Abstract
Interleukin (IL)-17 plays an important role in the pathogenesis in a number of immune inflammatory disorders. This study aims to investigate the mechanism by which microbial product flagellin is involved in the development of T helper type (Th)17 cells. Serum levels of IL-17 and CXCL9-11 in patients with ulcerative colitis (UC) were evaluated. The source and mechanism of CXC11 release in intestinal mucosa were examined with colonic biopsies from UC patients and a colitis mouse model. The role of flagellin in the development of Th17 cells was studied with a cell co-culture system. High serum levels of CXCL11 and IL-17 were observed in UC. Flagellin could induce the production of CXCL11 in CD14+ cells that facilitated the development of Th17 cells. In a skewed Th1 response environment flagellin induces intestinal inflammation, with IL-17 expression predominant. CXCR3/CXCL11 pathway is involved in microbial product flagellin-induced intestinal inflammation in which the Th17 response plays an important role.
Keywords: flagellin, inflammatory bowel disease, intestine, Th17 cells
Introduction
Inflammatory bowel disease (IBD) is an idiopathic inflammatory disease of the intestine that includes ulcerative colitis (UC) and Crohn's disease. The pathogenesis of IBD is poorly understood. Because most IBD is located in the colon, where the highest density of commensal microbe is situated, a plausible aetiology of IBD is that the host immune system over-responds to microbial stimuli that trigger the immune effector cells to mistakenly attack the hosts' own cells, resulting in local inflammation [1]; however, the underlying mechanism remains largely unknown.
Published data indicate that activated Toll-like receptor (TLR)-bearing cells, such as dendritic cells (DC) and macrophages, are associated with the pathogenesis of IBD [2]. Several TLRs have a close relationship with IBD that includes TLR-2, TLR-3, TLR-4, TLR-5, TLR-7 and TLR-9 [3–5]. TLR-bearing cells recognize microbes and their products that elicit proper immune responses to prevent microbes from invading into the body or their products injuring cells and tissue in the body. However, abnormal expression of the TLRs may elicit a skewed immune response that has been proposed as one of the aetiologies inducing immune inflammation in the body. An example is the flagellin-specific immune response, which is involved in the pathogenesis of immune disorders [6–8]. Flagellin is a component of the flagella of a number of bacteria that is constantly shed off to be released to their vicinity, and is a ubiquitous protein in the intestinal lumen that may be absorbed into the subepithelial regions to contact immune cells. However, the mechanism by which flagellin is involved in the pathogenesis of immune disorders needs to be understood further.
Interleukin (IL)-17 is involved in a number of immune and autoimmune diseases such as IBD [9], rheumatoid arthritis [10], systemic lupus erythematosus [11] and psoriasis [12]. T helper type 17 (Th17) cells are a subtype of CD4+ effector T cells that release the proinflammatory cytokine IL-17 upon activation. Recent studies indicate that TLR activation is associated with IL-17 production [13]. However, the mechanism in the development of Th17 cells is not understood fully.
Chemokines are secreted by immune cells or epithelial cells [14]. Apart from guiding the migration of immune cells, the interaction of chemokines and their receptors also modulates the cellular activities of target cells [15,16]. Unusually high levels of chemokines such as CXCL9, 10 and 11 have been noted in patients with immune disorders [17,18]. The CXCL9–11/CXCR3 axis is involved in IL-6-related immune inflammation [19,20]. Inhibition of CXCR3 reduces the expression of IL-6; the latter plays a critical role in the expression of IL-17 [19,20]. Thus, based on the above information, we hypothesized that chemokines may be important in microbial product-related intestinal inflammation. In the present study, we observed the correlation between the serum levels of CXCL9, 10 and 11 and IL-17 in patients with IBD; examined the role of flagellin in the expression of CXCL11 in CD14+ cells; and with a cell co-culture system, the role of flagellin in the development of Th17 cells was investigated.
Materials and methods
Mice
Balb/c mice were purchased from Charles River Canada (St Constant, QC, Canada; Wansheng Experimental Animal Company, Shanghai, China). The animal experimental procedures were approved by the Animal Study Ethics Committee at McMaster University and Tongji University.
Reagents
Antibodies against IL-17, IL-6, transforming growth factor (TGF)-β, CD4, CD14, CD25, CXCL9-11, CXCR3, CD11c, immunoglobulin (Ig)G, TLR-5 and interferon (IFN)-γ were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Magnetic cell-sorting reagents were purchased from Miltenyi Biotec (Auburn, CA, USA). All the fluorescently labelled antibodies for flow cytometry assay and permeable reagent kits were purchased from BD Bioscience (Mississauga, ON, Canada). Enzyme-linked immunosorbent assay (ELISA) kits of IL-17, IL-6, CXCL9, CXCL10, CXCL11, myeloperoxidase (MPO) and neutralizing anti-CXCL11 antibody were purchased from R&D Systems (Burlington, ON, Canada). Trinitrobenzene sulphonic acid (TNBS) was purchased from Sigma Aldrich (Oakville, ON, Canada). Flagellin was purchased from Austral Biologicals (San Ramon, CA, USA). Anti-flagellin antibody was purchased from Biodesign (Saco, ME, USA). Lipopolysaccharide (LPS) levels in all reagents were detected using the limulus assay (limulus amoebocyte lysate QCL 1000 was purchased from Bio Whittaker, Walkersville, MD, USA). The reagents used in this study contained < 0·2U LPS/10 µg reagents.
Collection of intestinal mucosal specimens and sera from IBD patients
Diagnosis of IBD was made on the basis of the endoscopic, radiological, histological and clinical criteria provided by the World Health Organization (WHO) Council for International Organizations of Medical Sciences and the International Organization for the Study of Inflammatory Bowel Disease [21,22]. Patients with indeterminate colitis were excluded. The demographic data of IBD patients are presented in Table S1 (see Supporting Information at the end of the paper). Informed consent was obtained from each patient. The collection of colonic biopsy and sera followed routine procedures in our departments. Two pieces of colon tissue were taken from the edge of ulcers: one for immunohistochemistry, one for Western blotting. The study using human specimens was approved by the Human Study Ethic Committees at Tongji University and Huazhong University of Science and Technology.
Immunohistochemistry
Colonic biopsy tissue was snap-frozen and processed for immune staining. Sections were incubated with primary antibodies against IL-17 (1:100), CD14 (1:200), CXCL11 (1:50) or isotype IgG, used as control, overnight at 4°C. The sections were washed with phosphate-buffered saline (PBS) and incubated with fluorescence labelled second antibody (1:300) and mixed with propidium iodide (10 µg/ml) for 1 h at room temperature. The sections were then washed and mounted with coverslips and observed with a confocal microscope (LSM510). To avoid observer bias, all the slides were coded. The observers were not aware of the codes.
Protein extraction
Tissue proteins were extracted from colonic biopsies or mouse colon using Western blotting or ELISA. Tissue was homogenized with a mini homogenizer in cell lysis buffer (20 mM Tris-HCl, pH 7·5; 150 mM NaCl; 1 mM Na2 ethylenediamine tetraacetic acid (EDTA); 1 mM ethylene glycol tetraacetic acid (EGTA); 1% Triton; 2·5 mM sodium pyrophosphate; 1 mM beta-glycerophosphate; 1 mM Na3VO4; 1 µg/ml leupeptin) in a ice water-bath. The extracts were then centrifuged for 10 min at 14 000 g in a cold microfuge. The supernatant was collected for further experiments.
Western blotting
To determine the levels of CXCL9, CXCL10 and CXCL11 in colonic biopsies, Western blotting was performed. Tissue protein extracts (50 µg/well) were separated by sodium dodecyl sulphate (SDS)–polyacrylamide gels and transferred onto nitrocellulose membranes. The membranes were then probed with the primary antibodies at concentrations of 1:1000–2000. Secondary antibodies were horseradish peroxidise (HRP)-conjugated goat anti-mouse IgG (Fab). Signals were visualized using the ECL Western Blotting Detection Kit.
Induction of inflammation in mouse colon
Under light general anaesthesia, C57/B6 mice (6–8 weeks of age) were administered with 150 µl of trinitrobenzene sulphonic acid (TNBS, 2·5 mg/mouse in 50% ethanol) intrarectally via a 3·5-French catheter. The treatment was repeated 3 days later. Mice were killed on day 7. The colon was collected for further experiments. The assessment procedures for colitis are detailed in the Supporting Information (see the end of the paper). Control groups included naive mice and mice treated with ethanol alone.
The second colitis mouse model was also developed. Ovalbumin-T cell receptor (OVA-TCR) transgenic DO11·10 mice were treated with OVA (10 mg/mouse) and flagellin (100 ng/mouse) in 0·15 ml 50% ethanol via the intrarectal route daily for 7 days using procedures similar to the TNBS model.
Evaluation of colitis
Body weight was recorded before introduction of TNBS and immediately before killing. A 0–4 grading system was employed to evaluate the inflammatory scale of colitis with haematoxylin and eosin (H&E)-stained histology sections: grade 0, normal with no signs of inflammation; grade 1, very low level of leucocyte infiltration; grade 2, low level of mononuclear cell infiltration; grade 3, high level of infiltration of mononuclear cells, high vascular density and intestinal wall thickening; and grade 4, transmural infiltrates with loss of goblet cells, high vascular density, wall thickening and disruption of normal intestinal structure.
Flow cytometry
Cells were collected and incubated with fluorescence labelled antibodies (0·5–1 µg/ml, or using isotype IgG as control) on ice for 30 min (for intracellular staining, cells were fixed with 1% paraformaldehyde on ice for 30 min and incubated with permealization reagents for 30 min on ice). The stained cells were analysed using a fluorescence activated cell sorter (FACS) array (BD Bioscience, San Jose, CA, USA). Data were analysed with FlowJo software.
ELISA
Levels of cytokines and MPO were measured by ELISA with commercial reagent kits following the manufacturer's instructions.
Isolation of lamina propria mononuclear cells (LPMC)
The colon was excised from mice at the time of killing, cut into small pieces and treated with predigestion solution [1 × Hanks's balanced salt solution (HBSS) containing 5 mM EDTA and 1 mM dithiothreitol (DTT)] at 37°C for 30 min under slow rotation. The tissue was collected by centrifugation (200 g, 10 min) and incubated in digestion solution (dissolved in 0·05 g of collagenase D, 0·05 g of DNase I and 0·3 g of dispase II in 100 ml of 1 × PBS) at 37°C for 60 min under slow rotation. Cells were filtered with a cell strainer. Cells were cultured for further experiments.
Isolation of CD4+ CD25- T cells
CD4+ CD25- T cells were isolated from LPMC with a commercial cell sorting reagent kit following the manufacturer's instruction.
Generation of CD14+ CXCL11+ cells
The bone marrow was obtained from mouse femurs. After lysis of red blood cells, the mononuclear cells were collected and cultured in RPMI-1640 culture medium in the presence of granulocyte–macrophage colony-stimulating factor (20 ng/ml) and flagellin (20 ng/ml). On the seventh day, cells were collected; the CD14+ CXCL11+ cells were isolated by magnetic affinity cell sorting (MACS) with a commercial reagent kit for further experiments.
Histology
The colon segments were excised from experimental mice, fixed with 4% paraformaldehyde overnight and processed for paraffin embedding and H&E staining. Section slides were coded. The observers were not aware of the codes to avoid observer bias.
Statistics
All values were expressed as the means ± standard deviation (s.d.) of at least three independent experiments. The values were analysed using the two-tailed unpaired Student's t-test when data consisted of two groups or by analysis of variance (anova) when three or more groups were compared. The correlation between variables was analysed using Pearson's correlation coefficient. P < 0·05 was accepted as statistically significant.
Results
Serum levels of CXCL11 are increased in patients with IBD
The CXCR3/CXCL11 axis is involved in the pathogenesis of a number of immune diseases [17]. We predict that CXCL11 may also be involved in the intestinal immune inflammation such as IBD. Thus, serum samples were collected from 26 patients with UC and 10 healthy subjects. As shown by ELISA data, the levels of CXCL9 and CXCL10 were slightly higher than the healthy control group, but did not reach the significant criteria; the levels of CXCL11 were significantly higher in the IBD group than in healthy controls (Fig. 1a–c). A correlation assay was performed between CXCL11 and IBD disease activities. However, no significant correlation was identified (P > 0·05). The data indicate that IBD patients have high serum levels of CXCL11 that may be involved in the pathogenesis of IBD.
Fig. 1.
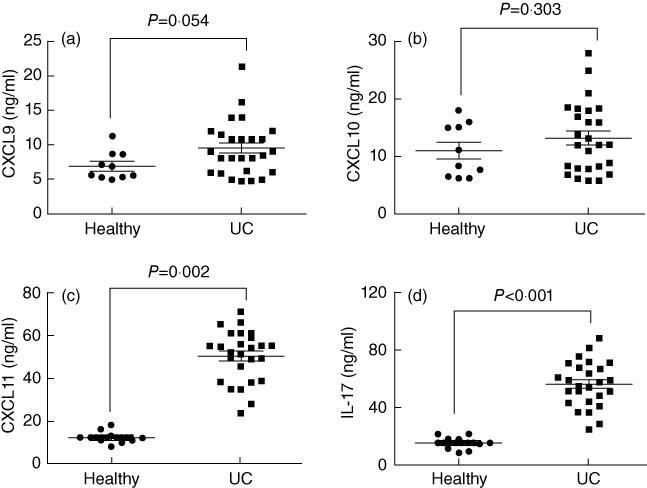
Serum levels of CXCL9-11 and interleukin (IL)-17 in inflammatory bowel disease (IBD) patients. The sera were collected from 26 ulcerative colitis (UC) patients and 10 healthy subjects. The levels of CXCL9, CXCL10, CXCL11 and IL-17 were determined by enzyme-linked immunosorbent assay. The scatter dot-plots indicate levels of CXCL9 (a), CXCL10 (b), CXCL11 (c) and IL-17 (d) in the sera. Each dot represents a datum from one subject.
Expression of CXCL11 is correlated with IL-17 levels in the colon of IBD patients
IL-17 is one of the proinflammatory cytokines in immune disorders such as IBD. As we observed that serum levels of CXCL11 were increased in IBD patients, we speculated as to whether there is an association between CXCL11 and IL-17. Therefore, we also measured the levels of IL-17 in collected sera by ELISA. The results showed that the levels of IL-17 were significantly higher in IBD patients than in the healthy control group (Fig. 1d). A correlation assay was performed between the serum CXCL9-11 and IL-17. The results showed a positive correlation existed between serum CXCL11 and IL-17 (r = 0·677; P = 0·006), but were not in CXCL9 and IL-17 (r = 0·153, P = 0·363) or CXCL10 and IL-17 (r = 0·206, P = 0·188). The results show a positive correlation between serum CXCL11 and IL-17 levels, implying that the increases in CXCL11 may contribute to the increase in IL-17 production in the body.
The increase in levels of CXCL11 and IL-17 in the serum imply that these molecules may be also increased in the colon of patients with IBD. To test the hypothesis, a piece of colonic tissue was processed for assessing the frequency of CXCL11+ and IL-17+ cells by immunohistochemistry. The results showed that higher frequencies of CXCL11+ and IL-17+ cells were observed in IBD colonic mucosa than in non-IBD samples (Fig. 2a–c). To confirm the results further, a piece of tissue was processed for Western blotting. The immune blots showed that significantly higher levels of CXCL11 and IL-17 were detected in samples from IBD colonic biopsies than those from non-IBD samples. The levels of CXCL9 and CXCL10 in UC samples were similar to those in non-IBD samples (Fig. 2d). The results show that the levels of CXCL11 and IL-17 in the colon are consistent with that in the serum.
Fig. 2.
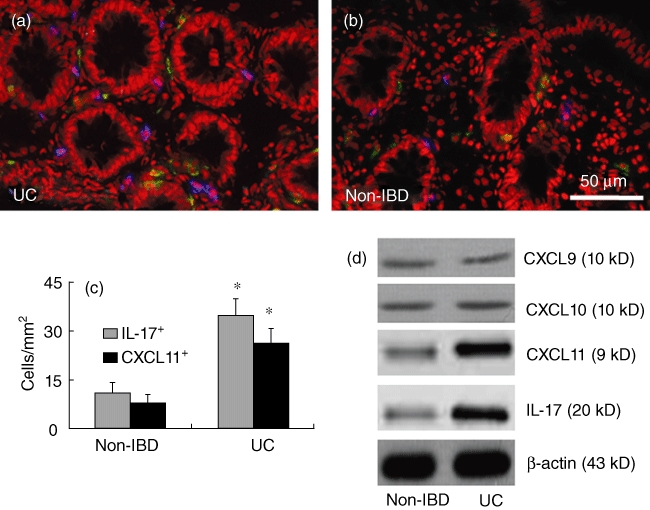
CXCL11 is correlated with the increase in interleukin (IL)-17 in inflammatory bowel disease (IBD) patients. Colonic biopsies were obtained from the 16 ulcerative colitis (UC) patients (a) as described in the text. Six non-IBD samples (b) were obtained from the marginal tissue of surgically removed colonic cancer (proved by a pathologist). (a–c) Cryosections were prepared with the colonic samples and stained with anti-CXCL11 and anti-IL-17 antibodies. The sections were observed under a confocal microscope. The representative confocal images show CXCL11+ cells (in blue) and IL-17+ cells (in green) in colonic tissue. The positive cell counts were presented in (c) as cells per mm2 (mean ± standard deviation; averaged from 20 fields of each subject). *P < 0·05, compared with non-IBD group. (d) A piece of colonic tissue from biopsies [as described in (a–c)] was processed for Western blotting to determine the protein levels of CXCL9, CXCL10, CXCL11 and IL-17. The representative immune blots show the levels of CXCL9, CXCL10, CXCL11 and IL-17 in colonic tissue of non-IBD subjects and UC patients. β-actin was used as internal control.
CXCL11+ cells are also CD14+ in inflamed colon of TNBS-induced colitic mice and IBD patients
To characterize further the CXCL11+ cells we noted in IBD colonic mucosa, we developed a mouse colitis model with TNBS. CXCL11+ cells were isolated from the colon of colitic mice and examined by flow cytometry. The results showed that more than 10% CXCL11+ CD14+ cells were detected in LPMCs from colitic mice, while only a few more than 2% CXCL11+ CD14+ cells were found in control mice (Fig. 3a–d). The data imply that a subset of CD14+ cell expresses CXCL11 in immune inflammation such as IBD. To confirm if this phenomenon also existed in IBD patients, we examined CXCL11+ CD14+ cells in human colonic tissue with immunohistochemistry. Indeed, abundant CXCL11+ CD14+ cells were observed in colonic samples from IBD patients while it was scarce in non-IBD colonic samples (Fig. 3e–g).
Fig. 3.
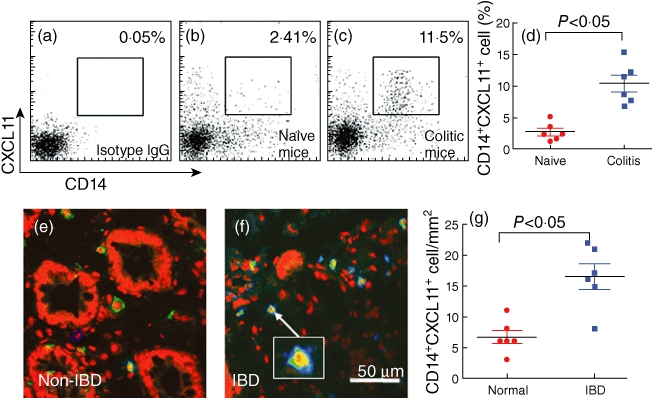
CD14+ cells express CXCL11. (a–d) A trinitrobenzene sulphonic acid (TNBS) colitic mouse model was developed. Lamina propria mononuclear cells (LPMCs) were isolated from mouse colon, stained with anti-CXCL11 and anti-CD14 antibodies and analysed by flow cytometry. The flow cytometry dot-plots show frequency of CD14+ CXCL11+ cells (the gated cells). (d) Summarized data of flow cytometry (each dot represents an individual datum). (e–g) Inflammatory bowel disease (IBD) patients' colonic biopsy tissue was processed for immunohistochemistry to examine the CD14+ CXCL11+ cells. The representative confocal images show CD14+ cells (in blue), CXCL11+ cells (in green) or CD14+ CXCL11+ cells (cells were stained in both green and blue or light blue; see the insert). (g) The immune-positive cell counts (each dot represents a datum from one sample that was averaged from 20 high-power fields, × 200).
Activation of TLR-5 is involved in the up-regulation of CXCL11 in CD14+ cells
Based on published data that the production of CXCL11 can be induced by flagellin-induced TLR-5 activation [21], that also plays a role in the pathogenesis of intestinal immune inflammation [5,6,22], we next investigated the mechanism by which the expression of CXCL11 was increased in colonic CD14+ cells in mice with colitis. Initially we isolated LPMCs from naive mice to be stimulated by flagellin; however, it did not induce the expected production of CXCL11 by these cells (Fig. 4a1). We then isolated LPMCs from colitic mice; the levels of CXCL11 were slightly higher than naive controls, but did not reach the significance criteria (data not shown). We then exposed the LPMCs from colitic mice and exposed them to flagellin; the CXCL11-expressing CD14+ cell number increased significantly (Fig. 4a2). Considering that colitic mice-derived LPMCs might have been conditioned by IFN-γ (TNBS-induced colitis has high levels of IFN-γ), IFN-γ is involved in the production of CXCL11; as mentioned previously [23], we exposed LPMCs from naive mice to both flagellin and IFN-γ. Indeed, marked increase in CXCL11 was observed in CD14+ cells (Fig. 4a4) that was abolished by pretreating the LPMCs with neutralizing TLR-5 antibody (Figs 4 and 5). The individual data are presented in Fig. 4b. In addition, we also measured the levels of CXCL11 in culture supernatant. The results showed that the CXCL11 levels in supernatant were in parallel to the frequency of CD14+ CXCL11+ cells (Fig. 4c). The results imply that in the Th1 polarization environment, flagellin is capable of inducing the production of CXCL11 by CD14+ cells in the intestine.
Fig. 4.
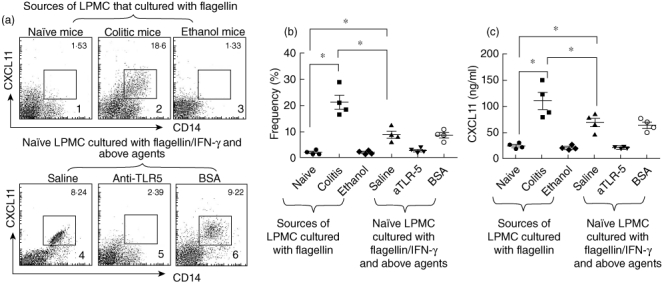
Toll-like receptor (TLR)-5 activation facilitates CD14+ cells to express CXCL11. (a) Flow cytometry dot-plots show CD14+ CXCL11+ cell population (the gated cells) in lamina propria mononuclear cells (LPMCs) that were isolated from naive mice (a1), colitic mice [a2, induced by trinitrobenzene sulphonic acid (TNBS)] or ethanol-treated mice (a3, control); cells were cultured in the presence of flagellin (40 ng/ml) for 72 h, or naive LPMCs were exposed to flagellin plus interferon (IFN)-γ (50 pg/ml; the dosage was tested in preliminary studies) and saline (a4), or pretreated with anti-TLR-5 antibody (a5, 100 ng/ml) or bovine serum albumin (BSA) (a6) for 72 h. Cells stained with isotype immunoglobulin (Ig)G did not show positive staining (data not shown). (b) Scatter dot-plots show individual datum in (a). (c) The scatter dot-plots show CXCL11 levels in culture supernatant that were measured by enzyme-linked immunosorbent assay. *P < 0·01.
Fig. 5.
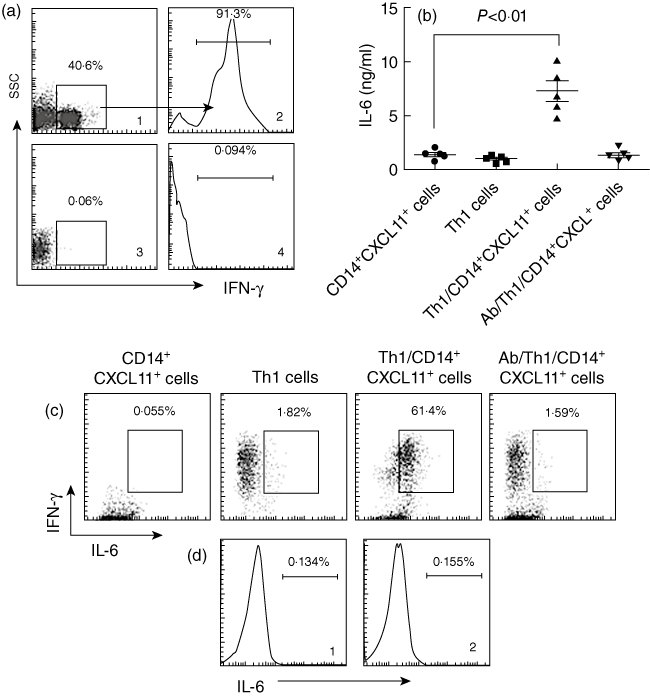
CXCL11 triggers T helper type 1 (Th1) cells to produce interleukin (IL)-6. (a) DO11·10 spleen mononuclear cells were isolated from the spleen and cultured in the presence of ovalbumin (OVA) (323–339; 20 ng/ml), interferon (IFN)-γ (50 pg/ml) and anti-IL-4 (20 pg/ml) for 96 h. The cells were collected and stained with anti-CD4 and anti-IFN-γ antibodies and analysed by flow cytometry. (a1) Representative dot-plot shows the frequency of CD4+ T cells (the gated cells). (a2) Histogram indicates the frequency of IFN-γ+ T cells (the gated area) in the gated CD4+ T cells in (a1). Cells in (a3) were from DO11·10 mice, but not stimulated by IFN-γ/IL-4/OVA; (a4) is an isotype control. (b) CD14+ CXCL11+ cells were prepared as described in the text; CD4+ CD25+ T cells were isolated by magnetic affinity cell sorter (MACS) from the cells in (a). The two cell populations were co-cultured at a ratio of 1:10 (CD14+ CXCL11+ cells : T cells) for 72 h. The scatter dot-plots indicate the levels of interleukin (IL)-6 in supernatant that were measured by enzyme-linked immunosorbent assay. (c) Cells in (b) were collected and stained with anti-IFN-γ and IL-6 antibodies and analysed by flow cytometry. The representative dot-plots indicate the IFN-γ+ IL-6+ cell population (the gated cells). (d) CD14+ CXCL11+ cells were prepared as described in the text and stained with isotype IgG (d1, used as a negative control) or anti-IL-6 antibody (d2). The histograms show the levels of IL-6.
CXCL11 triggers Th1 cells to produce IL-6
IL-6 is involved in the pathogenesis of IBD [24]. However, over-production of IL-6 in the body needs to be understood further. Because Th1 cells express the receptor of CXCL11 (CXCR3) [25], we speculated as to whether CD14+ CXCL11+ cell-released CXC11 can bind CXCR3 on Th1 cells to activate them to release IL-6. To this end, we first generated a batch of Th1 cells. Mononuclear cells were isolated from DO11·10 mouse spleen by MACS. The cells were cultured under a Th1 polarization condition in the presence of specific antigen OVA peptide (323–339), IFN-γ and anti-IL-4 antibody for 96 h. As shown by flow cytometry, more than 90% of the gated CD4+ T cells were IFN-γ+ T cells (Fig. 5a). The cells (mixed cells) were washed by fresh culture medium and co-cultured with flagellin-primed CD14+ cells (more than 90% cells express CXCL11 as evaluated by flow cytometry) for 72 h. As shown by ELISA (Fig. 5b) and flow cytometry (Fig. 5c), the levels of IL-6 in culture supernatant increased markedly compared with those co-cultured with naive mononuclear cells (Fig. 5b and c) that could be blocked by pretreatment with neutralizing anti-CXCL11 antibody (Fig. 5b and c). The results indicate that CD14+ CXCL11+ cells can trigger Th1 cells to produce IL-6.
CXCL11 facilitates the development of Th17 cells
Th17 cells play an important role in IBD [26]. Although we have known that IL-6 and TGF-β acting in concert trigger Th17 cell lineage commitment [27], the development of Th17 is not yet understood fully. Because CD14+ CXCL11+ cells can trigger polarized Th1 cells to produce IL-6 (Fig. 5) and TGF-β and IL-6 up-regulated the IL17 gene transcription factor retinoid-related orphan receptor-γt (RORγt) [28], we postulate that CXCL11 may facilitate the development of Th17 cells under the Th1 environment. To test this hypothesis, we treated DO11·10 mice with OVA daily for 7 days. After killing, we isolated LPMCs and examined by flow cytometry for the phenotypes of CD4+ CD25+ T cells. The results showed that the gated CD4+ T cells consisted of IL-4+ T cells (27·8% ± 2·3%), IFN-γ+ T cells (24·8% ± 4·6%), forkhead box P3 (FoxP3+) T cells (14·8% ± 3·2%) and IL-17 T cells (4·8% ± 1·5%), respectively (Fig. 6a). The results indicate that the OVA-specific immune response can activate the ‘pan’ T cells in the intestine of DO11·10 mice. We then isolated CD4+ CD25- T cells from the spleen of naive DO11·10 mice by MACS and co-cultured with flagellin-primed CD14+ cells in the presence of OVA peptide (323–339) for 96 h. The cells were then analysed by flow cytometry. The results showed that a significant increase in the frequency of IL-17 cells was induced that was abolished by pretreatment with neutralizing anti-CXCL11 antibody (Fig. 6b). The results indicate that CXCL11 plays a critical role in converting CD4+ CD25- T cells to Th17 cells.
Fig. 6.
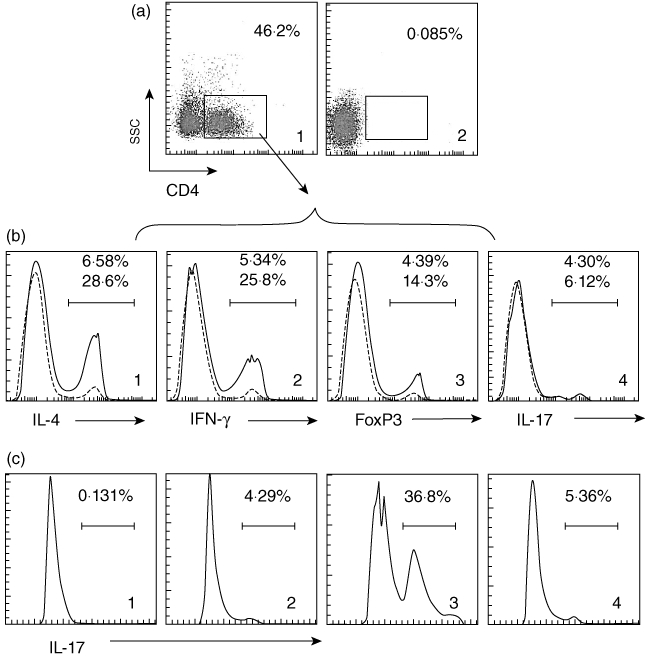
CXCL11 plays a critical role in the induction of T helper type 17 (Th17) cells. (a) Ovalbumin-T cell receptor (OVA-TCR) gene transgenic mice (DO11·10 mice) were gavage-fed with OVA (10 mg/mouse) daily for 7 days. Lamina propria mononuclear cells (LPMCs) were isolated by magnetic affinity cell sorter (MACS), stained with indicated antibodies and analysed by flow cytometry. A1, dot-plots show the frequency of CD4+ T cells in LPMCs. A2, dot-plots show isotype immunoglobulin (Ig)G staining used as a negative control. (b1–4) Histograms show frequencies of positively stained cells of indicated markers (annotated below each histogram). Histograms with broken lines show results of mice treated with saline (control group). Histograms with continuous lines show results of mice treated with OVA. The gated area indicates positive cell population in the OVA-treated group (also numerated inside histograms). (c) CD4+ CD25- T cells from the spleen of naive DO11·10 mice were isolated by MACS and co-cultured with flagellin-primed CD14+ cells (more than 90% cells express CXCL11 as checked by flow cytometry) at a ratio of 10:1 (T cell : CD14 CXCL11 cells) in the presence of OVA peptide (323–339; 20 ng/ml) for 96 h. Cells were collected, stained with anti-interleukin (IL)-17 antibody and analysed by flow cytometry. The histograms show the frequencies of IL-17+ cells (the gated area). C1, cells stained with isotype IgG used as a negative control. C2-4, cells were stained with anti-IL-17 antibody. C2, naive CD4+ CD25- T cells. C3, CD4+ CD25- T cells were co-cultured with flagellin-primed CD14+ cells. C4, pretreated with anti-CXCL11 antibody, CD4+ CD25- T cells were then co-cultured with flagellin-primed CD14+ cells.
CXCL11 plays a critical role in the induction of inflammation in the intestine
Next, we developed a mouse model to test the hypothesis that microbial product flagellin plays a role in the induction of intestinal inflammation. DO11·10 mice were treated with OVA plus flagellin via the intrarectal route with or without pretreatment with anti-CXCL11 antibody. As depicted in Fig. 7, the results showed that exposure to OVA and flagellin concurrently induced modest to severe colitis that could be blocked by pretreatment with anti-CXCL11 antibody. The data indicate that, under certain conditions such as under skewed immune response, the microbial product flagellin can initiate intestinal inflammation; CXCL11 plays an important role in the process. For comparison, we also applied anti-CXCL11 antibody to C57/B6 mice treated with TNBS. As predicted, the intestinal inflammation was also blocked.
Fig. 7.
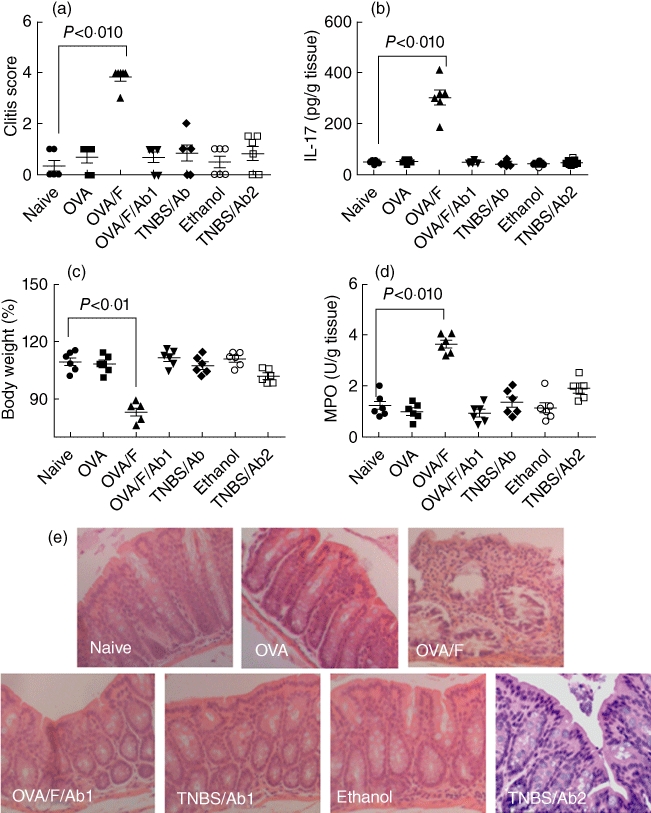
Flagellin induces intestinal inflammation. To determine the role of flagellin and CXCL11 in the pathogenesis of intestinal inflammation, mouse intestinal inflammation model was developed by treating C57/B6 mice with trinitrobenzene sulphonic acid (TNBS) in 50% ethanol or treating DO11·10 mice with ovalbumin (OVA) plus flagellin (in 50% ethanol) as detailed in the text. After killing, the indicated parameters in (a–e) were evaluated. Each group consisted of six mice. (a) The inflammatory condition of mouse colon was assessed by a semi-quantification scoring system as detailed in the text. The dot-plots indicate colon inflammatory score; each dot represents the score from one mouse (a–d). (b) Colon proteins were extracted and analysed by enzyme-linked immunosorbent assay (ELISA) to determine the levels of interleukin (IL)-17. The dot-plots show protein levels of IL-17 in colon protein extracts. (c) Mouse body weight was recorded before treatment (day 0) and immediately before killing (day 7); body weight was calculated and expressed as the reduction rate of body weight. The dot-plots show the body weight reduction rate after treatment. (d) The levels of myeloperoxidase (MPO) in colon tissue protein extracts were measured by ELISA. The dot-plots show MPO levels in the colon. (e) Representative photomicrographs show colonic histology of experimental mice. Original magnification: × 100. Naive, naive control mice; OVA, DO11·10 mice were treated with OVA (10 mg/mouse) daily for 7 days; OVA/F, DO11·10 mice were treated with OVA (10 mg/mouse) and flagellin (100 ng/mouse) daily for 7 days; OVA/F/Ab1, the ‘Ab1’ indicates that the mice were treated with anti-CXCL11 antibody (300 ng/mouse, intraperitoneally) daily for 7 days; TNBS/Ab1, mice were treated with TNBS via the intrarectal route as well as with anti-CXCL11 antibody; ethanol, mice were treated with vehicle ethanol used as controls; TNBS/Ab2, mice were treated with TNBS via the intrarectal route as well as with anti-Toll-like receptor-5 antibody (to avoid redundant datum presentation, we do not show the results from mice treated with TNBS/ethanol alone).
Discussion
The present study has provided further evidence that a chemokine, CXCL11, was increased in IBD intestinal CD14+ cells as well as in sera. The production of CXCL11 by CD14+ cells could be up-regulated by exposure to microbial stimuli. In co-operation with Th1 cytokine IFN-γ, CXCL11 acted on CD4+ T cells to induce the production of IL-6 that is involved in induction of immune inflammation in the intestine. The present study has revealed a novel pathway by which microbial stimuli facilitate the development of Th17 cells.
The main function of chemokines is to direct immune cell movement. However, chemokines also bind their receptors on target cells to activate a series of signal transduction pathways; some of them are the same as those activated in inflammatory responses such as p38 mitogen-activated protein kinases and phosphatidylinositol-3-kinase signalling pathways [29]. The present data indicate that CXCL11 has a close relation with the intestinal immune disorder IBD. Published studies also demonstrate that the activities of some chemokines are associated with the induction of proinflammatory genes, such as TNF, IL-1β, IL-6 and IL-1β[30]. The data imply that some chemokines such as CXCL11 may be involved in the pathogenesis of immune inflammation such as IBD. Others also propose that CXCL11 is involved in autoimmune disorders and creates local amplification loops of inflammation in target organs [17].
The endogenous sources of CXCL11 include fibroblasts [23], dendritic cells [31] and macrophages [32,33]. The present data show that CD14+ cells are one of the major sources of CXCL11 in inflamed intestinal tissue. The results are in line with the published data, as CD14 is expressed mainly by macrophages and a subset of dendritic cells. CXCL11 is not expressed constitutively in these cells. They can be up-regulated by inflammatory stimuli. The present data provide information not described previously on this point, that activation of TLR-5 can facilitate the expression of CXCL11 in CD14+ cells. The data thus link microbial stimuli to strengthening the expression of CXCL11 in inflammatory cell (such as macrophages). Others also have noted that the skewed CD14 gene expression in IBD patients is associated with an increased susceptibility to developing IBD comparing with healthy subjects [34].
Th1 inflammation is featured as high levels of IFN-γ in the serum and local tissue. Our data are in line with these observations. One of the functions of IFN-γ is to increase the expression of CXCL11 in other cells, such as in human gingival fibroblasts, as described in a recent report [23]. Our data expand this notion by showing that IFN-γ is also required in the expression of CXCL11 in CD14+ cells. The data imply that under a skewed Th1 polarization environment, the high levels of IFN-γ may be a trigger to increase CXCL11 production by CD14+ cells. As Th1 cells express CXCR3, the receptor of CXCL11, the CD14+ cell-derived CXCL11 can then bind CXCR3 on Th1 cells that have the potential to activate Th1 cells. Indeed, our data show that the interaction of CXCL11 and CXCR3 increases the production of IL-6 by Th1 cells.
Previous studies have recognized that IL-6 is an important mediator of gut dysfunction in IBD. Antibody neutralization of IL-6 improves gut function and lessens the severity of patient symptoms [35,36]. In recent years it has been recognized that the major role of IL-6 in IBD is required in the development of Th17 cells; the process requires IL-6 and TGF-β acting in concert [28]. The source of IL-6 in the intestine is therefore of significance. Our data provide important information about the sources of IL-6 in the intestine. The intestinal tract is full of microbial products. Disturbance of epithelial barrier integrity (such as by psychological stress) may cause absorption of microbial products into the subepithelial region [37] to trigger immune cells to produce CXCL11, as shown by the present data; the latter induces Th1 cells to produce IL-6.
Regulatory T cell (Treg)-derived TGF-β is crucial in immune regulation; however, TGF-β is also involved in the induction of proinflammatory cytokine IL-17 [28]. Higher Treg frequency is recognized in the normal intestine compared to other organs in the body, serving as one of the major cellular components of oral tolerance; the latter is usually suppressed in most immune disorders. However, the frequency of CD4+ FoxP3+ T cells may not always be reduced in IBD patients, as we have observed recently (unpublished data); the phenomenon has also been mentioned by others [38]. Therefore, these CD4+ FoxP3+ T cells can be an important source of TGF-β to facilitate the development of Th17 cells.
Skewed Th1 polarization plays an important role in intestinal inflammation of most IBD cases. IL-17 is a critical cytokine in the pathogenesis of IBD. Our data have bridged these two phenomena by showing the production of CXCL11 by CD14+ cells that requires a skewed Th1 environment. Thus, if this ‘bridge’ is broken, the over-expression of IL-17 may be abrogated. Indeed, our further results support the hypothesis that pretreatment with anti-CXCL11 antibody abolishes the development of IL-17+ cells, as well as the induction of intestinal inflammation. The data suggest that CXCL11 has the potential to be a novel therapeutic target in the treatment of IBD.
In summary, the present study shows that high serum levels of CXCL11 in IBD patients that correlate with the serum levels of IL-7. Microbial product flagellin plays a critical role in the induction of CXCL11 in CD14+ cells. The stimulation of CXCL11 induces Th1 cells to express IL-6 that, in co-operation with co-existing TGF-β, facilitates the development of Th17 cells.
Acknowledgments
This study was supported by grants from the Canadian Institute of Health Research (CIHR), Natural Sciences and Engineering Research Council of Canada (NSERC) and the Natural Science Foundation of China (no. 30770988, 30971358). Dr P.-C. Yang holds a New Investigator Award of CIHR.
Disclosure
The authors do not have any conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Table S1. Patient demographics.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8:564–77. doi: 10.1038/nrmicro2403. [DOI] [PubMed] [Google Scholar]
- 2.Cario E. Toll-like receptors in inflammatory bowel diseases: a decade later. Inflamm Bowel Dis. 2010;16:1583–97. doi: 10.1002/ibd.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szebeni B, Veres G, Dezsõfi A, et al. Increased expression of Toll-like receptor (TLR)-2 and TLR-4 in the colonic mucosa of children with inflammatory bowel disease. Clin Exp Immunol. 2008;151:34–41. doi: 10.1111/j.1365-2249.2007.03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cario E, Podolsky DK. Differential alteration in intestinal epithelial cell expression of Toll-like receptor 3 (TLR3) and TLR4 in inflammatory bowel disease. Infect Immun. 2000;68:7010–7. doi: 10.1128/iai.68.12.7010-7017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Feng BS, Zheng PY, et al. Fc gamma receptor signaling in mast cells links microbial stimulation to mucosal immune inflammation in the intestine. Am J Pathol. 2008;173:1647–56. doi: 10.2353/ajpath.2008.080487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erridge C, Duncan SH, Bereswill S, Heimesaat MM. The induction of colitis and ileitis in mice is associated with marked increases in intestinal concentrations of stimulants of TLRs 2, 4, and 5. PLoS ONE. 2010;5:e9125. doi: 10.1371/journal.pone.0009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vijay-Kumar M, Gewirtz AT. Role of flagellin in Crohn's disease: emblematic of the progress and enigmas in understanding inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:789–95. doi: 10.1002/ibd.20734. [DOI] [PubMed] [Google Scholar]
- 9.Munitz A, Seidu L, Cole ET, Ahrens R, Hogan SP, Rothenberg ME. Resistin-like molecule alpha decreases glucose tolerance during intestinal inflammation. J Immunol. 2009;182:2357–63. doi: 10.4049/jimmunol.0803130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR. Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum. 2008;58:875–87. doi: 10.1002/art.23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crispín JC, Oukka M, Bayliss G, et al. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J Immunol. 2008;181:8761–6. doi: 10.4049/jimmunol.181.12.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, et al. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J Immunol. 2008;181:7420–7. doi: 10.4049/jimmunol.181.10.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uematsu S, Fujimoto K, Jang MH, et al. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–76. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 14.Lukacs-Kornek V, Engel D, Tacke F, Kurts C. The role of chemokines and their receptors in dendritic cell biology. Front Biosci. 2008;13:2238–52. doi: 10.2741/2838. [DOI] [PubMed] [Google Scholar]
- 15.Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004;279:30219–27. doi: 10.1074/jbc.M403595200. [DOI] [PubMed] [Google Scholar]
- 16.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in beta-arrestin2- and GRK6-deficient mice. Proc Natl Acad Sci USA. 2002;99:7478–83. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lacotte S, Brun S, Muller S, Dumortier H. CXCR3, inflammation, and autoimmune diseases. Ann NY Acad Sci. 2009;1173:310–7. doi: 10.1111/j.1749-6632.2009.04813.x. [DOI] [PubMed] [Google Scholar]
- 18.Zeremski M, Dimova R, Brown Q, Jacobson IM, Markatou M, Talal AH. Peripheral CXCR3-associated chemokines as biomarkers of fibrosis in chronic hepatitis C virus infection. J Infect Dis. 2009;200:1774–80. doi: 10.1086/646614. [DOI] [PubMed] [Google Scholar]
- 19.Tokuyama H, Ueha S, Kurachi M, et al. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol. 2005;17:1023–34. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- 20.Bao CR, Tang GY, Zhang XP, Quan ZW. Lentivirus-mediated gene transfer of small interfering RNA against the chemokine receptor CXCR3 suppresses cytokine indicators of acute graft rejection in a rat model. J Int Med Res. 2010;38:1113–20. doi: 10.1177/147323001003800340. [DOI] [PubMed] [Google Scholar]
- 21.Means TK, Hayashi F, Smith KD, Aderem A, Luster AD. The Toll-like receptor 5 stimulus bacterial flagellin induces maturation and chemokine production in human dendritic cells. J Immunol. 2003;170:5165–75. doi: 10.4049/jimmunol.170.10.5165. [DOI] [PubMed] [Google Scholar]
- 22.Rhee SH, Im E, Riegler M, Kokkotou E, O'brien M, Pothoulakis C. Pathophysiological role of Toll-like receptor 5 engagement by bacterial flagellin in colonic inflammation. Proc Natl Acad Sci USA. 2005;102:13610–5. doi: 10.1073/pnas.0502174102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. TNFSF14 coordinately enhances CXCL10 and CXCL11 productions from IFN-gamma-stimulated human gingival fibroblasts. Mol Immunol. 2010;47:666–70. doi: 10.1016/j.molimm.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geiger B, Wenzel J, Hantschke M, Haase I, Ständer S, von Stebut E. Resolving lesions in human cutaneous leishmaniasis predominantly harbour chemokine receptor CXCR3-positive T helper 1/T cytotoxic type 1 cells. Br J Dermatol. 2010;162:870–4. doi: 10.1111/j.1365-2133.2009.09573.x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 27.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fina D, Sarra M, Fantini MC, et al. Regulation of gut inflammation and Th17 cell response by interleukin-21. Gastroenterology. 2008;134:1038–48. doi: 10.1053/j.gastro.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 29.Shahabuddin S, Ji R, Wang P, et al. CXCR3 chemokine receptor-induced chemotaxis in human airway epithelial cells: role of p38 MAPK and PI3K signaling pathways. Am J Physiol Cell Physiol. 2006;291:C34–9. doi: 10.1152/ajpcell.00441.2005. [DOI] [PubMed] [Google Scholar]
- 30.Zhai Y, Shen XD, Gao F, et al. CXCL10 regulates liver innate immune response against ischemia and reperfusion injury. Hepatology. 2008;47:207–14. doi: 10.1002/hep.21986. [DOI] [PubMed] [Google Scholar]
- 31.Jin P, Han TH, Ren J, et al. Molecular signatures of maturing dendritic cells: implications for testing the quality of dendritic cell therapies. J Transl Med. 2010;8:4. doi: 10.1186/1479-5876-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benson SA, Ernst JD. TLR2-dependent inhibition of macrophage responses to IFN-gamma is mediated by distinct, gene-specific mechanisms. PLoS ONE. 2009;4:e6329. doi: 10.1371/journal.pone.0006329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–49. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 34.Gazouli M, Mantzaris G, Kotsinas A, et al. Association between polymorphisms in the Toll-like receptor 4, CD14, and CARD15/NOD2 and inflammatory bowel disease in the Greek population. World J Gastroenterol. 2005;11:681–5. doi: 10.3748/wjg.v11.i5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuda R, Koide T, Tokoro C, et al. Quantitive cytokine mRNA expression profiles in the colonic mucosa of patients with steroid naïve ulcerative colitis during active and quiescent disease. Inflamm Bowel Dis. 2009;15:328–34. doi: 10.1002/ibd.20759. [DOI] [PubMed] [Google Scholar]
- 36.Danese S, Gao B. Interleukin-6: a therapeutic Jekyll and Hyde in gastrointestinal and hepatic diseases. Gut. 2010;59:149–51. doi: 10.1136/gut.2008.173534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang PC, Jury J, Söderholm JD, Sherman PM, McKay DM, Perdue MH. Chronic psychological stress in rats induces intestinal sensitization to luminal antigens. Am J Pathol. 2006;168:104–14. doi: 10.2353/ajpath.2006.050575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastaff-Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol. 2010;30:80–9. doi: 10.1007/s10875-009-9345-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


