Abstract
This study was designed to replicate and extend past research examining the impact of the serotonin transporter gene-linked polymorphic region (5HTTLPR) on neural activation during emotional processing. Six women with at least one short allele were compared to six age-matched women with long/long alleles of the 5HTTLPR. Participants were shown 36 positive and 36 negative slides from the International Affective Picture Set, while functional images were acquired using a 4-T magnetic resonance imaging scanner. Although we were unable to replicate past research demonstrating relatively increased amygdala activation among individuals with an “s” allele to negative stimuli, women with an s allele evidenced decreased left fusiform gyrus activation to positive emotional stimuli (as expected). We suggest that women with a short allele may be either less attentive or less “expert” with regard to positive emotional stimuli, and ideas for future research are presented.
Keywords: 5HTTLPR, fMRI, Emotion, Processing, Depression, Anxiety
1. Introduction
While it is clear that neurotransmission within serotonergic (5-HT) brain pathways play an important role in major depressive disorder and generalized anxiety disorder [1–5], very little is known about the pathogenesis of such disorders. Research has focused heavily on environmental factors, particularly on early life stressors such as childhood neglect, physical or sexual abuse, and early parental loss [6]. More recently, however, the central issue has been one of susceptibility. Why is it that some people are more prone to developing a mood disorder than others? Why do some people who experience early life stressors appear to adjust reasonably well while others develop disorders that interfere with everyday functioning?
Based on extensive longitudinal data, Caspi et al. [7] have outlined a compelling body of evidence that an individual’s sensitivity to major life stressors is significantly impacted by their genetic makeup. Specifically, the functional polymorphism in the regulatory region of the serotonin transporter gene-linked polymorphic region (5HTTLPR) has been implicated in the etiology of both anxiety and depression [8–10]. Descriptively, the polymorphism is a 44-bp insertion (designated as the “l” allele) or deletion (designated as the “s” allele) in the transcriptional control region on chromosome 17q11.1-q12 [9]. This particular polymorphism is of great interest in part because of the widespread pharmacological treatments known as selective serotonin reuptake inhibitors, used to treat depression [11] and anxiety [12,13].
There are 3 possible genotypes for the 5HTTLPR – long-long (l/l), short-long (s/l), and short-short (s/s). Pathogenically, the 5HTTLPR has been implicated in both anxiety and depression. For example, Lesch et al. [10] found that individuals with either one or two copies of the s allele showed greater levels of personality traits (e.g., neuroticism, hostility, and depression) implicated in anxiety and depression compared to those who were homozygous for the l allele. These data have been subsequently replicated and extended [14,15], including research suggesting a positive relationship between the s allele and suicidal behavior [16,17]. These relationships were further strengthened by suicide postmortem studies which showed a diffuse reduction of serotonergic transporter binding in the prefrontal cortex of individuals with depression [18].
Given that animal and human research has implicated the s/s and s/l 5HTT polymorphisms with anxiety [10,19–21] and depression [7,19,21–23], it is not surprising that the 5HTTLPR also predicts affective response to emotional stimuli. Hariri et al. [24], e.g., compared individuals with l/l homozygous alleles (n=14) to persons with at least one s-allele (n=14) using blood oxygen level-dependent functional magnetic resonance imaging (fMRI). They found that, during an emotional processing task, those with an s allele experienced significantly greater amygdaloid activation compared to the l/l homozygous group. Because both fear-and anger-inducing stimuli, relative to neutral stimuli, elicit greater amygdaloid activation among healthy individuals (e.g., Refs. [25–27]), these data are consistent with the notion that persons with the s allele experience greater emotional responding to negative stimuli. Moreover, because increased amygdala activation to negative stimuli has been documented among high- versus low-anxious individuals within both healthy [28] and clinical populations [29–32], Hariri et al.’s [24] data are also consistent with the notion that persons with the s allele are more anxious/threat-sensitive than their l/l homozygous counterparts.
The glaring void in this literature is the lack of research investigating the impact of the 5HTT polymorphism on neural processing of positive emotional stimuli, despite the fact that the s allele predicts depression symptomatology [7,19,21–23]. Investigating the depression-related aspects of the 5HTT polymorphism is of critical importance. First, depression is the single leading cause of disability worldwide [33] and affects about 9.5% of Americans aged 18 years or older in any given year [34]. Second, although other mechanisms may underlie disease onset [35–38], depression symptomatology has been linked to altered 5-HT neurotransmission or its postsynaptic cellular events [39–44]. Last, anxiety and depression disorders are highly comorbid (>50% [45,46]), highlighting the importance of the 5HTT polymorphism because it predicts both types of symptomatology. Of note, both depression and anxiety disorders appear to be caused by abnormal neural serotonergic/noradrenergic functioning (for a review, see Ref. [47]).
In addition to having increased neural response to negative stimuli, persons with the s/s or s/l 5HTTLPR may be expected to have reduced neural reactivity to positive stimuli because of its role in producing depressive symptomatology [7,19,21–23]. Persons with elevated levels of depression symptomatology (both at clinical and subsyndromal levels) experience reduced response to reward (e.g., Refs. [48,49]) and have a blunted affective response to pleasant stimuli [48,50–54]. Moreover, depressed individuals are more apt to identify neutral faces as sad [55,56] and happy faces as neutral [56,57].
Only one study to date has investigated the impact of the 5HTT polymorphism on neural activation in response to positive stimuli. Specifically, Heinz et al. [58] compared amygdaloid activation among healthy s/s, s/l and l/l participants in response to positive, negative and neutral slides. After controlling for activation in response to neutral stimuli, persons with an s allele were found to experience significantly greater amygdala activation to negative (but not positive) stimuli relative to the l/l homozygous group. A limitation of this study, however, is that analyses were limited to data collected within the amygdala: This is especially problematic when one considers that amygdaloid activation has been much more closely associated with the processing of negative [25–27] relative to positive stimuli (e.g., Ref. [59]). Other recently presented data, however, suggest that persons with at least one s allele evidence increased and decreased neural response to the presentation of negative and positive words, respectively [60].
Positive mood induction has been associated with neural activation within several brain regions in addition to the amygdala. One meta-analysis incorporating 106 positron emission tomography or fMRI studies revealed that happy mood induction reliably elicits activation within the rostral supracallosal anterior cingulate cortex (ACC) and dorsomedial prefrontal cortex (DPFC) as well as within the amygdala [61,62]. To date, one study has compared neural activation among healthy versus depressed individuals to positive stimuli (faces) [63]. Interestingly, unlike healthy persons, Surguladze et al. [57,63] found that depressed individuals failed to show activation of the right putamen and bilateral fusiform gyri (responsible for facial perception) to happy faces. Accordingly, each of these regions is included as a region of interest (ROI) in determining the impact of the 5HTT polymorphism on negative and positive emotion induction.
The purpose of the present study was to determine whether persons with an s allele experience greater neural activation to negative visual stimuli and decreased neural activation to positive stimuli. By better understanding neural processing among persons with different genotypes, the present research may aid in understanding both genotypic and phenotypic differences with regard to anxiety/depression susceptibility. Specific hypotheses included the following: persons with an ‘s’ allele will evidence greater activation within the amygdala to negative/threat stimuli relative to those who are l/l homozygous, and relative to those who are l/l homozygous, persons with an ‘s’ allele will evidence decreased activation to positive stimuli within the amygdala, rostral supracallosal anterior cingulate cortex (ACC), dorsomedial prefrontal cortex (DPFC), right putamen and bilateral fusiform gyri.
2. Experimental procedures
This research was approved by the University Hospitals of Cleveland Institutional Review Board. Twelve right-handed, female adult participants were included in the fMRI portion of the experiment. Only women were incorporated into the present research because men and women have been found to differentially process emotional stimuli (e.g., Ref. [64]). Participants were recruited via fliers placed around a University community and were compensated $100 for their time and effort during the research. Participants first completed the Coren, Porac and Duncan Laterality questionnaire (cutoff of +5, Coren et al. [65]) to determine hemi-body preference. If the participant did not have sufficient right hemi-body preference, she was excluded from the study. All other participants were administered the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision [66] to ascertain diagnostic status or lack thereof. In addition, the Beck Depression Inventory [67] (cutoff of 16) and State Trait Anxiety Inventory [68] (cutoff of 45) were completed. Based on these psychiatric measures, if the participant evidenced symptomatology consistent with a psychiatric disorder, her participation in the experiment ceased (the participant was informed of her symptomatology and, if she was interested, treatment referrals were offered). Otherwise, approximately 5 ml of blood was collected at the General Clinical Research Center from eligible women for genetic testing. From the original sample of 17 participants, 12 were enrolled into the fMRI portion of the study — six participants with at least one short allele of the 5HTTLPR polymorphism and six participants with two long alleles. Participants were matched with regard to age and ethnicity.
3. fMRI protocol
Images were acquired on a 4-T Bruker Whole Body Medspec MR system. The 4T scanner was equipped with an MRI-compatible audio/visual system (Avotec) developed for stimulus presentation used in the fMRI studies. Stimuli were back-projected onto a screen in the scanner, and participants viewed pictures through an angled mirror attached to the head coil. High-resolution T1-weighted (T1W) 3D anatomical data were first acquired using a 3D magnetization prepared rapid gradient echo. Repetition time (TR)=2500 ms, echo time (TE)=3.52 ms, inversion time = 1200 ms; excitation flip angle 12°, field of view=256×224 mm, 256×224 data matrix, 176 slices, slice/partition thickness of 1 mm, resulting in voxel size of 1×1×1 mm. Next, high resolution T1W 2D radio frequency spoiled gradient echo (GE) images were acquired in an obliqued axial plane aligned so as to be parallel to the anterior commissure/posterior commissure line at slice locations identical to those used in the subsequent fMRI studies. Acquisition parameters were as follows: TR=340 ms, TE=3.77 ms; 240 mm×240 mm field of view, 256×256 matrix, excitation flip angle=45°, 44 slices, slice thickness of 3 mm contiguous (no gap) slices, resulting in voxel size of 0.9375×0.9375×3 mm. These images were used during post processing to facilitate echo planar imaging (EPI) to 3D coregistration. Lastly, during the affective fMRI portion of the study, functional images were acquired using a T2*-weighted GE EPI sequence (TR=2000 ms, TE=18 ms; field of view=240 mm×240 mm, 64×64 data matrix, slice thickness of 3 mm, no gap between slices, resulting in voxel size of 3.75×3.75×3 mm; flip angle=90°). Forty-four slices, with scan plane orientation and slice locations identical to those used to collect the high resolution 2D T1W images, were acquired in an interleaved fashion. In total, 122 44-slice volumes were collected for each functional run.
The affective portion of the experiment consisted of four runs, each 4 min and 4 s in duration, with a 2-min rest in between runs. In each run, six blocks of three affective stimuli were presented for 6 s each (i.e., each block=18 s) with an 18-s fixation point (a “+”) presented in between. There was also a 10-s blank screen at the beginning of each functional run. Two runs included pictures that were only positive in valence, whereas the other two runs included pictures only negative in valence (please see Fig. 1 for a schematic representation of a run with positively valenced pictures). Half of the participants (within each genetic group) saw the runs in the positive-negative-positive-negative sequence, whereas the others saw it in the reverse sequence. The 36 affectively positive and 36 affectively negative slides were selected from the International Affective Picture System [69]. The positive and negative slides were selected to maximize affective valence (scale from 1=unpleasant to 9=pleasant; positive pictures: 7.88±0.22; negative pictures: 1.74±0.15).
Fig. 1.
A schematic representation of a positively valenced run.
Prior to the experiment, participants partook in a training session at a mock scanner to get acclimated to the neuroimaging environment and become familiarized with the task. Participants were instructed to passively maintain their focus on the slides presented.
4. Results
4.1. Participants
Six women with an s allele and six women with the l/l genotype participated in the fMRI portion of the experiment. All women were Caucasian and were matched with regard to age [F(1,10)=.014, P>.90 (‘s’ group mean=28.50, S.D.=14.49, range=21–58; l group mean=27.50, S.D.=14.65, range=18–57)].
4.2. fMRI Results
Imaging data were analyzed with BrainVoyager QX (Version 1.7). Preprocessing included 3D motion correction, intrascan spatial realignment, spatial filtering (full-width half-max 7.5 mm), linear trend removal and normalization into Talairach Space.
In the first step of hypothesis testing, brain areas activated in response to negative and positive pictures were identified using whole brain analysis. t Test contrasts between picture condition and fixation condition was performed for negative and positive picture conditions, respectively. Data from all twelve participants were all included in the analyses. Compared to the baseline (fixation point) condition, watching negative pictures activated bilateral fusiform gyrus, right lingual gyrus, left middle occipital gyrus and left inferior occipital gyrus. Bilateral amygdala were also activated but at a less significant level (please see Table 1).
Table 1.
Details of brain regions showing more activation under negative-picture condition relative to baseline condition (all uncorrected Ps<.0006)
| VOI | No. of voxels | Mean t value | Mean Talairach coordinates
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Left fusiform gyrus 1** | 87 | 16.492747 | −32 | −46 | −15 |
| Left fusiform gyrus 2** | 53 | 14.695091 | −29 | −66 | −20 |
| Right fusiform gyrus 1** | 156 | 14.686855 | 26 | −42 | −16 |
| Right fusiform gyrus 2** | 37 | 13.885775 | 26 | −85 | −12 |
| Right fusiform gyrus 3** | 112 | 14.6054 | 33 | −51 | −18 |
| Right fusiform gyrus 4** | 39 | 14.855031 | 26 | −77 | −20 |
| Right lingual gyrus** | 752 | 17.523182 | 2.9 | −80 | −11 |
| Left middle occipital gyrus** | 214 | 15.057266 | −39 | −81 | −7.6 |
| Left inferior occipital gyrus** | 634 | 11.923223 | −34 | −85 | −11 |
| Right amygdala* | 29 | 4.863636 | 24 | −4.4 | −14 |
| Left amygdala* | 369 | 5.680529 | −21 | −3.4 | −11 |
VOI, voxels of interest.
P<.0006.
P<3.24e-08.
Compared to the baseline condition, positive pictures involved activation of the bilateral lateral geniculate nuclei, bilateral lingual gyri, bilateral fusiform gyri, right middle occipital gyrus and right middle temporal gyrus (see Table 2). Again, bilateral amygdala were activated but at a less significant level.
Table 2.
Details of brain regions showing more activation under positive-picture condition relative to baseline condition (all uncorrected Ps<.0003)
| VOI | No. of voxels | Mean t value | Mean Talairach coordinates
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| LGN** | 547 | 11.716177 | 22 | −28 | −2.4 |
| Left LG** | 314 | 11.148729 | −24 | −25 | −2.5 |
| Right lingual gyru** | 366 | 13.483659 | 5.1 | −80 | −12 |
| Left lingual gyru** | 274 | 12.640711 | −9.4 | −71 | −12 |
| Right fusiform gyrus 1** | 44 | 11.301743 | 47 | −69 | −14 |
| Right fusiform gyrus 2** | 31 | 11.436368 | 36 | −49 | −20 |
| Right fusiform gyrus 3** | 33 | 11.527119 | 27 | −75 | −20 |
| Right fusiform gyrus 4** | 425 | 11.888366 | 26 | −40 | −11 |
| Left fusiform gyrus** | 49 | 11.336004 | −33 | −46 | −14 |
| Right middle occipital gyrus** | 185 | 11.679367 | 34 | −79 | 7.7 |
| Right middle temporal gyrus 1** | 225 | 10.749715 | 44 | −62 | 4 |
| Right amygdala* | 376 | 7.985857 | 22 | −2.4 | −12 |
| Left amygdala* | 312 | 5.370686 | −24 | −0.95 | −18 |
LGN, right lateral geniculate nucleus.
P<.0003.
P<4.9891e-07.
Next, in order to test group differences of brain activity in response to different emotional pictures, t test comparisons between the two genotype groups (s/s and s/l vs. l/l) were performed using data from positive- and negative-picture conditions, respectively.
Compared to participants in the l/l homozygous group, participants with at least one short allele (s/s or s/l) showed greater activation in left lingual gyrus, right frontal gyri, right precentral gyrus and right precuneus, but less activation in left middle frontal gyrus and right middle temporal gyrus in response to negative pictures (please see Table 3). Contrary to a priori expectations, there was no group difference in amygdaloid activation. It is important to note that none of these brain areas were significantly affected by negative picture viewing (see Table 1), and hence, these group differences are not believed to be associated with differential emotional response.
Table 3.
Details of brain regions showing significant activation difference (all uncorrected Ps<.0023) across two groups (s/s and s/l vs. l/l) in response to negative pictures
| VOI | No. of voxels | Mean t value | Mean Talairach coordinates
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Left lingual gyrus | 40 | 4.409747 | −8.5 | −67 | −14 |
| Left middle frontal gyrus | 52 | −4.231740 | −29 | 43 | 24 |
| Right superior frontal gyrus | 92 | 4.298643 | 13 | 44 | 39 |
| Right middle frontal gyrus | 104 | 4.816371 | 39 | 41 | 15 |
| Right inferior frontal gyrus | 48 | 4.228783 | 56 | 14 | 15 |
| Right precentral gyrus | 85 | 4.438008 | 51 | −0.25 | 45 |
| Right middle temporal gyrus | 113 | −4.340642 | 63 | −47 | −5.2 |
| Right precuneus 1 | 295 | 4.396760 | 15 | −62 | 25 |
| Right precuneus 2 | 34 | 4.077823 | 8.1 | −66 | 44 |
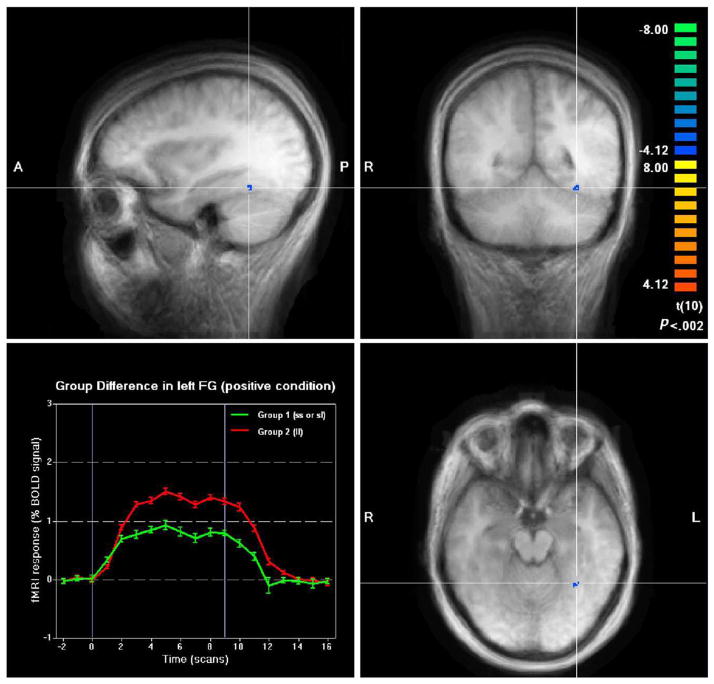
For positive stimuli, participants with at least one short allele (s/s or s/l) showed less activation in left fusiform gyrus, left superior temporal gyrus and left supramarginal gyrus, but greater activation in right insula and right medial frontal gyrus compared to their l/l counterparts (please see Table 4). Among those brain areas, only the left fusiform gyrus was found to be activated to positive pictures (see Table 2), and thus appears to be a region where positive emotion differentially affected people with the s/s and s/l versus l/l genotypes. The direction of the effect on the left fusiform gyrus is consistent with a priori hypotheses. Specifically, relative to those who are l/l homozygous, persons with an s allele exhibited decreased activation to positive stimuli (please see Fig. 2). Contrary to expectations, however, no significant group differences were found within the amygdala, rostral supracallosal ACC, DPFC and right putamen.
Table 4.
Details of brain regions showing significant differences in activation (all uncorrected Ps<.002) between two genotypes (s/s and s/l vs. l/l) in response to positive pictures
| VOI | No. of voxels | Mean t value | Mean Talairach coordinates
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Left fusiform gyrus | 52 | −4.265178 | −32 | −49 | −16 |
| Left superior temporal gyrus | 18 | −4.571856 | −40 | 9.8 | −14 |
| Right insula | 22 | 4.495863 | 42 | −22 | 18 |
| Right medial frontal gyrus | 123 | 4.745180 | 4 | 48 | 17 |
| Left supramarginal gyrus | 90 | −5.043974 | −42 | −38 | 34 |
Fig. 2.
Group differences in left FG under positive-picture condition. FG, fusiform gyrus.
5. Discussion
The present research was designed to replicate and extend past research investigating the impact of the 5HTTLPR on neural activation in response to emotional stimuli. Although we were unable to find differences between s/s and s/l versus l/l groups within regions of interest in response to negative stimuli, our ability to detect such differences may have been attenuated by small sample size.
Our most important finding was in response to positive emotional stimuli. Specifically, relative to l/l homozygous individuals, participants with an s allele evidenced decreased activation in the left fusiform gyrus while attending to positive affective pictures. The fusiform gyrus, commonly known as the “fusiform face area” (FFA), is a region that shows robust activation among persons viewing faces [70,71]. It is important to note that a large number of faces were included among the 40 negative and 40 positive slides used in this study (negative: 35 human faces, 2 animal faces; positive: 25 human, 7 animal). Two subsequent discoveries involving the FFA may help us appreciate why persons with an s allele show reduced activation in that region while viewing positive faces.
First, it has been demonstrated that the FFA is more sensitive to stimuli that can be discretely categorized at a subordinate level (as opposed to stimuli which may only be broadly labeled, e.g., Refs. [72,73]). Of note, one’s ability to discretely categorize objects is associated with his/her expertise in the area. For example, FFA activation is increased the more expert the person is with regard to the object, be they cars, birds, or face-like, but nonhuman, “Greebles” (e.g., Refs. [74–76]). This raises the possibility that persons with an s-allele may be less “expert” with regard to positive (but not negative) emotional faces and are perhaps relatively unable to categorize such faces into discrete emotions (e.g., happy, amused, excited, etc.). Behavioral work assessing the impact of the 5HTTLPR on positive and negative face recognition and categorization would help test this notion. Second, FFA activation has been found to depend on one’s level of attention towards the visual stimuli. For example, decreased FFA activation to faces among autistic versus typically developing individuals results from the former group’s decreased gaze towards faces (e.g., Ref. [77]). Thus, another possibility is that persons with an s allele do not attend to positive (but not negative) stimuli as much as their l/l counterparts. Either as part of fMRI procedures or as behavioral research, the use of eye tracking instrumentation would test this link. It is important to note, however, that these two possibilities are not mutually exclusive and, in fact, may result from the other: For example, decreased attention towards positive faces may reduce one’s development of expertise for such stimuli.
The present work has both strengths and limitations. In terms of strengths, the participants in this research were age- and ethnicity-matched and had no reported psychiatric illness. Moreover, fMRI procedures included both positive and negative affective stimuli, with a priori ROIs involving areas heavily recruited by emotional stimuli. In terms of limitations, power was relatively low, all participants were Caucasian, no eye tracking device was used and behavioral data (e.g., self-reported affective response or identification of stimuli) were not collected. Given the relatively low power, even significant findings in the present research should be interpreted with caution. Each of these limitations should be rectified as part of future research, with the latter two being especially important to determine the primary cause of the present findings.
Footnotes
This research was made possible by a generous grant to HAD by the Department of Radiology, University Hospitals of Cleveland. In addition, this publication was made possible by Grant Number M01 RR00080 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH.
References
- 1.Asberg M, Thoren P, Traskman L, Bertilsson L, Ringberger V. “Serotonin depression” — a biochemical subgroup within the affective disorders? Science. 1976;191:478–80. doi: 10.1126/science.1246632. [DOI] [PubMed] [Google Scholar]
- 2.Coppen A, Eccleston EG, Peet M. Total and free tryptophan concentration in the plasma of depressive patients. Lancet. 1973;2:60–3. doi: 10.1016/s0140-6736(73)93259-5. [DOI] [PubMed] [Google Scholar]
- 3.Cowen PJ, Parry-Billings M, Newsholme EA. Decreased plasma tryptophan levels in major depression. J Affect Disord. 1989;16:27–31. doi: 10.1016/0165-0327(89)90051-7. [DOI] [PubMed] [Google Scholar]
- 4.Healy D, Leonard BE. Monoamine transport in depression: kinetics and dynamics. J Affect Disord. 1987;12:91–103. doi: 10.1016/0165-0327(87)90001-2. [DOI] [PubMed] [Google Scholar]
- 5.Meltzer HY, Maes M. Effects of ipsapirone on plasma cortisol and body temperature in major depression. Biol Psychiatry. 1995;38:450–7. doi: 10.1016/0006-3223(94)00370-i. [DOI] [PubMed] [Google Scholar]
- 6.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 7.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–90. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 8.Heils A, Teufel A, Petri S, Stober G, Riederer P, Bengal D, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66:2621–4. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 9.Lesch KP, Balling U, Gross JJ, Strauss K, Wolozin BL, Murphy DL, et al. Organization of the human serotonin transporter gene. J Neural Transm. 1994;95:157–62. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- 10.Lesch KP, Bengal D, Heils A, Sabol SZ, Greenberg BD, Petri S, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–31. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 11.Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. [11C]-DASB, a tool for in vivo measurement of SSRI-induced occupancy of the serotonin transporter: PET characterization and evaluation in cats. Synapse. 2003;47:123–33. doi: 10.1002/syn.10155. [DOI] [PubMed] [Google Scholar]
- 12.Ball SG, Kuhn A, Wall D, Shekhar A, Goddard AW. Selective serotonin reuptake inhibitor treatment for generalized anxiety disorder: a double-blind, prospective comparison between paroxetine and sertraline. J Clin Psychiatry. 2005;66(1):94–9. doi: 10.4088/jcp.v66n0113. [DOI] [PubMed] [Google Scholar]
- 13.Kampman M, Keijsers GPJ, Hoogduin CAL, Jendriks GJ. A randomized, double-blind, placebo-controlled study of the effects of adjunctive paroxetine in panic disorder patients unsuccessfully treated with cognitive-behavioral therapy alone. J Clin Psychiatry. 2002;63(9):772–7. doi: 10.4088/jcp.v63n0904. [DOI] [PubMed] [Google Scholar]
- 14.Greenberg BD, Li Q, Lucas FR, Hu S, Sirota LA, Benjamin J, et al. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. Am J Med Genet. 2000;96:202–16. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Mazzanti CM, Lappalainen J, Long JC, Bengel D, Naukkarinen H, Eggert M, et al. Role of the serotonin transporter promoter polymorphism in anxiety related traits. Arch Gen Psychiatry. 1998;55:936–40. doi: 10.1001/archpsyc.55.10.936. [DOI] [PubMed] [Google Scholar]
- 16.Bellivier F, Szoke A, Henry C, Lacoste J, Bottos C, Nosten-Bertrand M, et al. Possible association between serotonin transporter gene polymorphism and violent suicidal behavior in mood disorders. Biol Psychiatry. 2000;48:319–22. doi: 10.1016/s0006-3223(00)00891-x. [DOI] [PubMed] [Google Scholar]
- 17.Bondy B, Erfurth A, de Jonge S, Kruger M, Meyer H. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Mol Psychiatry. 2000;5:193–5. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- 18.Mann JJ, Huang YY, Underwood MD, Kassir SA, Oppenheim S, Kelly TM, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal binding in major depression and suicide. Arch Gen Psychiatry. 2000;57:729–38. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- 19.Holmes A, Murphy DL, Crawley JN. Abnormal behavior phenotypes of serotonin transporter knockout mice: parallels with human anxiety and depression. Biol Psychiatry. 2003;54:953–9. doi: 10.1016/j.biopsych.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Jacob CP, Strobel A, Hohenberger K, Ringel T, Gutknecht L, Reif A, et al. Association between allelic variation of serotonin transporter function and neuroticism in anxious cluster C personality disorders. Am J Psychiatry. 2004;161:569–72. doi: 10.1176/appi.ajp.161.3.569. [DOI] [PubMed] [Google Scholar]
- 21.Neumeister A, Young T, Stastny J. Implications of genetic research on the role of the serotonin in depression: emphasis on the serotonin type 1A receptor and the serotonin transporter. Psychopharmacology. 2004;174:512–24. doi: 10.1007/s00213-004-1950-3. [DOI] [PubMed] [Google Scholar]
- 22.Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002;59(7):613–20. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- 23.Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, Goodwin GM, et al. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–3. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- 24.Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, et al. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297(5580):400–2. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- 25.Kessler-West ML, Andersen AH, Smith CD, Avison MJ, Davis CE, Kryscio RJ, et al. Neural substrates of facial emotion processing using fMRI. Cogn Brain Res. 2001;11(2):213–26. doi: 10.1016/s0926-6410(00)00073-2. [DOI] [PubMed] [Google Scholar]
- 26.Stark R, Schienle A, Walter B, Kirsch P, Sammer G, Ott U, et al. Hemodynamic responses to fear and disgust-inducing pictures: an fMRI study. Int J Psychophysiol. 2003;50(3):225–34. doi: 10.1016/s0167-8760(03)00169-7. [DOI] [PubMed] [Google Scholar]
- 27.Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1(1):70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- 28.Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. J Neurosci. 2004;24(46):10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birbauer N, Grodd W, Diedrich O, Klose U, Erb M, Lotze M, et al. FMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–6. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- 30.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldsteain M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2004;57:464–73. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 31.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 32.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. The World Health Report 2001 — mental health: new understanding, new hope. Geneva: World Health Organization; 2001. [Google Scholar]
- 34.National Institute of Mental Health. The invisible disease: depression. Bethesda (Md): National Institute of Mental Health, National Institutes of Health, US Department of Health and Human Services; 2001. [Google Scholar]
- 35.Bunney WE, Jr, Davis JM. Norepinephrine in depressive reactions: a review. Arch Gen Psychiatry. 1965;13:483–94. doi: 10.1001/archpsyc.1965.01730060001001. [DOI] [PubMed] [Google Scholar]
- 36.Fava M. The role of serotonergic and noradrenergic neurotransmitter systems in the treatment of psychological and physical symptoms of depression. J Clin Psychiatry. 2003;64(13):26–9. [PubMed] [Google Scholar]
- 37.Nelson JC, Mazure CM, Jatlow PI, Bowers MB, Jr, Price LH. Combining norepinephrine and serotonin reuptake inhibition mechanisms for treatment of depression: a double-blind, randomized study. Biol Psychiatry. 2004;55(3):296–300. doi: 10.1016/j.biopsych.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. Am J Psychiatry. 1965;33:811–91. doi: 10.1176/ajp.122.5.509. [DOI] [PubMed] [Google Scholar]
- 39.Ashcroft GW, Crawford TBB, Eccleston D, Sharman DF, MacDougall EJ, Stanton JB, et al. 5-Hydroxyindole compounds in the cerebrospinal fluid of patients with psychiatric or neurological diseases. Lancet. 1966;ii:1049–52. doi: 10.1016/s0140-6736(66)92028-9. [DOI] [PubMed] [Google Scholar]
- 40.Duman RS. Pathophysiology of depression: the concept of synaptic plasticity. Eur Psychiatry. 2002;17(3):306–10. doi: 10.1016/s0924-9338(02)00654-5. [DOI] [PubMed] [Google Scholar]
- 41.Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromol Med. 2004;5(1):11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- 42.Duman RS, Charney DS. Cell atrophy and loss in major depression. Biol Psychiatry. 1999;45(9):1083–4. doi: 10.1016/s0006-3223(99)00057-8. [DOI] [PubMed] [Google Scholar]
- 43.Goodwin FK, Post RM. 5-Hydroxytryptamine and depression: a model for the interaction of normal variance pathology. Br J Clin Psychopharmacol. 1983;15(Suppl 3):393S–405S. doi: 10.1111/j.1365-2125.1983.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28(9):1562. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- 45.Hirschfield RMA. The comorbidity of major depression and anxiety disorders: Recognition and management in primary care. Prim Care Companion J Clin Psychiat. 2001;3(6):244–54. doi: 10.4088/pcc.v03n0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kessler RC, Nelson CB, McGonagle KA, Liu J, Swartz M, Blazer DG. Comorbidity of DSM-III-R major depressive disorder in the general population: results from the US National Comorbidity Survey. Br J Psychiatry. 2005;168:17–30. [PubMed] [Google Scholar]
- 47.Gorman JM. Comorbid depression and anxiety spectrum disorders. Depress Anxiety. 2005;4:160–8. doi: 10.1002/(SICI)1520-6394(1996)4:4<160::AID-DA2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 48.Henriques JB, Davidson RJ. Decreased responsiveness to reward in depression. Cogn Emotion. 2000;14(5):711–24. [Google Scholar]
- 49.Pizzagalli D, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry. 2004;57:319–27. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crews WD, Harrison DW. Sex differences and cerebral asymmetry in facial affect perception as a function of depressed mood. Psychobiology. 1994;22(2):112–6. doi: 10.2466/pms.1994.79.3f.1667. [DOI] [PubMed] [Google Scholar]
- 51.Henriques JB, Glowacki JM, Davidson RJ. Reward fails to alter response bias in depression. J Abnorm Psychol. 1994;103:237–48. doi: 10.1037//0021-843x.103.3.460. [DOI] [PubMed] [Google Scholar]
- 52.Sloan DM, Bradley MM, Dimoulas E, Lang PJ. Looking at facial expressions: dysphoria and facial EMG. Biol Psychology. 2002;60:79–90. doi: 10.1016/s0301-0511(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 53.Sloan DM, Strauss ME, Wisner KL. Diminished response to pleasant stimuli by depressed women. J Abnorm Psychol. 2001;110:488–527. doi: 10.1037//0021-843x.110.3.488. [DOI] [PubMed] [Google Scholar]
- 54.Suslow T, Junghanns K, Arolt V. Detection of facial expressions of emotions in depression. Percept Mot Skills. 2001;92:857–68. doi: 10.2466/pms.2001.92.3.857. [DOI] [PubMed] [Google Scholar]
- 55.Bouhuys AL, Geerts E, Gordijn MCM. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 56.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- 57.Surguladze SA, Young AW, Senior C, Brebion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18(2):212–8. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- 58.Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8(1):20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- 59.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3(3):207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 60.Heitzig MM, Zucker RA, Stoltenberg SF, Sliwerska E, Burmeister M, Zubieta J. 5-HTTLPR polymorphism affects hippocampal and prefrontal BOLD responses to positive and negative lexical stimuli. Paper presented at the Annual Meeting of the Society for Neuroscience; Washington, DC. 2005. [Google Scholar]
- 61.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 62.Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, et al. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13(14):1737–41. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- 63.Surguladze SA, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, et al. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2004;57:201–9. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 64.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- 65.Coren SP, Porac C, Duncan P. A behaviorally validated self-report inventory to assess 4 types of lateral preferences. J Clin Neuropsychol. 1979;1:55–64. [Google Scholar]
- 66.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCIP-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 67.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 68.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs AG. Manual for the State-Trait Anxiety Inventory. Palo Alto (Calif): Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- 69.Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): technical manual and affective ratings. Gainesville (Fla): The Center for Research in Psychophysiology, University of Florida; 1999. [Google Scholar]
- 70.Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17(11):4302–11. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McCarthy G, Puce A, Gore JC, Allison T. Face-specific processing in the human fusiform gyrus. J Cogn Neurosci. 1997;9(5):605–10. doi: 10.1162/jocn.1997.9.5.605. [DOI] [PubMed] [Google Scholar]
- 72.Gauthier I, Anderson AW, Tarr MJ, Skudlarski P, Gore JC. Levels of categorization in visual recognition studies using functional magnetic resonance imaging. Curr Biol. 1997;7(9):645–51. doi: 10.1016/s0960-9822(06)00291-0. [DOI] [PubMed] [Google Scholar]
- 73.Gauthier I, Tarr MJ, Moylan J, Anderson AW, Skudlarski P, Gore JC. Does visual subordinate-level categorisation engage the functionally defined fusiform face area? Cogn Neuropsychol. 2000;17(1–3):143–63. doi: 10.1080/026432900380544. [DOI] [PubMed] [Google Scholar]
- 74.Gauthier I, Skudlarski P, Gore JC, Anderson AW. Expertise for cars and birds recruits brain areas involved in face recognition. Nat Neurosci. 2000;3:191–7. doi: 10.1038/72140. [DOI] [PubMed] [Google Scholar]
- 75.Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nat Neurosci. 1999;2:568–73. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- 76.Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci. 2000;3(8):764–9. doi: 10.1038/77666. [DOI] [PubMed] [Google Scholar]
- 77.Dalton KM, Nacewicz BM, Johnstone T, Schaefer HS, Gernsbacher MA, Goldsmith HH, et al. Gaze fixation and the neural circuitry of face processing in autism. Nat Neurosci. 2005;8(4):519–26. doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]