Abstract
We were interested in the question of whether the congenital lack of B cells actually had any influence on the development of the T cell compartment in patients with agammaglobulinaemia. Sixteen patients with X-linked agammaglobulinaemia (XLA) due to mutations in Btk, nine patients affected by common variable immune deficiency (CVID) with <2% of peripheral B cells and 20 healthy volunteers were enrolled. The T cell phenotype was determined with FACSCalibur and CellQuest Pro software. Mann–Whitney two-tailed analysis was used for statistical analysis. The CD4 T cell memory compartment was reduced in patients with XLA of all ages. This T cell subset encompasses both CD4+CD45RO+ and CD4+CD45RO+CXCR5+ cells and both subsets were decreased significantly when compared to healthy controls: P = 0·001 and P < 0·0001, respectively. This observation was confirmed in patients with CVID who had <2% B cells, suggesting that not the lack of Bruton's tyrosine kinase but the lack of B cells is most probably the cause of the impaired CD4 T cell maturation. We postulate that this defect is a correlate of the observed paucity of germinal centres in XLA. Our results support the importance of the interplay between B and T cells in the germinal centre for the activation of CD4 T cells in humans.
Keywords: CD4+ T memory cells, CD4+CD45RO+CXCR5+ cells, TFH cells, XLA
Introduction
It has been widely accepted that CD4 T cell help is required for B cells to produce high-affinity antibodies to protein antigens and mature into memory cells (T cell-dependent immune response) [1,2]. Conversely, many studies in mice [3–6] have demonstrated that B cells play a critical role in presenting antigen to T cells during the priming phase in lymph nodes (LN). This interaction in the germinal centre is mediated by co-stimulatory molecules such as B7/CD28, CD40/CD40 ligand, OX40 ligand/OX40 and inducible co-stimulator ligand (ICOS)/ICOS ligand [7–12].
Many studies on B cell-depleted mice (µMT mice) demonstrated that both dendritic cells (DC) and B cells are involved in CD4+ T cell activation [13–15]. In µMT mice, where the B cell development is blocked after the pre-B cell stage, the absence of a mature B cell compartment also affected other parts of the immune system, such as a remarkable reduction in thymocyte numbers and diversity [16,17], defects within splenic DC and T cell compartments [18,19] and the absence of Peyer's patches [20].
Recent studies, depleting B cells from normal adult mice, demonstrated that B cells are essential for optimal CD4+ T cell activation, sharing this function with DC. The role of DC is crucial when antigen levels are low, while B cells are not required for CD8 T cell activation [21,22].
X-linked agammaglobulinaemia (XLA) is one of the most frequent monogenetic immunodeficiency diseases in man and is characterized by an almost complete arrest of B cell differentiation in the bone marrow at the pre-B cell stage. The gene defective in XLA encodes the cytoplasmic signalling molecule Bruton's tyrosin kinase (BTK) in B cells. XLA is characterized by a marked reduction of serum immunoglobulins of all subclasses and inability to generate specific antibody responses. Patients have very low B cell numbers in peripheral blood which exhibit an immature IgM phenotype, and an absence of B cell-dependent lymphoid tissue [23,24]. As XLA patients lack almost all B cells in blood, this condition represents a valuable human model of T cell evolution in the absence of B cells.
We were interested in the question of whether the congenital lack of B cells in the periphery actually had any influence on the development of the T cell compartment in patients with XLA as observed in mice. Currently there are few and contrasting data about the role of B cells in maintaining long-term T cell memory in humans [25–28].
Materials and methods
Patients and healthy controls
Human peripheral blood samples were taken from 13 adults with XLA (age range 19–58 years, median age 40·3), three children with XLA (aged 8, 11 and 11 years, respectively) and nine subjects with common variable immune deficiency (CVID) and B cells <2% of total lymphocyte count (five females and four males, age range 17–61 years, median age 44·1) (Table 1). Within those nine CVID patients, one had a mutation in transmembrane activator and CAML interactor (TACI) (C193X), two had polymorphisms in the B cell activating receptor (BAFF) and one had a polymorphism in B lymphocyte-induced maturation protein (BLIMP)-1. Btk mutations were excluded in all males with CVID. All patients were free of infection (C-reactive protein level was taken as surrogate marker) when T cell analysis was performed.
Table 1.
Patient demographic, age, gender and T cell phenotyping
| Patient | Age | Gender M/F | TLC Cells/mcl | CD3 % TLC | CD4 % TLC | CD8 % TLC | CD4+CD45RO+ (%CD4) | CD4+CD45RO+CXCR5+ (%CD4+CD45RO+) | CD4+CD45RO+CXCR5- (%CD4+CD45RO+) | CD4+CD45RA+ (%CD4) | CD8+CD27-CD28- (%CD8) | CD8+CD27+CD28- (%CD8) | CD3+CD4-CD8- (%TCRαβ) | CD4+CD45RA+CD31+ (%CD4 naive) | CD127lowCD25+ (%CD4) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XLA1 | 44 | M | 1813 | 82 | 47 | 31 | 38 | 9 | 91 | 58 | 25 | 10 | <1 | 48 | |
| XLA2 | 32 | M | 1967 | 93 | 51 | 37 | 27 | 8 | 92 | 72 | 6 | 9 | 3 | 85 | 5 |
| XLA3 | 19 | M | 737 | 63 | 50 | 13 | 73 | 6 | 94 | 32 | 19 | 10 | <1 | 52 | 5 |
| XLA4 | 48 | M | 1467 | 86 | 55 | 27 | 48 | 10 | 90 | 55 | 5 | 6 | 3 | 65 | 3 |
| XLA5 | 28 | M | 2049 | 91 | 65 | 23 | 26 | 6 | 94 | 74 | 15 | 6 | 3 | 74 | 4 |
| XLA6 | 30 | M | 2006 | 91 | 51 | 36 | 45 | 8 | 92 | 49 | 16 | 13 | 5 | 41 | 6 |
| XLA7 | 40 | M | 1923 | 84 | 62 | 21 | 38 | 4 | 96 | 64 | 54 | 8 | 1 | 49 | 3 |
| XLA8 | 58 | M | 2491 | 86 | 68 | 22 | 50 | 8 | 92 | 45 | 27 | 3 | <1 | 70 | 4 |
| XLA9 | 39 | M | 3797 | 88 | 77 | 11 | 17 | 6 | 94 | 81 | 6 | 3 | <1 | 54 | 3 |
| XLA10 | 40 | M | 1170 | 81 | 59 | 17 | 42 | 4 | 96 | 61 | 27 | 6 | 1 | 49 | 5 |
| XLA11 | 56 | M | 2319 | 79 | 49 | 28 | 36 | 6 | 94 | 62 | 15 | 8 | 2 | 63 | 5 |
| XLA12 | 43 | M | 1408 | 72 | 43 | 28 | 56 | 3 | 97 | 41 | 64 | 4 | 1 | 69 | 6 |
| XLA13 | 48 | M | 1625 | 81 | 58 | 18 | 64 | 3 | 97 | 33 | 16 | 4 | 1 | 38 | 9 |
| XLA14 | 8 | M | 1270 | 76 | 45 | 27 | 11 | 13 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| XLA15 | 11 | M | 2550 | 92 | 57 | 31 | 12 | 11 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| XLA16 | 11 | M | 2430 | 93 | 65 | 32 | 10 | 7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| CVID1 | 32 | M | 482 | 82 | 34 | 45 | 99 | 21 | 79 | 1 | 49 | 13 | 2 | 54 | 3 |
| CVID2 | 58 | F | 5399 | 97 | 12 | 86 | 92 | 14 | 86 | 2 | 27 | 12 | 1 | 54 | 8 |
| CVID3 | 61 | F | 3995 | 96 | 21 | 71 | 89 | 17 | 83 | 7 | 67 | 11 | 2 | 72 | 4 |
| CVID4 | 33 | F | 747 | 90 | 35 | 52 | 76 | 22 | 78 | 23 | 52 | 10 | 1 | 77 | 8 |
| CVID5 | 45 | M | 1139 | 79 | 53 | 27 | 82 | 17 | 83 | 12 | 5 | 13 | 1 | 80 | 3 |
| CVID6 | 47 | M | 1420 | 94 | 26 | 55 | 71 | 15 | 85 | 24 | 47 | 27 | <1 | n.d. | 4 |
| CVID7 | 17 | F | 2130 | 91 | 54 | 29 | 23 | 23 | 77 | 71 | 0 | 8 | 1 | n.d. | 1 |
| CVID8 | 54 | F | 3620 | 86 | 56 | 20 | 25 | 21 | 79 | 68 | 61 | 19 | 1 | n.d. | 2 |
| CVID9 | 50 | M | 530 | 74 | 39 | 34 | 93 | 18 | 82 | 4 | 48 | 28 | <1 | n.d. | 0 |
TLC, total lymphocyte count; n.d., not done; XLA, X-linked agammaglobulinaemia; F, female; M, male.
Twenty healthy volunteers (nine females and 11 males) were used as control subjects (age range 25–59 years, median age 36). Healthy controls were tested repeatedly and results were stable over time. However, repeat data on the same subject were not included in the statistical analysis.
Informed consent was obtained from all contributing individuals according to the declaration of Helsinki.
Antibodies and flow cytometry
Peripheral blood samples were prepared using a ‘whole blood lyse no wash’ method with OptiLyse B lysing solution (Beckman Coulter, Brea, CA, USA). Whole blood was cell surface-stained with mixtures of the following antibodies at optimal concentrations: fluorescein isothiocyanate (FITC)-conjugated anti-CD4 and anti-CD27, phycoerythrin (PE)-conjugated anti-CD28, anti-CD31, anti-CD25 and anti-T cell receptor (TCR)αβ, phycoerythrin cyanin 5 (PE-Cy5)-conjugated anti-CD3, peridinin chlorophyll protein (PerCP)-conjugated anti-CD4, allophycocyanin (APC)-conjugated anti-CD8, anti-CD45RO and anti-CD45RA (all obtained from Becton Dickinson, Oxford, UK), FITC-conjugated anti-CD127 (eBioscience, San Diego, CA, USA) and PE-conjugated anti-CXCR5 (R&D Systems, Minneapolis, MN, USA). Cells were processed using four-colour acquisition on a FACSCalibur (Becton Dickinson) and data analysed using CellQuest Pro software (Becton Dickinson).
Analysis was performed by forward versus side-scatter gating on lymphocytes in combination with gating on CD3+ cells and was used to identify the following populations in both patients and healthy controls: CD3+ T cells, CD3+CD4+ T helper cells, CD3+CD8+ cytotoxic T cells, CD4+CD45RO+ memory cells, CD4+CD45RO+CXCR5+ circulating CXCR5+ memory T cells, CD4+CD45RA+ naive cells, CD4+CD45RA+CD31+ recent thymic emigrants, CD8+CD27+CD28- effector and CD8+CD27-CD28- late effector cells, CD3+TCRαβ+CD4/8- double-negative T cells and CD4+CD45RO+CD127lowCD25+ regulatory T cells.
Statistical analysis
Comparison between healthy volunteers and XLA or CVID subjects, as well as between XLA and CVID patients, were analysed using Mann–Whitney two-tailed analysis with GraphPad Prism software. A P-value less than or equal to 0·05 was considered to be statistically significant.
Results
Most interestingly, within the CD4 T cell compartment, only the CD4 T memory cells were reduced in patients with XLA (Fig. 1a–d). This T cell subset encompasses both CD4+CD45RO+ and CD4+CD45RO+CXCR5+ cells and both subsets obtained a significant P-value when compared to healthy controls: P = 0·01 (Fig. 1d) and P < 0·0001 (Fig. 2c), respectively.
Fig. 1.
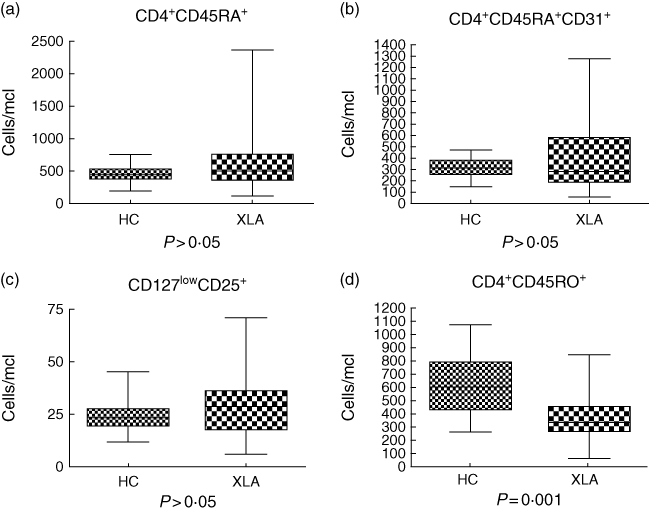
CD4 T cell subsets in X-linked agammaglobulinaemia (XLA). (a) Naive CD4 T cell numbers in XLA patients (CD4+CD45RA+) and (b) the CD4 recent thymic emigrant numbers were comparable to healthy controls (P > 0·05). We also analysed (c) the number of regulatory T cells, defined as CD127lowCD25+ cells, and obtained comparable results between cohorts (P > 0·05). Conversely, the CD4 T memory compartment (CD4+CD45RO+) was reduced significantly (P = 0·001) (d).
Fig. 2.
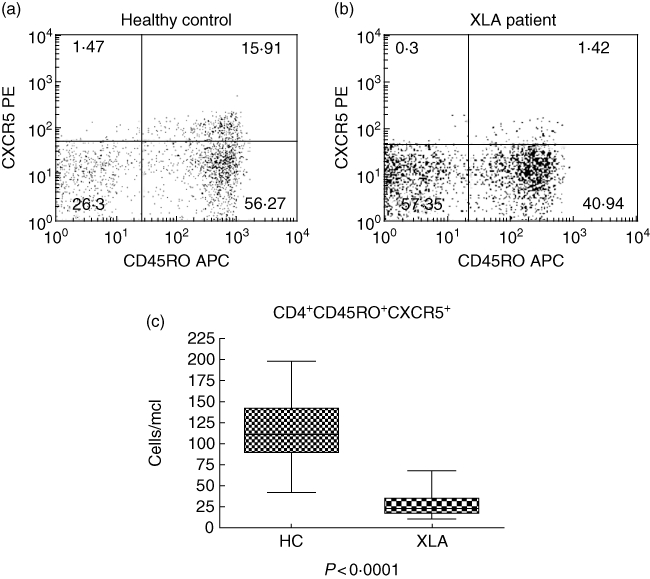
Lack of circulating CXCR5+ memory T cells in X-linked agammaglobulinaemia (XLA). As shown in this representative FACS plot from a healthy donor (a), circulating CXCR5+ memory T cells (CD4+CD45RO+CXCR5+) represent usually 5–15% of total CD4+CD45RO+ memory CD4 T cells. In XLA subjects a representative example is shown in (b), where we observed a dramatically lower number of these cells. This difference was statistically significant (P < 0·0001) (c).
Despite a degree of variability within the CD3+ T cells count (CD3 range from 464 to 3351 cells/mcl, median value 1618 cells/mcl in XLA), other subsets of the T cell compartment were, however, generally comparable to controls; in fact, we found no additional significant difference between XLA patients and controls while analysing CD4+ and CD8+ T cells.
Dividing the former population in different subsets, we found that naive CD4 T cells (CD4+CD45RA+) in XLA patients were comparable to controls (P > 0·05) (Fig. 1a), as well as the CD4 recent thymic emigrant numbers (P > 0·05) (Fig. 1b).
We also analysed the number of regulatory T cells, defined as CD127lowCD25+ cells, and this was comparable to healthy controls (P > 0·05) (Fig. 1c).
In the peripheral blood of XLA patients, CD8 T cells were unaffected by the lack of B cells, as we found comparable results of total CD8 T cells (P > 0·05) as well as normal subsets of activated CD8 T cells: CD8 effector cells (CD8+CD27+CD28-) and late CD8 effector cells (CD8+CD27-CD28-) (P > 0·05, respectively). Double-negative T cells (CD3+ CD4-/CD8-) and the subset of CD4+CD45RO+CXCR5- cells in the peripheral blood also showed no significant difference (P > 0·05, respectively) compared to healthy controls.
Considering that XLA is an inborn B cell defect, we asked whether the CD4 T memory compartment was affected in adults with XLA as a consequence of a progressive alteration. Therefore we analysed the CD4+CD45RO+ and CD4+CD45RO+CXCR5+ T cells in three children with XLA. We found the same profound defect of these subsets compared to age-matched donors (Table 1).
Moreover, we asked whether the defect observed in the CD4 T memory subset was due to the lack of B cells only, or whether it was an effect of the mutation in Btk. To this end, we analysed the T cell subsets in nine patients with CVID who had <2% B cells (B cell count ranged from 0 to 41 cells/mcl). We found reduced numbers of CD4 T memory cells (P = 0·01) and CD4+CD45RO+CXCR5+ (P = 0·002) in all nine CVID subjects compared to controls (Fig. 3a,b). As expected, no significant statistical difference was observed between the T subsets of patients with XLA and those with CVID, despite a considerable degree of variability within the CD3+ T cell counts (CD3 range from 464 to 3351 cells/mcl, with a median value of 1618 cells/mcl in XLA, and CD3 range from 397 to 5242 cells/mcl with a median value of 1335 in CVID). Therefore, we concluded that CD4+CD45RO+ and CD4+CD45RO+CXCR5+ cell numbers were comparable in XLA and CVID (P > 0·05) (Fig. 3c,d), even though the percentages of these subsets were much reduced in XLA (P = 0·02 and P = 0·001, respectively).
Fig. 3.
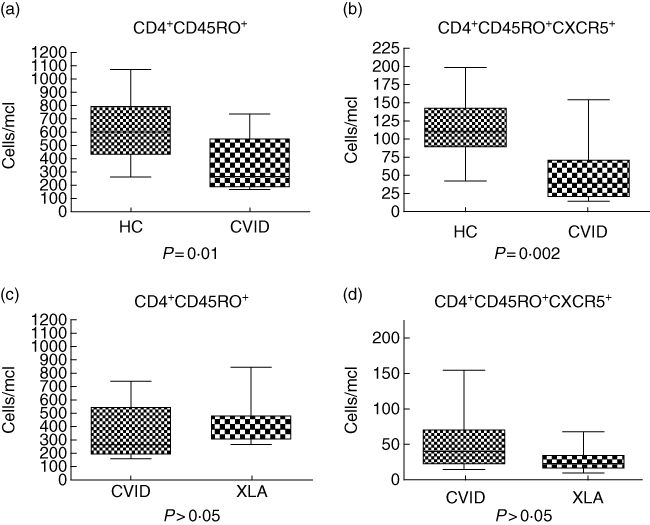
Common variable immune deficiency (CVID) without B cells: defective CD4 T memory compartment. Reduced (a) CD4+CD45RO+ and (b) CD4+CD45RO+CXCR5+ T cell numbers were observed in CVID patients without B cells when compared to healthy controls (HC) (P = 0·01 and P = 0·002, respectively). Conversely, no significant difference was found between the T cell subsets of subjects with X-linked agammaglobulinaemia (XLA) and patients with CVID without B cells. In particular (c) CD4+CD45RO+ and (d) CD4+CD45RO+CXCR5+ cell numbers were comparable (P > 0·05).
Discussion
While DC prime naive CD4+ T cells, B cells are thought to control CD4+ T cell expansion after their initial activation by DC [29]. However, many studies on mice with a congenital lack of B cells at birth (µMT mice), as well as studies depleting B cells from normal adult mice, demonstrated that B cells are essential for optimal CD4+ T cell activation. They share this function with DC, and their role is crucial when antigen levels are low, while B cells are not required for CD8 T cell activation [13–16].
A human model to study T cell development in the absence of B cells is represented by recent studies on patients after treatment with the anti-CD20 antibody rituximab, showing some effects on the T cell compartment. In particular, a change in the T helper type 1 (Th1)/Th2 balance of CD4+ T cells skewing towards Th1 [30,31], but without significant changes in CD4+CD45RA and CD4+CD45RO subsets [31], and an increase of CD4+CD25+ and CD25bright T cells [32] were found in several autoimmune diseases treated with the anti-CD20 monoclonal antibody rituximab. However, this transient B cell depletion might not effect T cell development profoundly.
Bruton's agammaglobulinaemia (X-linked agammaglobulinaemia, XLA) is another model to study T cell development in the absence of B cells, as its genetic cause, the mutation in Bruton's tyrosine kinase (Btk), blocks the maturation of B cells at the pre-B stage leading to peripheral B cell numbers of <1% and a consecutive agammaglobulinaemia.
In addition to the B cell lineage, Btk is also expressed in the myeloid lineage [33,34]. In recent years, several lines of evidence in mice have indicated that Btk plays a significant role at multiple points in the development and function of both macrophages and neutrophils, and that its absence results in compromised inflammatory responses in vivo[35–37]. Moreover, mast cell activation through immunoglobulin E receptors appears to be dependent on Btk function [38–40]. Some platelet responses also appear to involve Btk[41,42].
As XLA patients represent an interesting congenital human model of B cell depletion, we asked whether the absence of B cells interferes with the CD4 T cell differentiation in humans as described in mice [13–16]. We found that the T cell compartment was intact, except for CD4+CD45RO- T cells which were reduced significantly but not absent in XLA patients.
There are contrasting data on T cell memory in XLA patients, and the conditions required to maintain this T cell memory pool are still subject of controversy. On one hand, Plebani et al. showed normal T cell proliferation in vitro and cytokine production in response to either mitogens or tetanus toxoid (TT) in XLA patients up to 6 months after a TT booster immunization [26]; moreover, a good in vitro T cell response to HBV was demonstrated in nine XLA patients up to 24 months after vaccination [27]. On the other hand, Crockard et al. reported an impaired delayed cutaneous hypersensitivity reaction in XLA patients [25], and recently an impaired maintenance of T cell memory to Neisseria meningitidis was shown in patients with XLA and CD40L deficiency [28]. These results in particular confirm that the mucosa-associated T memory compartment is affected, showing the importance of the pathogen entrance in the maintenance of long-term memory. This might explain why we found a reduction but not a complete lack in the T cell memory pool.
Moreover, we found a significant depletion of a CD4+CD45RO+CXCR5+ T cells. This population has been described as circulating CXCR5+ memory T cells, which usually represent 5–15% of CD4+CD45RO+ T cells. The origin of these cells is still debated. It has been suggested that they derive from germinal centre T follicular helper cells (TFH) and that they correlate with the presence of germinal centres [43–45]. TFH are activated CD4 T cells with up-regulated CXCR5 which attracts them into the germinal centre, where they provide cognate help for B cells [46–48].
At this point we asked whether the lack of cognate B–T cell interaction interferes with the maturation of CD4 T cell compartment or whether the Btk mutation was the cause of the described results per se. We analysed the T cell compartment of nine subjects with CVID and fewer than 2% peripheral B cells, and we found no significant difference in the number of CD4+CD45RO+ or CD4+CD45RO+CXCR5+ T cells when compared to the XLA cohort. Therefore, we concluded that a lack of Btk itself is most probably not the cause of the impaired CD4 T cell subset maturation.
Moreover, we assume that the severe reduction of circulating CXCR5+ memory T cells described in XLA correlates with an important decrease of TFH cells and the lack of germinal centre formation in our patients, as described in mice [43]. In fact, the lack of germinal centre in lymph nodes has already been documented in XLA subjects [49,50]. Interestingly, TFH cells were also reduced strongly in ICOS-deficient and CD40L-deficient patients [44], in whom germinal centre formation is also impaired severely. This suggests that circulating CD4+CD45RO+CXCR5+ T cells depend on the presence of B cells and co-stimulatory signals via ICOS and CD40. Even though this might occur in germinal centre reaction, the origin of circulating CXCR5+ T cells remains unknown.
In conclusion, our results support the importance of the interaction between B and T cells for the differentiation of memory CD4 T cells in humans, as demonstrated by reduced peripheral CD4+CD45RO+ T cells and circulating CXCR5+ memory T cells in XLA subjects, and CVID patients without B cells.
Acknowledgments
We would like to thank Viviane Knerr for her assistance, Fariba Tahami and Rita Carsetti for their contribution to the flow cytometry and their advice, and Walter Morgani for his support. We also appreciate the support of the patients and controls for agreeing to take part in the study. The study was funded by the EURO-PADnet HEALTH-F2-2008-1549.
Disclosure
The authors declare no competing financial interests.
References
- 1.Lanzavecchia A. Receptor-mediated antigen uptake and its effect on antigen presentation to class II-restricted T lymphocytes. Annu Rev Immunol. 1990;8:773–93. doi: 10.1146/annurev.iy.08.040190.004013. [DOI] [PubMed] [Google Scholar]
- 2.Parker DC. T cell-dependent B cell activation. Annu Rev Immunol. 1993;11:331–60. doi: 10.1146/annurev.iy.11.040193.001555. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Ron J, Katz ME. The B cell is the initiating antigen-presenting cell in peripheral lymph nodes. J Immunol. 1987;138:1051–5. [PubMed] [Google Scholar]
- 4.Ron Y, Sprent J. T cell priming in vivo: a major role for B cells in presenting antigen to T cells in lymph nodes. J Immunol. 1987;138:2848–56. [PubMed] [Google Scholar]
- 5.Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 6.Garside P, Ingulli E, Merica RR, Johnson JG, Noelle RJ, Jenkins MK. Visualization of specific B and T lymphocyte interactions in the lymph node. Science. 1998;281:96–9. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Pinto D, Moreno J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154–CD40-dependent manner. Eur J Immunol. 2005;35:1097–105. doi: 10.1002/eji.200425732. [DOI] [PubMed] [Google Scholar]
- 8.Linton PJ, Bautista B, Biederman E, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–83. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sfikakis PP, Boletis JN, Lionaki S, et al. Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52:501–13. doi: 10.1002/art.20858. [DOI] [PubMed] [Google Scholar]
- 10.Tafuri A, Shahinian A, Bladt F, et al. ICOS is essential for effective T-helper-cell responses. Nature. 2001;409:105–9. doi: 10.1038/35051113. [DOI] [PubMed] [Google Scholar]
- 11.McAdam AJ, Greenwald RJ, Levin MA, et al. ICOS is critical for CD40-mediated antibody class switching. Nature. 2001;409:102–5. doi: 10.1038/35051107. [DOI] [PubMed] [Google Scholar]
- 12.Dong C, Juedes AE, Temann UA, et al. ICOS co-stimulatory receptor is essential for T-cell activation and function. Nature. 2001;409:97–101. doi: 10.1038/35051100. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Wu Y, Ramarathinam L, et al. Gene-targeted B-deficient mice reveal a critical role for B cells in the CD4 T cell response. Int Immunol. 1995;7:1353–62. doi: 10.1093/intimm/7.8.1353. [DOI] [PubMed] [Google Scholar]
- 14.Linton PJ, Harbertson J, Bradley LM. A critical role for B cells in the development of memory CD4 cells. J Immunol. 2000;165:5558–65. doi: 10.4049/jimmunol.165.10.5558. [DOI] [PubMed] [Google Scholar]
- 15.Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- 16.Joao C, Ogle BM, Gay-Rabinstein C, Platt JL, Cascalho M. B cell-dependent TCR diversification. J Immunol. 2004;172:4709–16. doi: 10.4049/jimmunol.172.8.4709. [DOI] [PubMed] [Google Scholar]
- 17.AbuAttieh M, Rebrovich M, Wettstein PJ, et al. Fitness of cell-mediated immunity independent of repertoire diversity. J Immunol. 2007;178:2950–60. doi: 10.4049/jimmunol.178.5.2950. [DOI] [PubMed] [Google Scholar]
- 18.Moulin V, Andris F, Thielemans K, Maliszewski C, Urbain J, Moser M. B lymphocytes regulate dendritic cell (DC) function in vivo: increased interleukin 12 production by DCs from B cell-deficient mice results in T helper cell type 1 deviation. J Exp Med. 2000;192:475–82. doi: 10.1084/jem.192.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ngo VN, Cornall RJ, Cyster JG. Splenic T zone development is B cell dependent. J Exp Med. 2001;194:1649–60. doi: 10.1084/jem.194.11.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golovkina TV, Shlomchik M, Hannum L, Chervonsky A. Organogenic role of B lymphocytes in mucosal immunity. Science. 1999;286:1965–8. doi: 10.1126/science.286.5446.1965. [DOI] [PubMed] [Google Scholar]
- 21.Bouaziz JD, Yanaba K, Venturi GM, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20882–7. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiu Y, Wong CP, Bouaziz JD, et al. B lymphocytes depletion by CD20 monoclonal antibody prevents diabetes in NOD mice despite isotype-specific differences in FcγR effector functions. J Immunol. 2008;180:2863–75. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- 23.Conley ME. B cells in patients with X-linked agammaglobulinemia. J Immunol. 1985;134:3070–4. [PubMed] [Google Scholar]
- 24.Campana D, Farrant J, Inamdar N, Webster AD, Janossy G. Phenotypic features and proliferative activity of B cell progenitors in X-linked agammaglobulinemia. J Immunol. 1990;145:1675–80. [PubMed] [Google Scholar]
- 25.Crockard AD, Boyd NA, McNeill TA, McCluskey DR. CD4 lymphocyte subset abnormalities associated with impaired delayed cutaneous hypersensitivity reactions in patients with X-linked agammaglobulinaemia. Clin Exp Immunol. 1992;88:29–34. doi: 10.1111/j.1365-2249.1992.tb03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plebani A, Fischer MB, Meini A, Duse M, Thon V, Eibl MM. T cell activity and cytokine production in X-linked agammaglobulinemia: implications for vaccination strategies. Int Arch Allergy Immunol. 1997;114:90–3. doi: 10.1159/000237649. [DOI] [PubMed] [Google Scholar]
- 27.Paroli M, Accapezzato D, Francavilla V, et al. Long-lasting memory-resting and memory-effector CD4+ T cells in human X-linked agammaglobulinemia. Blood. 2002;99:2131–7. doi: 10.1182/blood.v99.6.2131. [DOI] [PubMed] [Google Scholar]
- 28.Morales-Aza B, Glennie SJ, Garcez TP, et al. Impaired maintenance of naturally acquired T-cell memory to the meningococcus in patients with B-cell immunodeficiency. Blood. 2009;113:4206–12. doi: 10.1182/blood-2008-08-171587. [DOI] [PubMed] [Google Scholar]
- 29.Ronchese F, Hausmann B. B lymphocytes in vivo fail to prime naive T cells but can stimulate antigen-experienced T lymphocytes. J Exp Med. 1993;177:679–90. doi: 10.1084/jem.177.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamimoto Y, Horiuchi T, Tsukamoto H, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans' syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology. 2008;47:821–7. doi: 10.1093/rheumatology/ken071. [DOI] [PubMed] [Google Scholar]
- 31.Stasi R, Del Poeta G, Stipa E, et al. Response to B-cell depleting therapy with rituximab reverts the abnormalities of T-cell subsets in patients with idiopathic thrombocytopenic purpura. Blood. 2007;110:2924–30. doi: 10.1182/blood-2007-02-068999. [DOI] [PubMed] [Google Scholar]
- 32.Vallerskog T, Gunnarsson I, Widhe M, et al. Treatment with rituximab affects both the cellular and the humoral arm of the immune system in patients with SLE. Clin Immunol. 2007;122:62–74. doi: 10.1016/j.clim.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 33.de Weers M, Verschuren MC, Kraakman ME, et al. The Bruton's tyrosine kinase gene is expressed throughout B cell differentiation, from early precursor B cell stages preceding immunoglobulin gene rearrangement up to mature B cell stages. Eur J Immunol. 1993;23:3109–14. doi: 10.1002/eji.1830231210. [DOI] [PubMed] [Google Scholar]
- 34.Muller S, Sideras P, Smith CI, Xanthopoulos KG. Cell specific expression of human Bruton's agammaglobulinemia tyrosine kinase gene (Btk) is regulated by Sp1- and Spi-1/PU.1-family members. Oncogene. 1996;13:1955–64. [PubMed] [Google Scholar]
- 35.Lachance G, Levasseur S, Naccache PH. Chemotactic factor-induced recruitment and activation of Tec family kinases in human neutrophils: implication of phosphatidynositol 3-kinases. J Biol Chem. 2002;277:21537–41. doi: 10.1074/jbc.M201903200. [DOI] [PubMed] [Google Scholar]
- 36.Gilbert C, Levasseur S, Desaulniers P, et al. Chemotactic factor-induced recruitment and activation of Tec family kinases in human neutrophils, II: effects of LFM-A13, a specific Btk inhibitor. J Immunol. 2003;170:5235–43. doi: 10.4049/jimmunol.170.10.5235. [DOI] [PubMed] [Google Scholar]
- 37.Mangla A, Khare A, Vineeth V, et al. Pleiotropic consequences of Bruton tyrosine kinase deficiency in myeloid lineages lead to poor inflammatory responses. Blood. 2004;104:1191–7. doi: 10.1182/blood-2004-01-0207. [DOI] [PubMed] [Google Scholar]
- 38.Hata D, Kawakami Y, Inagaki N, et al. Involvement of Bruton's tyrosine kinase in FcepsilonRI dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–47. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kawakami Y, Kitaura J, Satterthwaite AB, et al. Redundant and opposing functions of two tyrosine kinases, Btk and Lyn, in mast cell activation. J Immunol. 2000;165:1210–19. doi: 10.4049/jimmunol.165.3.1210. [DOI] [PubMed] [Google Scholar]
- 40.Setoguchi R, Kinashi T, Sagara H, Hirosawa K, Takatsu K. Defective degranulation and calcium mobilization of bone-marrow derived mast cells from Xid and Btk-deficient mice. Immunol Lett. 1998;64:109–18. doi: 10.1016/s0165-2478(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 41.Quek LS, Bolen J, Watson SP. A role for Bruton's tyrosine kinase (Btk) in platelet activation by collagen. Curr Biol. 1998;8:1137–40. doi: 10.1016/s0960-9822(98)70471-3. [DOI] [PubMed] [Google Scholar]
- 42.Tibbles H, Vassilev A, Uckun FM. A dual function anti-leukemic agent with anti-thrombotic activity. Leuk Lymph. 2002;43:1121–7. doi: 10.1080/10428190290021650. [DOI] [PubMed] [Google Scholar]
- 43.Akiba H, Takeda K, Kojima Y, et al. The role of ICOS in the CXCR5 follicular B helper T cell maintenance in vivo. J Immunol. 2005;175:2340–8. doi: 10.4049/jimmunol.175.4.2340. [DOI] [PubMed] [Google Scholar]
- 44.Bossaller L, Burger J, Draeger R, et al. ICOS deficiency is associated with a severe reduction of CXCR5+CD4 germinal center Th cells. J Immunol. 2006;177:4927–32. doi: 10.4049/jimmunol.177.7.4927. [DOI] [PubMed] [Google Scholar]
- 45.Hardtke S, Ohl L, Forster R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymphnode follicles and is essential for efficient B cell help. Blood. 2005;106:1924–31. doi: 10.1182/blood-2004-11-4494. [DOI] [PubMed] [Google Scholar]
- 46.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–62. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breitfeld D, Ohl L, Kremmer E, et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med. 2000;192:1545–52. doi: 10.1084/jem.192.11.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG. In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med. 1999;190:1123–34. doi: 10.1084/jem.190.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Good RA. Clinical investigations in patients with agammaglobulinaemia. J Lab Clin Med. 1954;44:803. [Google Scholar]
- 50.Sideras P, Smith CIE. Molecular and cellular aspects of X-linked agammaglobulinaemia. Adv Immunol. 1995;59:135–223. doi: 10.1016/s0065-2776(08)60631-8. [DOI] [PubMed] [Google Scholar]


