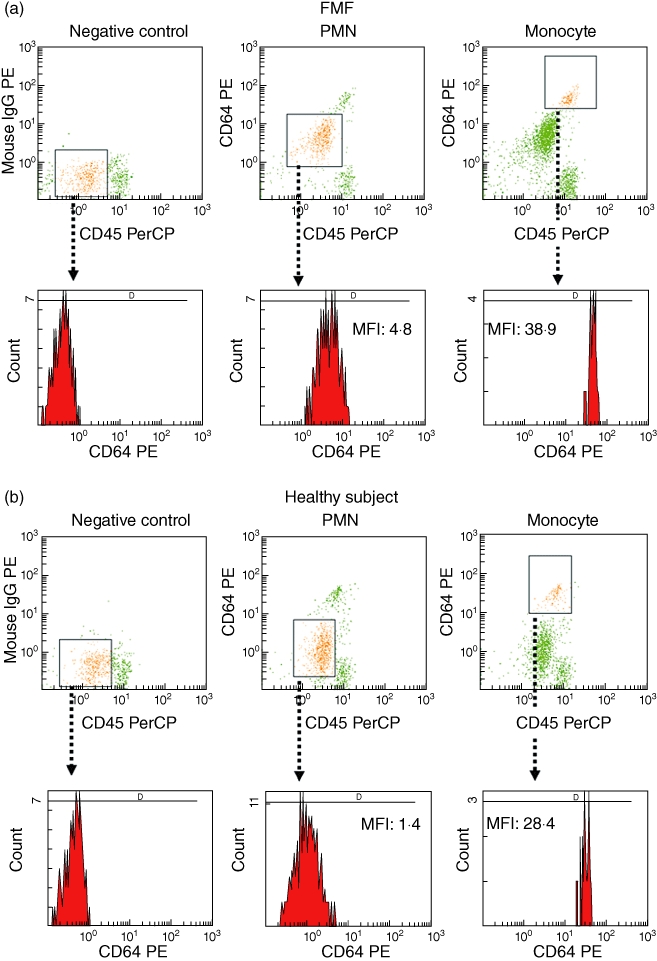
Representative histogram showing the increased CD64 expression on polymorphonuclear neutrophils (PMNs) in familial Mediterranean fever (FMF) patients. Determination of PMNs and monocytes in peripheral blood from a FMF patient (a) and a healthy subject (b) by double-staining using pre-captopril (preCP)-conjugated anti-CD45 PreCP-labelled antibodies and phycoerythrin (PE)-conjugated anti-CD64 antibodies. Negative control samples were stained with peridinin chlorophyll (PerCP)-conjugated anti-CD45 antibodies plus PE-conjugated mouse immunoglobulin (Ig)G (upper panel). Lower panel shows CD64 expression on PMNs and monocytes.
Fig. 3.
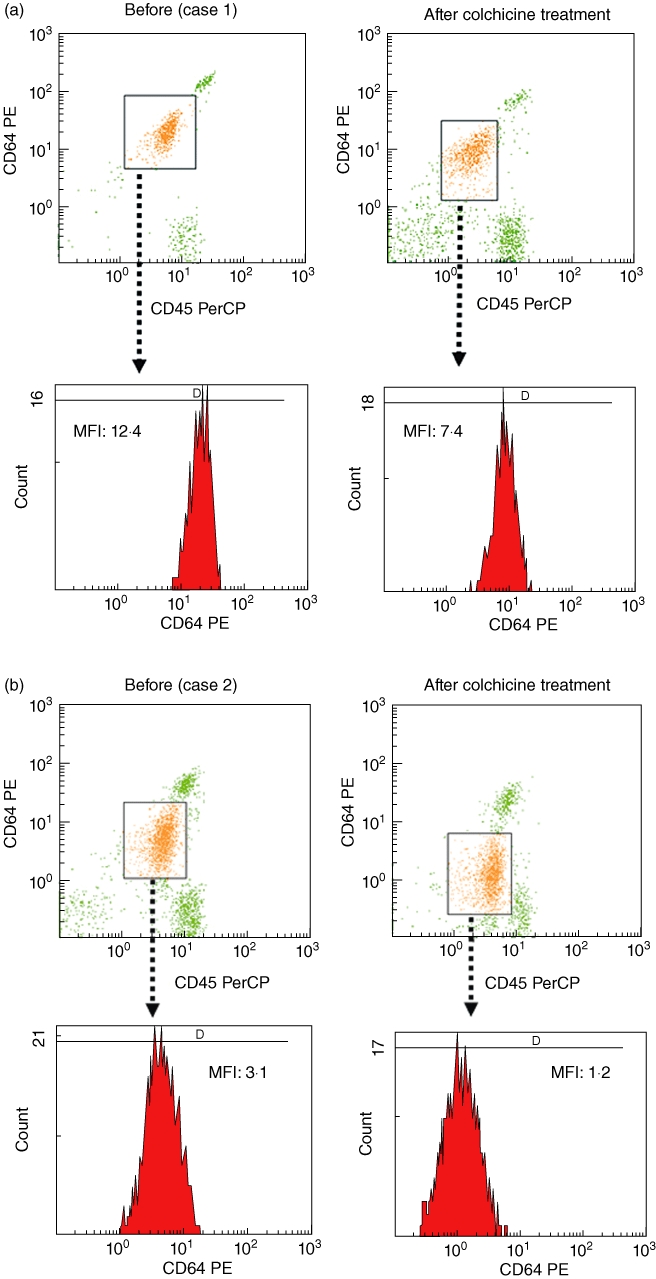
Histogram of CD64 expression on polymorphonuclear neutrophils (PMNs) in blood samples of untreated familial Mediterranean fever (FMF) patients (a, case 1; b, case 2) before and after colchicine treatment.
Effects on NACHT-LRR-PYD-containing protein 3 (NLRP3) activation on CD64 expression in PMNs
Dysregulation of the proinflammatory NLRP-3-dependent pathway has been thought to be involved in the autoinflammation of FMF [12]. Recent studies have documented that MDP, a NOD2 ligand, is an activator of the NLRP-3 inflammasome [13]. We asked whether stimulation with an NLRP-3 activator, MDP, modulated the expression of CD64 in PMNs. We stimulated PMNs from healthy control individuals with MDP. As shown in Fig. 4a, incubation with MDP significantly increased CD64 expression on PNMs rapidly (6 h), whereas MDP-negative control peptide did not affect the surface expression of CD64. This increased CD64 expression on PMNs was maintained after overnight incubation (12 h) and down-regulated after 24 h (Fig. 4b). We confirmed this increased CD64 expression on PMNs activated by MDP in multiple blood samples from healthy subjects (Fig. 4c). We also analysed CD16 expression on PMNs after MDP stimulation. CD16 was expressed highly on PMNs and its expression level was affected marginally by MDP stimulation (Fig. 5).
Fig. 4.
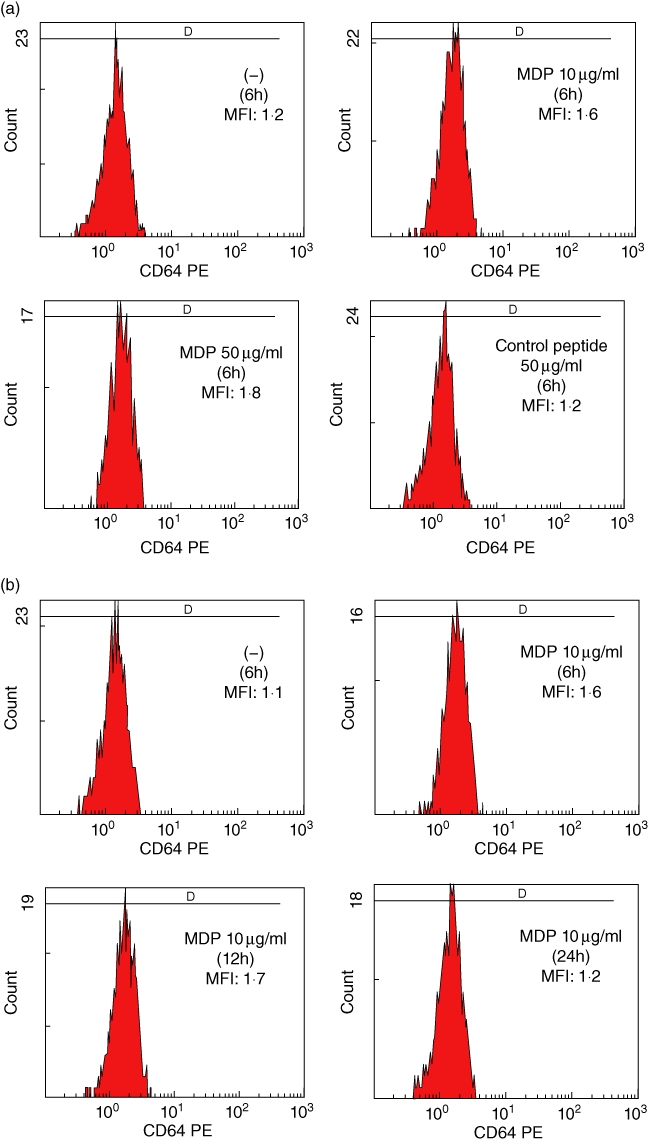
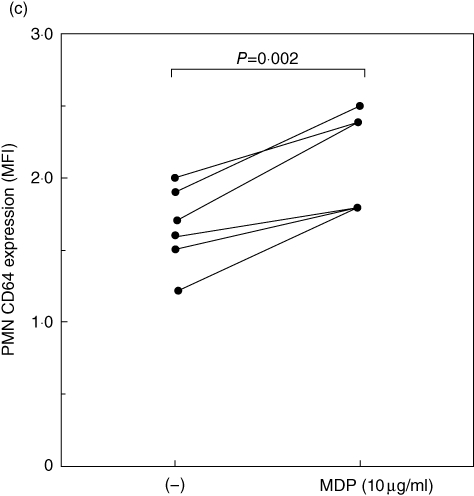
Expression of CD64 on polymorphonuclear neutrophils (PMNs) stimulated with MurNAc-L-Ala-D-isoGln (MDP). (a) PMNs isolated from a healthy subject were stimulated with non-obese diabetic (NOD)2 ligand, MDP or MDP control peptide for 6 h. The expression of CD64 is indicated as the mean fluorescence intensity (MFI). (b) PMNs isolated from a healthy subject were stimulated with MDP (10 µg/ml) for the periods indicated. The expression of CD64 is indicated as the MFI. (c) PMNs isolated from healthy subjects (n = 6) were cultured with or without MDP (10 µg/ml) for 6 h. CD64 expression levels on PMNs were elevated significantly by MDP stimulation.
Fig. 5.
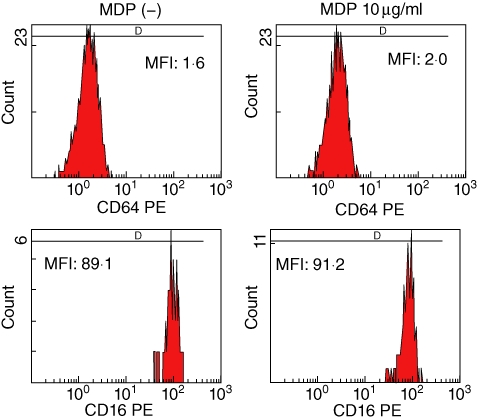
The expression of CD64 and CD16 on polymorphonuclear neutrophils (PMNs) stimulated with MurNAc-L-Ala-D-isoGln (MDP). PMNs isolated from a healthy subject were stimulated with MDP (10 µg/ml) for 6 h. The expression of CD64 and CD16 is indicated as the mean fluorescence intensity (MFI). Three experiments were performed using different PMNs and a representative result is shown.
Discussion
FMF is a genetic autoinflammatory disease characterized by recurrent episodes of fever, accompanied by serositis and arthritis [3]. The disease is prevalent among populations surrounding the Mediterranean Sea. However, an increasing number of cases have been reported in countries not related to this area, including Japan [14]. In countries where FMF is rare, the clinical diagnosis of FMF may not be easy and the role of genetic testing is crucial. However, in 20% of FMF patients, mutations of MEFV genes cannot be identified [15]. During the periodic attack of FMF, laboratory markers, such as white blood cell counts, CRP and ESR, are elevated. However, these markers have low specificity and show limitations in the diagnosis.
In the present study, we demonstrated that CD64 expression on PMNs was elevated significantly in FMF patients, compared to healthy subjects and in patients with common autoimmune diseases, including RA and SLE. CD64 (FCγRI) is expressed constitutively on monocytes and cytokines can induce expression of CD64 on PMNs and induces increased monocyte expression of CD64 [16]. Up-regulation of CD64 expression on PMNs occurs within several hours after stimulation with inflammatory cytokines, such as interferon (IFN)-γ, IL-8 and granulocyte–macrophage colony-stimulating factor (GM-CSF) [8,16]. High concentrations of inflammatory cytokines have been shown to be associated with the periodic attack phase of FMF [17]. Our data are consistent with the possibility that over-production of these inflammatory cytokines during the periodic fever phase seen in FMF patients contributes to the up-regulation of CD64 expression by PMNs. However, up-regulated expression of CD64 in PMNs was also observed in FMF patients who were in remission after colchicine treatment. Therefore, dysregulation of PMN activation, due probably to altered pyrin function, can persist during the subclinical phase and elevated CD64 expression reflects the activation state of PMNs in treated FMF patients in remission. Interestingly, elevated CD64 expression on PMNs in untreated FMF patients was down-regulated by colchicine treatment, concomitant with the clinical improvement. These findings also suggested that PMN expression of CD64 correlated with the autoinflammation seen in FMF. Up-regulated expression of CD64 on PMN has been observed in acute bacterial infection and systemic inflammatory response syndrome. More recently, an elevated expression of CD64 was observed in patients with inflammatory bowel disease and Behçet's disease [18,19], which are non-hereditary autoinflammatory diseases. Taken together, our results demonstrating the increased expression of CD64 in PMNs in FMF patients suggest that PMN expression of CD64 appears to be ideally suited as a diagnostic indicator of autoinflammatory disease.
Neutrophils play an essential role in the initial response to pathogens. Neutrophils recognize pathogens through pathogen-recognition receptors (PPRs) [20]. The nucleotide binding domain [non-obese diabetic (NOD)-like receptors (NLRs)] are a group of PPRs whose role in neutrophils has not been well characterized. It has been reported that NLR activation may cause an inflammatory response that attracts neutrophils to the site of inflammation [21]. FMF is caused by inherited mutations in MEFV, which encodes pyrin. Pyrin regulates caspase-1 activation and consequently IL-1β production by affecting NLRP-3-mediated proinflammatory cascades [22]. MDP was first shown to be a specific activator of a NLR family member, NOD2; however, recent studies have indicated that MDP induces IL-1β release via a NLRP-3-dependent pathway [13]. The expression of NOD2 and NLRP3 protein has been demonstrated in PMNs [23]. Therefore, the effects of NLRP3 activation on CD64 expression were investigated by stimulating PMNs with MDP. Our data indicate that MDP stimulation up-regulates CD64 expression on PMNs, suggesting that CD64 expression could be modulated in response to NLRP-3 activation. It is possible that dysregulated NLRP-3-mediated proinflammatory cascade, probably by altered pyrin function, contributed to the up-regulation of CD64 in PMNs from patients with FMF.
In conclusion, our data indicate that PMN expression of CD64 was elevated abnormally in patients with FMF. These findings suggest that PMN activation, due probably to impaired pyrin function, could contribute to the pathophysiology of FMF and that CD64 is a suitable marker to differentiate between FMF, a hereditary autoinflammatory disease, and autoimmune disease.
Acknowledgments
This work was supported by a Grant-in-Aid for Research on intractable diseases from Ministry of Health, Labour and Welfare of Japan.
Disclosures
None.
References
- 1.Masters SL, Simon A, Aksentijevich I, Kastner DL. Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease. Annu Rev Immunol. 2009;27:621–68. doi: 10.1146/annurev.immunol.25.022106.141627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chitkara P, Stojanov S, Kastner DL. The hereditary autoinflammatory syndromes. Pediatr Infect Dis J. 2007;26:353–4. doi: 10.1097/01.inf.0000258777.86510.da. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Chetrit E, Levy M. Familial Mediterranean fever. Lancet. 1998;351:659–64. doi: 10.1016/S0140-6736(97)09408-7. [DOI] [PubMed] [Google Scholar]
- 4.French FMF Consortium. A candidate gene for familial Mediterranean fever. Nat Genet. 1997;17:25–31. doi: 10.1038/ng0997-25. [DOI] [PubMed] [Google Scholar]
- 5.International FMF Consortium. Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 6.Papin S, Cuenin S, Agostini L, et al. The SPRY domain of Pyrin, mutated in familial Mediterranean fever patients, interacts with inflammasome components and inhibits proIL-1beta processing. Cell Death Differ. 2007;14:1457–66. doi: 10.1038/sj.cdd.4402142. [DOI] [PubMed] [Google Scholar]
- 7.Nuutila J. The novel applications of the quantitative analysis of neutrophil cell surface FcgammaRI (CD64) to the diagnosis of infectious and inflammatory diseases. Curr Opin Infect Dis. 2010;23:268–74. doi: 10.1097/QCO.0b013e32833939b0. [DOI] [PubMed] [Google Scholar]
- 8.Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony-stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood. 1993;82:3265–72. [PubMed] [Google Scholar]
- 9.Matsui T, Ohsumi K, Ozawa N, et al. CD64 on neutrophils is a sensitive and specific marker for detection of infection in patients with rheumatoid arthritis. J Rheumatol. 2006;33:2416–24. [PubMed] [Google Scholar]
- 10.Kallinich T, Wittkowski H, Keitzer R, Roth J, Foell D. Neutrophil-derived S100A12 as novel biomarker of inflammation in familial Mediterranean fever. Ann Rheum Dis. 2010;69:677–82. doi: 10.1136/ard.2009.114363. [DOI] [PubMed] [Google Scholar]
- 11.Livneh A, Langevitz P, Zemer D, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40:1879–85. doi: 10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 12.Chae JJ, Aksentijevich I, Kastner DL. Advances in the understanding of familial Mediterranean fever and possibilities for targeted therapy. Br J Haematol. 2009;146:467–78. doi: 10.1111/j.1365-2141.2009.07733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Q, Mathison J, Fearns C, et al. MDP-induced interleukin-1beta processing requires Nod2 and CIAS1/NALP3. J Leukoc Biol. 2007;82:177–83. doi: 10.1189/jlb.1006627. [DOI] [PubMed] [Google Scholar]
- 14.Tsuchiya-Suzuki A, Yazaki M, Nakamura A, et al. Clinical and genetic features of familial Mediterranean fever in Japan. J Rheumatol. 2009;36:1671–6. doi: 10.3899/jrheum.081278. [DOI] [PubMed] [Google Scholar]
- 15.Cazeneuve C, Hovannesyan Z, Geneviève D, et al. Familial Mediterranean fever among patients from Karabakh and the diagnostic value of MEFV gene analysis in all classically affected populations. Arthritis Rheum. 2003;48:2324–31. doi: 10.1002/art.11102. [DOI] [PubMed] [Google Scholar]
- 16.Buckle AM, Hogg N. The effect of IFN-gamma and colony-stimulating factors on the expression of neutrophil cell membrane receptors. J Immunol. 1989;143:2295–301. [PubMed] [Google Scholar]
- 17.Aypar E, Ozen S, Okur H, Kutluk T, Besbas N, Bakkaloglu A. Th1 polarization in familial Mediterranean fever. J Rheumatol. 2003;30:2011–3. [PubMed] [Google Scholar]
- 18.Tillinger W, Jilch R, Jilma B, et al. Expression of the high-affinity IgG receptor FcRI (CD64) in patients with inflammatory bowel disease: a new biomarker for gastroenterologic diagnostics. Am J Gastroenterol. 2009;104:102–9. doi: 10.1038/ajg.2008.6. [DOI] [PubMed] [Google Scholar]
- 19.Ureten K, Ertenli I, Oztürk MA, et al. Neutrophil CD64 expression in Behçet's disease. J Rheumatol. 2005;32:849–52. [PubMed] [Google Scholar]
- 20.Kantari C, Pederzoli-Ribeil M, Witko-Sarsat V. The role of neutrophils and monocytes in innate immunity. Contrib Microbiol. 2008;15:118–46. doi: 10.1159/000136335. [DOI] [PubMed] [Google Scholar]
- 21.Chen G, Shaw MH, Kim YG, Nuñez G. NOD-like receptors: role in innate immunity and inflammatory disease. Annu Rev Pathol. 2009;4:365–98. doi: 10.1146/annurev.pathol.4.110807.092239. [DOI] [PubMed] [Google Scholar]
- 22.Chae JJ, Wood G, Masters SL, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci USA. 2006;103:9982–7. doi: 10.1073/pnas.0602081103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekman AK, Cardell LO. The expression and function of Nod-like receptors in neutrophils. Immunology. 2010;130:55–63. doi: 10.1111/j.1365-2567.2009.03212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


