Abstract
While Gr1+CD11b+ cells are known to regulate immune responses and accumulate in most cancer tissues, the function of Gr1+CD11b+ cells in inflammation is poorly understood. We investigated the role of Gr1+CD11b+ cells in a dextran sulphate sodium (DSS)-treated mouse model of ulcerative colitis (UC). C57BL/6 mice were treated with 2% DSS in drinking water for 5 days. Disease progression and recovery were assessed by body weight, disease activity index score (DAI) score and colon length. Splenic Gr1+CD11b+ cell number was greatly increased during the recovery phase of DSS-induced colitis. DSS-derived splenic Gr1+CD11b+ cells were administered intravenously to recipient (C57BL/6) mice during the early phase of DSS treatment. The transplanted splenic DSS-induced Gr1+CD11b+ cells improved DSS-induced colitis and promoted efficient colonic mucosal healing. We found that the CD11b+ single positive cells increased in the course of DSS-induced colitis in lamina propria. The transplantation of splenic Gr1+CD11b+ cells induced feedback suppression of myeloid-lineage cell development. Namely, the transplantation of splenic Gr1+CD11b+ cells greatly suppressed the migration of CD11b+ single positive cells to the lamina propria. Further, transplantation of Gr-1+CD11b+ cells greatly suppressed the increase of the same population, especially during the late phase of DSS colitis both in spleen and bone marrow.
Keywords: CD11b, DSS, Gr-1, myeloid-lineage, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) is a chronic and relapsing inflammation of the gastrointestinal tract that affects millions of people worldwide. The major forms of idiopathic IBD are ulcerative colitis (UC) and Crohn's disease (CD), and occur typically in the second and third decades of life, with the majority of affected individuals progressing to a relapsing and chronic disease [1]. Although the causes of IBD are still unknown, recent advances in the understanding of the molecular pathogenesis of IBD have been made owing to three related lines of investigation. First, in contrast to some other complex disorders, IBD has been amenable to the discovery of susceptibility genes. Secondly, it appears that commensal bacteria (or their products), rather than conventional pathogens, are the drivers for the dysregulated immunity observed typically in IBD cases. Thirdly, murine models that exhibit many of the features of UC seem to be driven bacterially and have helped to unravel the pathogenesis and/or mucosal immunopathology of IBD. Overall, it appears that an imbalance of the mucosal immune system leads to the overproduction of inflammatory cytokines, release of reactive oxygen metabolites and the infiltration of neutrophils into the intestine, resulting in uncontrolled intestinal inflammation and tissue damage [2–4].
Thus, neutrophils and macrophages may play important roles in disease progression and/or host defence. Both neutrophils and macrophages belong to the myeloid lineage, and multiply and differentiate in the bone marrow. In mice, surface expression of Gr-1 and CD11b defines granulocytes, macrophages and dendritic cells (DC), as well as immature myeloid cells (IMCs) having a marker of Gr1+CD11b+ phenotype. In normal mouse bone marrow 20–30% of the cells possess this phenotype, but these cells constitute only a small proportion (2–4%) of spleen cells and are absent from the lymph nodes. Myeloid-derived suppressor cells (MDSCs), which are also Gr1+CD11b+, have been shown to be up-regulated in several pathological conditions, including cancer [5,6] and infection [7–10].
Dextran sulphate sodium (DSS) is used commonly in rodent IBD models to induce acute intestinal inflammation chemically. DSS-induced colitis is characterized by weight loss, bloody diarrhoea, epithelial cell damage and immune cell infiltration. The DSS model faithfully reproduces many of the immunological disturbances observed in human IBD [11,12]. Here we asked whether DSS administration induces Gr1+CD11b+ myeloid cells in spleen and bone marrow.
Materials and methods
Experimental animals
All experiments used C57BL/6NCrSlc (C57BL/6) mice, 8–12 weeks old, purchased from SLC (Japan SLC Inc., Shizuoka, Japan) and maintained at the Animal Research Facility at Nagoya University Graduate School of Medicine under specific pathogen-free conditions. This work was approved by the ethical committee of Nagoya University.
Induction of DSS colitis
Colitis was induced by oral administration of DSS (ICN Biomedical, molecular weight (MW) = 36 000–50 000 Da) at 2% (w/v) in drinking water ad libitum for 5 days followed by normal drinking water. Mice were checked each day for morbidity and weights were recorded. After the mice were killed, colons were dissected, and colon length and spleen weight were measured. Day 0 represents the last day of DSS administration.
Colon histology
At autopsy, 1-cm sections of colon were fixed with octreotide (OCT) compound. Colon sections (5 µm) were stained with haematoxylin and eosin (H&E) according to standard protocol and sections were graded in a blinded fashion according to a scoring system based on a previous study [13,14]. Briefly, a combined score of inflammatory cell infiltration and tissue damage was determined as follows: normal colonic mucosa was considered zero, loss of the bottom one-third of the crypts was graded as one, loss of the bottom two-thirds of the crypts was scored as two, loss of the entire crypt area while retaining an intact surface epithelium was graded as three and loss of both the entire crypt area and surface epithelium (i.e. erosion) was graded as four.
Disease activity index (DAI) scores
To reflect the general condition of the mice, DAI scores were determined by an investigator blinded to the protocol, and based on the extent of body weight loss, stool guaiac positivity or gross bleeding and stool consistency, according to the method of Murthy et al. [13,14]. For each parameter a score of 0 to 4 was attributed, giving rise to a maximal DAI score of 12.
Flow cytometric analysis
Bone marrow and spleen tissues were homogenized to give single cell preparations, which were then isolated, counted and stained with antibodies against myeloid cell markers. All antibodies were purchased from Pharmingen (San Diego, CA, USA). Data were acquired (10 000 events) and lymphocytes separately gated on a side-scatter versus forward-scatter cytogram using a fluorescence activated cell sorter (FACS)Calibur and analysed using FlowJo software (BD Biosciences, Franklin Lake, NJ, USA), Fluorescein isothiocyanate (FITC)-labelled anti-mouseGr-1 (clone RB6-8C5, recognizing both Ly-6G and Ly-6C; BD Biosciences), phycoerythrin (PE)-labelled anti-mouse CD11b (clone M1/70; BD Biosciences), FITC-labelled anti-mouse Ly6G (clone 1A8; BD Biosciences), peridinin chlorophyll-cyananin (PerCP-Cy)5·5-labelled anti-mouse Ly6C (cloneHK1·4; eBioscience) were used in these experiments.
Granulocyte–macrophage colony-stimulating factor (GM-CSF) treatment in vivo
GM-CSF-producing Chinese hamster ovary cells (CHO–GM-CSF) were a gift from Dr T. Sudo, Toray Silicon, Tokyo, Japan. Mice received intraperitoneal (i.p.) injections of 0·1 ml of CHO–GM-CSF supernatant daily for 7 days.
Isolation of splenic Gr-1+/CD11b+ cell populations and lamina propria cells
Single splenocyte suspensions were obtained on day 11. Following red blood cell lysis, splenic Gr-1+/CD11b+ cell populations were purified and collected by flow cytometry (FACS Vantage; BD Biosciences) (>5 × 105 cells). Cells were resuspended at a density of 2·5 × 104 cells/ml in modified Eagle's medium (MEM) supplemented with 10% fetal bovine serum (FBS). In transplantation experiments, a total of 1·5 × 106 Gr1+CD11b+ myeloid-derived splenocytes were administered intravenously to recipient mice (C57BL/6J mice) cytospins. Sorted splenocytes (2 × 105) were resuspended in 200 µl phosphate-buffered saline (PBS) and centrifuged onto microscope slides using a Cytospin-4 (Shandon, Life Sciences International, Astmoor, UK). Slides were then stained by May–Grünwald Giemsa according to standard protocol. Lamina propria cells were isolated as described previously [15]. Briefly, colons were opened longitudinally, digested, and epithelial cells removed and collected into 5-mm ethylenediamine tetraacetic acid (EDTA) PBS. Cells were then incubated with Hanks' balanced salt solution (HBSS) containing 4% FBS, 1 mg/ml collagenase type II, 1 mg/ml dispase and 40 µg/ml DNase, and incubated for 15 min at 37°C. The cells then washed three times and suspended in PBS.
Real-time polymerase chain reaction (PCR) analysis
Total RNA was purified from sorted cells using an RNeasy mini kit (Qiagen, Tokyo, Japan). First-strand cDNA was synthesized using the high-capacity cRNA reverse transcription kit (Applied Biosystems). Real-time PCR reactions were performed using the MX3005p system (Stratagene, Santa Clara, CA, USA) using SYBR premix EX Taq II (Takara, Otsu, Japan). The expression of the β-actin gene was used to normalize the amount of the investigated transcript.
The following primers were used: β-actin (forward) 5′-AGTGTGACGTTGACATCCGT-3′, β-actin (reverse), 5′-GCAGCTCAGTAACAGTCCGC-3′, interleukin (IL)-1β (forward), 5′-GCCCATCCTCTGTGACTCAT-3′, IL-1β (reverse), 5′-AAGGCCAGGTATTTTGTCG-3′, transforming growth factor (TGF)-β (forward), 5′-AAGTGGATCCACGAGCCCAA-3′, TGF-β (reverse), 5′-CTGCACTTGCAGGAGCGCAC-3′, monocyte chemoattractant protein (MCP)-1 (forward), 5′-TGAATGTGAAGTTGACCCGT-3′, MCP-1 (reverse), 5′-AAGGCATCACAGTCCGAGTC-3′, M-CSF receptor (forward), 5′-GACCTGCTCCACTTCTCCAG-3′, M-CSF receptor (reverse), 5′-GGGTTCAGACCAAGCGAGAAG-3′, GM-CSF (forward), 5′-ATGCCTGTCACGTTGAATGA-3′, GM-CSF (reverse), 5′-CCGTAGACCCTGCTCGAATA-3′ and GM-CSF receptor (forward), 5′-ACGGAGGTCACAAGGTCAAG-3′, GM-CSF receptor (reverse), 5′-TGAGGGTCTCAGGGTTCACT-3′.
Results were evaluated on a Lumi Vision Analyzer (Aisinseiki Co., Ltd, Aichi, Japan).
Statistical analysis
Differences were analysed statistically by Student's t-test using StatView5·0 for Windows (SAS Institute, Cary, NC, USA). A P-value of <0·05 was considered to be statistically significant.
Results
Spleen and bone marrow Gr1+CD11b+ cell populations are increased in the spleen and bone marrow of mice with DSS-induced colitis
To investigate DSS-induced epithelial damages and its relationship to myeloid-lineage cells during the disease progression and recovery from DSS-induced colitis, C57BL/6 mice were treated with 2% DSS for 5 days. DAI scores changed during the DSS treatment (Fig. 1a) and colon lengths were found to shorten significantly during the progression of colonic inflammation (Fig. 1b). Disease progression peaked around 5 days after completion of the 5-day DSS treatment. The mice recovered gradually and colon length was back to the starting level at day 10 (Fig. 1b). These findings were associated with marked splenomegaly on day 11 (Fig. 1c). To investigate whether DSS could induce Gr1+CD11b+ cells in the spleen and bone marrow, splenocytes and bone marrow cells were subjected to flow cytometric analysis. The Gr1+CD11b+ cell population in spleens without stimulation was less than 5%. Gr1+CD11b+ cells normally constitute approximately 30–40% of the cells in bone marrow without stimulation. The Gr1+CD11b+ cell population in both the spleen and bone marrow increased steadily from day 11 to day 28. In spleen the Gr1+CD11b+ cell population reached 28·8% on day 28, while the bone marrow Gr1+CD11b+ cell population increased to 75·3% (Fig. 2a). We performed a morphological assay by cytospin and May–Grünwald Giemsa staining, and found that the splenic Gr1+CD11b+ cells from DSS-treated mice had a segmented nuclear morphology characteristic of myeloid-lineage cells and were a heterogeneous population in nature (Fig. 2b).
Fig. 1.
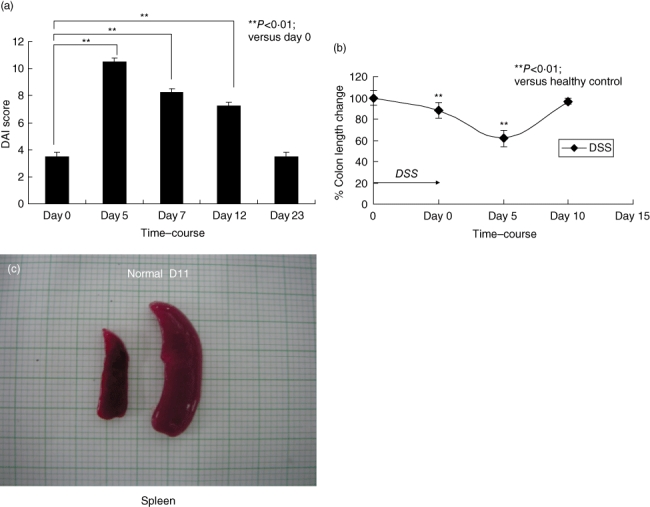
Time–course of dextran sulphate sodium (DSS)-induced acute colitis. (a) Disease activity index (DAI) in C57BL/6 mice treated with 2% DSS for 5 days. DAI is determined by body weight loss, stool consistency and the crypt damage index, as described in Materials and methods. Each value represents the mean ± standard deviation (s.d.) (n = 4 mice). **P < 0·01 (day 0 versus days 5, 7, 12 or 23 mice). (b) Colon length was determined on days 0, 5 and 10. Each value represents the mean ± s.d. (n = 4 mice). **P < 0·01 (normal versus DSS-treated mice). (c) Macroscopic examination of the spleen. Normal indicates untreated control. D11 indicates day 11 after DSS treatment.
Fig. 2.
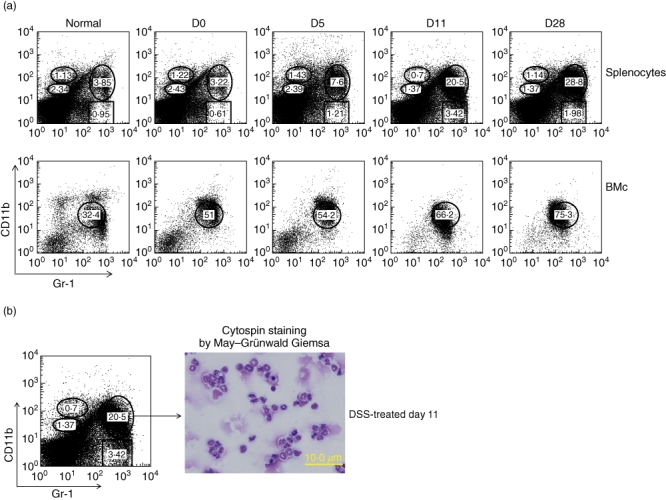
Gr1+CD11b+ cells were markedly increased after dextran sulphate sodium (DSS) treatment. (a) Splenocytes (upper) and bone marrow cells (lower) were isolated from normal and DSS-induced C57BL/6 mice. Cells were stained with fluorescein isothiocyanate (FITC)-labelled anti-Gr1 and phycoerythrin (PE)-labelled anti-CD11b and analysed by fluorescence activated cell sorter (FACS), as described in Materials and methods. Each value represents the mean ± standard deviation (s.d.) (n = 4 mice). **P < 0·01 (day 0 mice versus days 5, 7, 12 or 23 mice). (b) Splenic Gr1+CD11b+ cells were isolated on day 11 of DSS by cell sorting. They were cytospun and stained with May–Grünwald Giemsa. Data are representative of five independent experiments.
Transfer of splenic DSS-derived Gr1+CD11b+ cells into DSS-treated mice ameliorates colitis
Great increases of GR-1+ CD11b+ cells may enhance recovery from colitis. We performed a transplantation experiment whereby DSS-derived splenic Gr1+CD11b+ cells obtained at day 11 were transferred into mice at the end of DSS treatment (day 0) to investigate the function of the DSS-induced Gr1+CD11b+ cells. As shown in Fig. 3, the loss of body weight was less severe and the weight recovery faster in the transplanted mice than in the control DSS-treated mice that did not receive transplanted cells (Fig. 3a). The DAI score of the transplantation group was significantly lower than that of the control group on day 5 (Fig. 3b). The spleen was not enlarged in the transplanted mice (Fig. 3c) and colon length did not decrease in the transplanted group (Fig. 3d). Pathological examination showed that the recovery from epithelial destruction of non-transplanted mice was delayed compared to the transplanted group. Invasion of neutrophils and macrophages to the epithelial layer and lamina propria continued later in the non-transplanted group (Fig. 3e). These results indicated that DSS-treated splenic Gr-1+CD11b+ cells could ameliorate DSS-induced colitis.
Fig. 3.
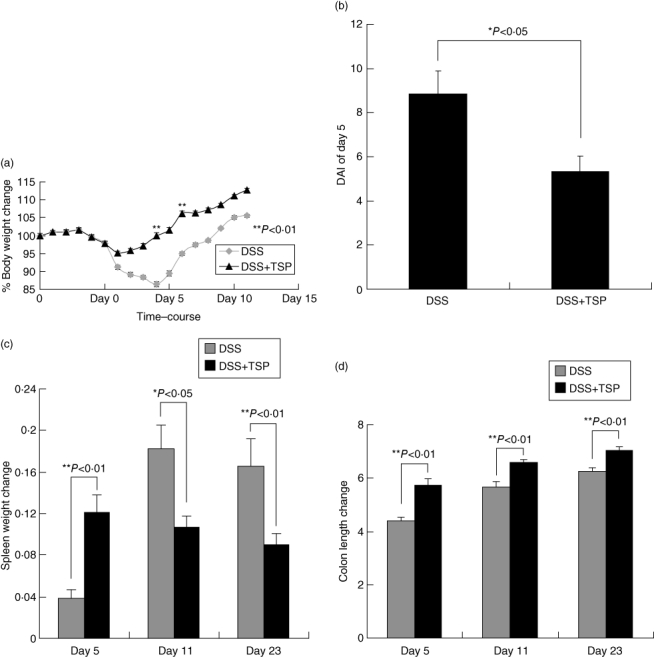
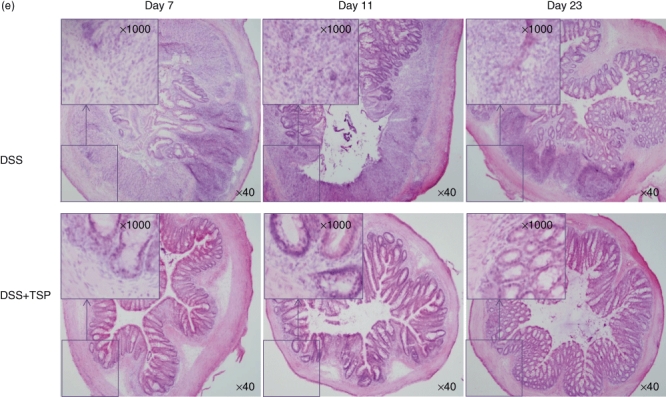
Transplantation of dextran sulphate sodium (DSS)-induced splenic Gr1+CD11b+ cells ameliorated DSS-induced colitis. Gr1+CD11b+ spleen cells from DSS-treated mice were purified on day 11 by sorting. They were administered intravenously to recipient mice on the last day of DSS-treatment at a dose of 1·5 × 106 Gr1+CD11b+ cells per mouse. (a) DAI scores, (b) body weight, (c) spleen weight (d) and colon lengths of non-transplanted and transplanted mice were measured. Each value represents the mean ± standard deviation (s.d.) (n = 4 animals); **P < 0·01; *P < 0·05. DSS versus DSS + TSP mice. (e) Haematoxylin and eosin-staining results of lower part of colon sections from non-transplanted and transplanted mice on days 7, 11 and 23. Results are representative of five independent experiments. Photographs were taken under ×40 and ×1000 magnification. DSS: 2% DSS-treated C57BL/6 mice. DSS + trimethylsilyl propionate (TSP): 2% DSS-treated C57BL/6 transplanted with 1·5 × 106 Gr1+CD11b+ cells.
Increased expression of GM-CSFR in DSS-treated spleen
In order to identify the cause of the expansion of Gr-1+CD11b+ cells, we used real-time PCR to examine the expression of GM-CSF receptor (GM-CSFR) in DSS-treated spleen by real-time PCR. We found that the spleens of day 11 of DSS-treated mice greatly increased their levels of GM-CSFR expression. While the expression of GM-CSFR was also observed in GM-CSF-treated mice, the expression of GM-CSFR was much higher in DSS-treated mice than that of GM-CSF-treated mice. Although GM-CSFR was high, GM-CSF was low in the spleens of day 11 of DSS-treeted mice (Fig. 4). Other cytokines, including IL-1β, TGF-β, chemokine MCP-1 and cytokine receptor MCSF-R, were low in DSS-treated spleens (Fig. 4).
Fig. 4.
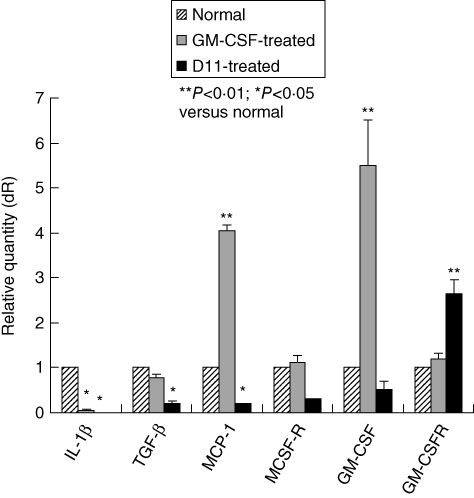
Increased expression of granulocyte–macrophage colony-stimulating factor receptor (GM-CSFR) by Gr1+CD11b+ cells in dextran sulphate sodium (DSS)-treated spleens. Total RNA from splenic Gr1+CD11b+ cells isolated at day 11 was extracted, DNase-treated and converted to cDNA. Real-time polymerase chain reaction (PCR) was performed according to the Materials and methods. Bars indicate mean values of three experiments. Normal indicates untreated control.
Possible mechanism of early recovery from colitis by Gr1+CD11b+ transplantation
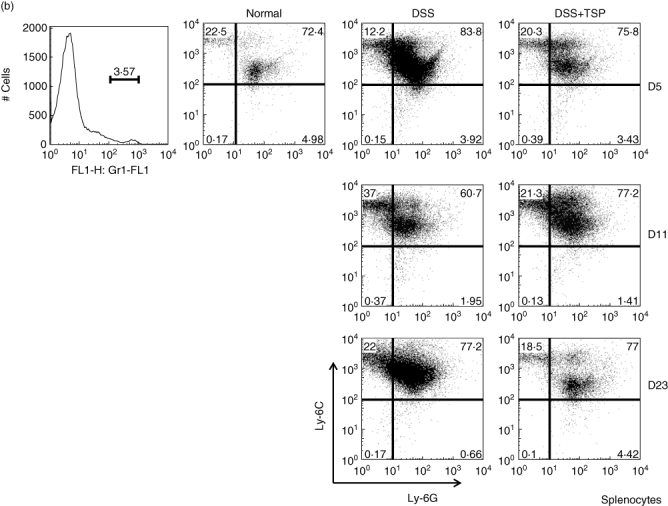
We measured the myeloid cell population in the lamina propria. We found that the CD11b+ single positive cells (CD11b+ Gr1neg) increased from day 5 to day 11 in DSS-treated non-transplanted mice, and decreased at day 23. Surprisingly, the transplantation of splenic Gr1+CD11b+ cells greatly suppressed the migration of CD11b+ single positive cells to the lamina propria (Fig. 5a). We confirmed that the CD11b+ single positive population consisted of Ly6Chigh Ly6Glowmacrophages, which were down-regulated by the transplantation of Gr1+CD11b+ cells (Fig. 5b).
Fig. 5.
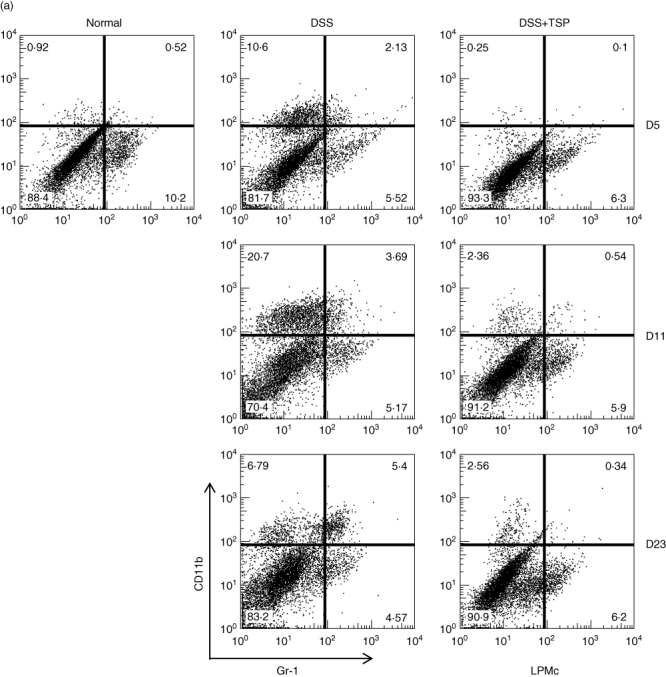
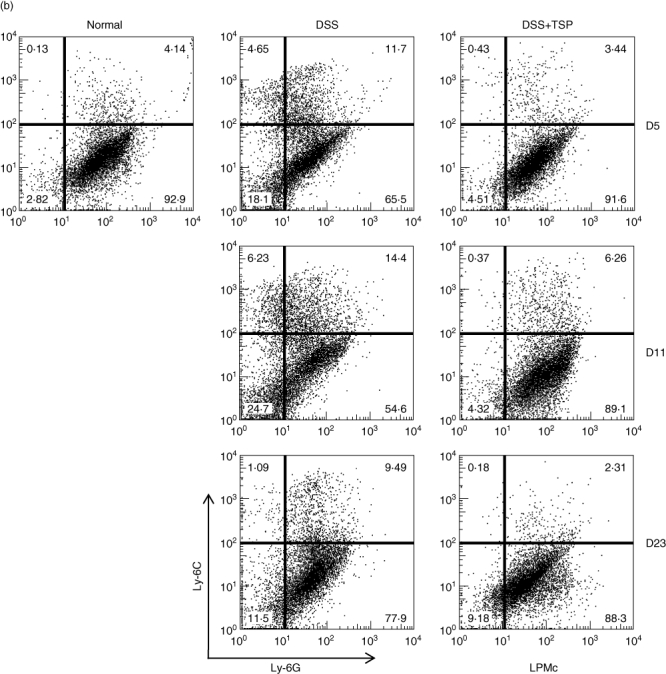
Analysis of lamina propria monocytes derived from non-transplanted and transplanted mice during the experimental time–course. Gr1+ and CD11b+ populations (a) and the Ly6G+ and Ly6C+ populations (b). Frequency fluorescence activated cell sorter (FACS) data are representative of four experiments. Normal: lamina propria cells from control age-matched C57BL/6. Dextran sulphate sodium (DSS): lamina propria cells from 2% DSS-treated C57BL/6 mice. DSS+trimethylsilyl propionate (TSP): lamina propria cells from 2% DSS-treated C57BL/6 transplanted with 1·5 × 106 Gr1+CD11b+ cells.
As shown in Fig. 6a, transplantation of Gr-1+CD11b+ cells greatly suppressed the increase of the same population, especially during the late phase of DSS colitis both in spleen and bone marrow (Fig. 6a and b). In contrast, high levels of Gr-1+CD11b+ cells were maintained in both the spleen and bone marrow of non-transplanted mice after DSS treatment.
Fig. 6.
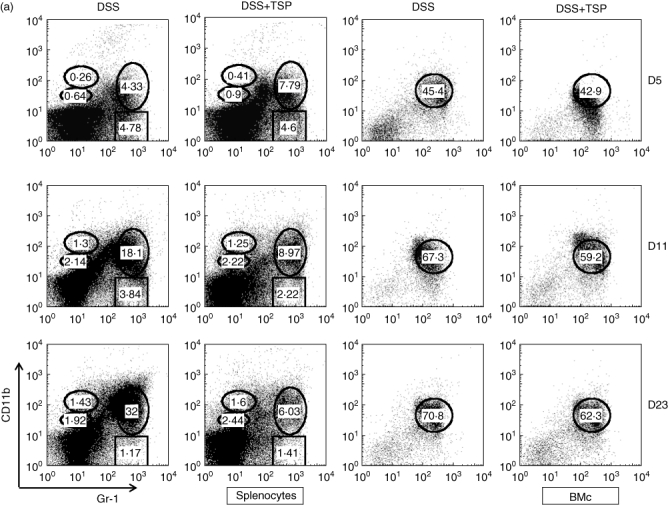
Splenic and bone marrow Gr1+CD11b+ cells from non-transplanted and transplanted mice during the experimental period were investigated by flow cytometry to determined the percentage of Gr1+CD11b+ cells were measured. (a) Spleen. (b) Bone marrow. Data are representative of five independent experiments. Dextran sulphate sodium (DSS): spleen cells from 2% DSS-treated C57BL/6 mice. DSS+trimethylsilyl propionate (TSP): spleen cells from 2% DSS-treated C57BL/6 transplanted with 1·5 × 106 Gr1+CD11b+ cells.
Discussion
Our results showed that the Gr1+CD11b+ cell population was greatly increased in the spleen and bone marrow after DSS treatment. Importantly, transplantation of splenic Gr1+CD11b+ cells to DSS-treated mice during the early phase of disease induction reduced inflammation and promoted efficient colonic mucosal healing. Gr1+CD11b+ cells constitute a heterogeneous population of myeloid-lineage cells, which include MDSC. Increased Gr1+CD11b+ cells in colitis has been reported by Haile et al. [16] in a T cell-dependent murine model of IBD. They also found an increase of CD14+ human leucocyte antigen D-related (HLA-DR)–/low (human homologue of Gr1+CD11b+ cells) in patients with IBD. Haile et al. reported that increased MDSC (Gr1+CD11b+ cells) suppresses T cell responses. However, they found that Gr1+CD11b+ cells were not increased in DSS-treated mice. They concluded that the Gr1+CD11b+ population was increased only in T cell-dependent colitis. It has been shown that acute DSS-induced colitis does not require T or B cells, as it occurs in severe combine immune deficiency (SCID) mice that lack these cell types [17]. Conflicting results between our experiments and those of Haile et al. might be due to the amount and duration of DSS administered and the strain of mice used. We administered 2% DSS in drinking water to C57BL/6 mice for 5 days, while Haile et al. administered DSS on days 0 (5%), 15 (7%) and 27 (7%) to VILLIN-haemagglutinin (HA) mice. The sensitivities of the murine strains to DSS differ greatly between the two strains [18]. We showed that the Gr1+CD11b+ population was increased even in DSS-treated T cell-independent colitis in some condition. MDSCs, which have a Gr1+CD11b+ cell phenotype, are a heterogeneous population of cells that expand during cancer, inflammation and infection, and that have a remarkable ability to suppress T cell responses [19]. Various mouse and human studies have found that MDSCs emerge in the peripheral blood and tumour microenvironment and often exhibit strong immunosuppressive capabilities. The accumulation of MDSCs within either the tumour microenvironment or peripheral blood correlates with a poor prognosis [20,21]. Most studies conducted in tumour-bearing mice or human indicate that Gr1+CD11b+ cells are immunosuppressive. It has been reported that the accumulation of myeloid cells in tumour-bearing mice decreased anti-tumour immune responses and promoted tumour growth [22,23]. Accumulating evidence has shown that Gr1+CD11b+ cells also regulate immune responses during bacterial and parasitic infections, acute and chronic inflammation, traumatic stress, sepsis and transplantation [19]. In experimental autoimmune encephalomyelitis and experimental autoimmune uveoretinitis, an increase in the number of MDSCs was observed in the spleen and blood [24,25]. However, the functions of Gr1+CD11b+ cells are not only immunosuppressive. It has been shown that Gr1+CD11b+ cells found in the ascites of epithelial ovarian cancer-bearing mice at advanced stages of disease are immunostimulatory rather than being immunosuppressive [26].
We showed that myeloid-lineage cells in the lamina propria in DSS-treated mice expressed consisted primarily of CD11b+ (Ly6C+) single positive (Gr-1neg) macrophages and a few Gr1+CD11b+ cells. These results suggest that Gr1+CD11b+ cells may differentiate to CD11b+ single positive cells and migrate to the lamina propria. In an enzyme immunoassay (EAE) model, among Gr1+CD11b+ cells only a small population of CD11b+Ly-6Chigh inflammatory monocytes (IMC) can suppress T cell proliferation efficiently. However, in our DSS-treated colitis, transplantation of Gr1+CD11b+ cells greatly suppressed the increase of CD11b+ cells in the colon. Suppression may be caused by feedback mechanism to inhibit the differentiation of myeloid-lineage cells in the bone marrow.
Splenic Gr1+CD11b+ cells express GM-CSFR during DSS-induced colitis. From these results, we speculate that the increased number of of spleenic Gr1+CD11b+ cells in immature myeloid-lineage cells may increased the number of Gr1+CD11b+ cells. Recent reports showed that GM-CSF-dependent stimulation of bone marrow-derived cells during DSS-induced colitis accelerates colonic tissue repair [27,28].
In summary, our results showed that Gr1+CD11b+ cell number was increased dramatically at the recovery phase of DSS-induced colitis. These cells accelerate the repair of colitis when transplanted at the early phase of colitis.
Acknowledgments
This study was supported entirely by grants from the Ministry of Education, Culture, Sports, Science and Technology, Japan. We thank M. Tanaka for technical support of FACS analysis and cell sorting.
Disclosure
The authors have no disclosures to declare.
References
- 1.Podolsky D. Inflammatory bowel disease. N Engl J Med. 2002;347:417–29. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Xavier R, Podolsky D. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 3.Sartor R. Cytokines in intestinal inflammation: pathophysiological and clinical considerations. Gastroenterology. 1994;106:533–9. doi: 10.1016/0016-5085(94)90614-9. [DOI] [PubMed] [Google Scholar]
- 4.Mahida Y, Wu K, Jewell D. Enhanced production of interleukin 1-beta by mononuclear cells isolated from mucosa with active ulcerative colitis of Crohn's disease. Gut. 1989;30:835–8. doi: 10.1136/gut.30.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ochoa A, Zea A, Hernandez C, Rodriguez P. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–6s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 6.Almand B, Clark J, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–89. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 7.Goñi O, Alcaide P, Fresno M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1(+))CD11b(+)immature myeloid suppressor cells. Int Immunol. 2002;14:1125–34. doi: 10.1093/intimm/dxf076. [DOI] [PubMed] [Google Scholar]
- 8.Giordanengo L, Guiñazú N, Stempin C, Fretes R, Cerbán F, Gea S. Cruzipain, a major Trypanosoma cruzi antigen, conditions the host immune response in favor of parasite. Eur J Immunol. 2002;32:1003–11. doi: 10.1002/1521-4141(200204)32:4<1003::AID-IMMU1003>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 9.Voisin M, Buzoni-Gatel D, Bout D, Velge-Roussel F. Both expansion of regulatory GR1+ CD11b+ myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect Immun. 2004;72:5487–92. doi: 10.1128/IAI.72.9.5487-5492.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delano M, Scumpia P, Weinstein J, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whittem C, Williams A, Williams C. Murine colitis modeling using dextran sulfate sodium (DSS) J Vis Exp. 2010;35:1652. doi: 10.3791/1652. http://www.jove.com/Details.stp?ID=1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okayasu I, Hatakeyama S, Yamada M, Ohkusa T, Inagaki Y, Nakaya R. A novel method in the induction of reliable experimental acute and chronic ulcerative colitis in mice. Gastroenterology. 1990;98:694–702. doi: 10.1016/0016-5085(90)90290-h. [DOI] [PubMed] [Google Scholar]
- 13.Murthy S, Cooper H, Shim H, Shah R, Ibrahim S, Sedergran D. Treatment of dextran sulfate sodium-induced murine colitis by intracolonic cyclosporin. Dig Dis Sci. 1993;38:1722–34. doi: 10.1007/BF01303184. [DOI] [PubMed] [Google Scholar]
- 14.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 15.Weigmann B, Tubbe I, Seidel D, Nicolaev A, Becker C, Neurath M. Isolation and subsequent analysis of murine lamina propria mononuclear cells from colonic tissue. Nat Protoc. 2007;2:2307–11. doi: 10.1038/nprot.2007.315. [DOI] [PubMed] [Google Scholar]
- 16.Haile L, von Wasielewski R, Gamrekelashvili J, et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology. 2008;135:871–81. 81.e1–5. doi: 10.1053/j.gastro.2008.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Dieleman L, Ridwan B, Tennyson G, Beagley K, Bucy R, Elson C. Dextran sulfate sodium-induced colitis occurs in severe combined immunodeficient mice. Gastroenterology. 1994;107:1643–52. doi: 10.1016/0016-5085(94)90803-6. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki S, Ishida Y, Nishio N, Ito S, Isobe K. Thymic involution correlates with severe ulcerative colitis induced by oral administration of dextran sulphate sodium in C57BL/6 mice but not in BALB/c mice. Inflammation. 2008;31:319–28. doi: 10.1007/s10753-008-9081-3. [DOI] [PubMed] [Google Scholar]
- 19.Gabrilovich D, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murdoch C, Muthana M, Coffelt S, Lewis C. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 21.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seung L, Rowley D, Dubey P, Schreiber H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc Natl Acad Sci USA. 1995;92:6254–8. doi: 10.1073/pnas.92.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pekarek L, Starr B, Toledano A, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–40. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr E, Raveney B, Copland D, Dick A, Nicholson L. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun. 2008;31:354–61. doi: 10.1016/j.jaut.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Tomihara K, Guo M, Shin T, et al. Antigen-specific immunity and cross-priming by epithelial ovarian carcinoma-induced CD11b(+)Gr-1(+) cells. J Immunol. 2010;184:6151–60. doi: 10.4049/jimmunol.0903519. [DOI] [PubMed] [Google Scholar]
- 26.Zhu B, Bando Y, Xiao S, et al. CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol. 2007;179:5228–37. doi: 10.4049/jimmunol.179.8.5228. [DOI] [PubMed] [Google Scholar]
- 27.Bernasconi E, Favre L, Maillard M, et al. Granulocyte–macrophage colony-stimulating factor elicits bone marrow-derived cells that promote efficient colonic mucosal healing. Inflamm Bowel Dis. 2010;16:428–41. doi: 10.1002/ibd.21072. [DOI] [PubMed] [Google Scholar]
- 28.Sainathan S, Hanna E, Gong Q, et al. Granulocyte–macrophage colony-stimulating factor ameliorates DSS-induced experimental colitis. Inflamm Bowel Dis. 2008;14:88–99. doi: 10.1002/ibd.20279. [DOI] [PMC free article] [PubMed] [Google Scholar]


