Abstract
BCR-ABL is a key mediator in the pathogenesis of all cases of chronic myelogenous leukemia (CML) and a subset of precursor B-acute lymphoblastic leukemia (Ph+ALL). Previous animal and cell-based studies have shown that the expression of members of the Forkhead family of tumor suppressors, including FoxO3, is suppressed in BCR-ABL-expressing cells. Furthermore, it has been reported that the proteasomal degradation pathway plays an important role in suppression of FoxO expression in BCR-ABL-transformed cells. In this study, a patient diagnosed with Ph+ALL and refractory to standard therapies was treated with a proteasome inhibitor (bortezomib)-based chemotherapy regimen. This treatment resulted in complete hematologic, cytogenetic and molecular remission with excellent performance status for >4 years since her initial diagnosis. FoxO3 was not detectable within the blasts of this patient at diagnosis and was ‘rescued’ following treatment with bortezomib therapy, leading to her recovery. As a next step, in the attempt to propose FoXO3 as a therapeutic target and a theranostic marker, we further validated FoxO3 expression in human bone marrow biopsy samples. Human core biopsy samples of Ph+ALL and Ph-negative-negative ALL, along with uninvolved controls, revealed that FoxO3 downregulation was specific to Ph+ALL. This study provides support that FoxO3 is a good biomarker for BCR-ABL-mediated leukemogenesis. Additionally, proteasomal inhibition by bortezomib may be a promising therapeutic option in Philadelphia-positive ALL, where FoxO3 is downregulated.
Key words: philadelphia positive precursor B-acute lymphoblastic leukemia (Ph+ALL), BCR-ABL, bortezomib, proteasomal inhibition
Introduction
Several downstream mediators of BCR-ABL, including the tumor suppressor FoxO3, a member of the Forkhead family, are targets of the proteasomal degradation pathway.1,2 Inhibition of FoxO3 has been implicated in the disease pathogenesis of Ph+ALL and CML, two diseases in which BCR-ABL mediates leukemogenesis. However, downregulation of FoxO3 in human disease and restoration of FoxO3 expression associated with clinical remission has not yet been studied.
The Philadelphia chromosome is seen in about 25% of adult patients with ALL and carries a poor prognosis,3 with a median survival of about 11 months, and long term survival of less than 10%. While the addition of tyrosine kinase inhibitors (TKI) to intensive chemotherapy has improved the response rate, their effect on overall survival remains unclear and the overall prognosis for adult patients, especially >55 years of age, with this subtype remains poor.4–7
The proteasome inhibitors, of which bortezomib is a prototype, were initially FDA approved for use in multiple myeloma but have shown activity in other diseases such as mantle cell lymphoma and refractory acute leukemias.6,8 Recently, we and others have demonstrated that bortezomib, alone or in combination with other chemotherapeutic agents, is effective in abrogating disease in animal models of chronic myelogenous leukemia.9–12
Despite these animal studies, unequivocal clinical evidence is lacking for FoxO3 downregulation in BCR-ABL-mediated disease in humans and the effectiveness of bortezomib in treatment of Ph+ALL patients. Here, we describe a patient with Ph+ALL who was treated with a bortezomib-based chemotherapy regimen and sustained a prolonged clinical, cytogenetic and molecular remission, along with restoration of FoxO3 levels. Moreover, in human bone marrow core biopsies, we show downregulation of FoxO3 within blasts and precursors in Ph+ALL, as compared to Ph-negative ALL and matched controls. This report thus provides clinical evidence that FoxO3 is involved in Philadelphia-positive disease and provides a valuable theranostic biomarker for treatment with bortezomib in BCR-ABL-induced disease.
Results
Ph+ALL patient achieves full molecular and cytogenetic remission and restoration of expression of FoxO3 in response to bortezomib.
Previous cell-based and animal studies demonstrated that FoxO proteins are involved in mediating BCR-ABL-induced cell survival.1,2 Additionally, we have found that in BCR-ABL-transformed cells and in a CML mouse model, FoxO3 expression is abrogated and bortezomib treatment causes restoration of FoxO3 expression.2,9 Our preclinical studies in a mouse model for Ph+ leukemia have shown that bortezomib induces regression of leukemia in both imatinib-sensitive (BCR-ABL unmutated) and imatinib-resistant (BCR-ABL T315I) cases.9,13 However, the efficacy of bortezomib in Ph+ leukemia cases has yet to be evaluated. In this study, a Ph+ALL patient with a number of poor prognostic factors including her age (56 years), high WBC count at presentation and presence of the Philadelphia chromosome was intolerant to standard hyper-CVAD chemotherapy and developed severe neurologic, infectious and cardiac toxicity. Her bone marrow at the time of diagnosis showed replacement by immature lymphoblasts (Fig. 1A). Analysis of FoxO3 expression within the blasts revealed that these blasts were negative for FoxO3 (Fig. 1B). The patient was then treated with bortezomib (1.3 mg/m2 twice a week) for 12 months as described earlier. She cleared leukemic lymphoblasts from her bone marrow and became transiently pancytopenic. Her bone marrow biopsy following this treatment showed hypocellularity (Fig. 1C). Interestingly, following bortezomib treatment, rare immature precursor cells within the bone marrow showed positive expression of FoxO3 (Fig. 1D). Subsequently, she was maintained on bortezomib along with dasatinib (70 mg BID), monthly rituximab and pulse steroids. The patient tolerated this combination therapy very well and had marked improvements in neurologic and cardiac function. Her white blood counts, platelets and hematocrit (Fig. 2) improved with no circulating blasts, indicating hematologic remission. Additionally, 3 months following bortezomib treatment, RT-PCR analysis of her blood/bone marrow specimens demonstrated the absence of Bcr-Abl transcripts (Fig. 2).
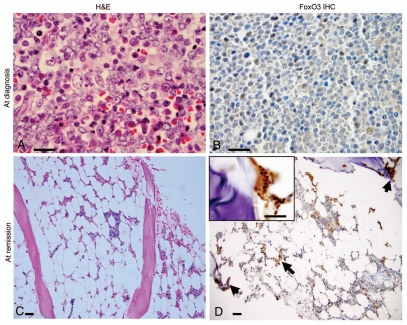
Figure 1.
Bone marrow findings and FoxO3 expression in a Ph+ALL patient at diagnosis and after treatment with bortezomib. (A) H&E stained slide of a bone marrow trephine core biopsy at diagnosis revealed marrow space occupied by immature precursor B-lymphoblasts. Blasts (indicated by arrows; ∼90% of marrow cellularity) have an open chromatin, sparse cytoplasm and high N:C ratio. Rare scattered hematopoietic elements were present (x1,000). (B) FoxO3 immunostains (1:100; Millipore, Billerica, MA) revealed that most of these immature blasts lacked FoxO3 (magnification x1,000). (C) H&E slide of bone marrow core biopsy after treatment with bortezomib. The marrow was hypocellular for age (∼10% cellularity). It revealed only rare blasts along with normal hematopoietic elements (x100). (D) FoxO3 stains revealed that FoxO3 expression was seen in small clusters of cells within the interstitium (arrows; x100), including precursor cells localized to the endosteal niche (inset; x1,000). Reticle bar represents 100 µ.
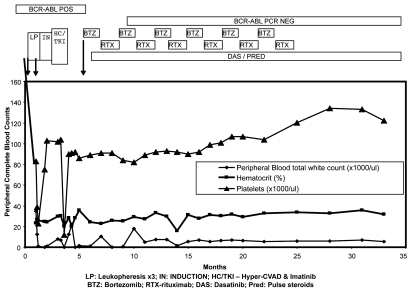
Figure 2.
Clinical course and remission in the patient with Ph+ALL treated with bortezomib. The graph shows persistence of BCR-ABL-positive disease despite correction of white counts in the initial phase of the disease, when the patient was treated with leukopheresis, followed by induction chemotherapy and TKI (Imatinib). Bortezomib was added at approximately 6 months following diagnosis. Bortezomib and rituxan are maintained at alternate months, while dasatinib is given orally every day. Her BCR-ABL expression levels, by molecular analysis (RT-PCR for p210 transcripts), turned negative following this combination regimen. The data is truncated at 35 months. The patient maintains a similar status at 48 months at last follow-up.
FoxO3 expression is significantly attenuated in Ph+ALL.
FoxO members of the Forkhead family of proteins have emerged as important tumor suppressors. The loss of expression of FoxO3 in BCR-ABL-transformed cells, in a mouse model for BCR-ABL-induced leukemia and in the Ph+All patient described above, suggested that the loss of FoxO expression may be a valuable biomarker in Philadelphia chromosome-positive leukemia. In order to examine the expression of FoxO3 in BCR-ABL-positive disease, we analyzed the expression of FoxO3 in bone marrow biopsies of 20 patients with ALL and 4 matched controls. Immunostains for FoxO3 revealed that there was downregulation of FoxO3 within blasts of most patients with Ph+All (Fig. 3A; n = 8/11). As opposed to this, patients with Ph-negative ALL (Fig. 3B) and normal controls (Fig. 3C) showed positive nuclear/cytoplasmic expression of FoxO3 (n = 11/13). The demographics and pattern of immunostains of FoxO3 expression are given in Table 1A. Comparison of FoxO3 expression between Philadelphia chromosome-positive and negative cases (Fig. 4), shows that there is a significant (two-tailed Fisher's exact probability test; p = 0.011) downregulation of FoxO3 expression in the former. The sensitivity and specificity analysis of FoxO3 as a (negative predictive) biomarker is presented in Table 1B. FoxO3 is negative in Philadelphia positive cases (true positives) and positive in Philadelphia negative cases and normal control (true negatives). The sensitivity for FoxO3 was calculated to be 73% and the specificity of FoxO3 for Ph+All was 85%.
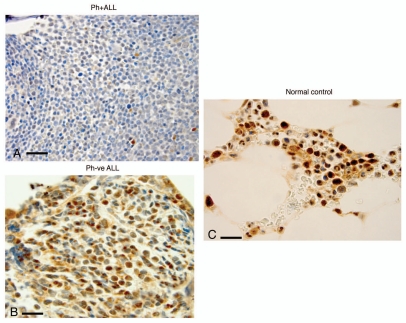
Figure 3.
Bone marrow expression of FoxO3 (IHC) (x1,000) in representative patient samples with with Ph+ALL, Ph−ALL and ‘normal’ controls. (A) Bone marrow trephine core biopsies of patients with Ph+ALL show marked downregulation of FoxO3 in all blasts (x1,000). Note the near-complete absence of maturation and absence of cytoplasmic and nuclear FoxO3. Rare myeloid elements show positive staining with FoxO3 (internal control). (B) A patient with Ph-negative ALL. The specimen is cellular with immature blasts. Most blasts are positive for FoxO3 expression in cytoplasm/nuclear localization. (C) Normal controls. The cellularity is sparser than leukemic marrows and shows hematopoietic cells in the insterstitium of normal marrow space and adipose tissue. The hematopoietic cells, specifically myeloid cells, show strong expression of FoxO3.
Table 1A.
Demographics and results of FoxO3 expression of the 24 bone marrow samples analyzed
| Diagnosis | Demographics | Pattern* |
| Ph+ALL | 54; male | C & N |
| Ph+ALL | 39; male | Negative |
| Ph+ALL | 76; male | N & C |
| Ph+ALL | 79; male | Negative |
| Ph+ALL | 41; male | Negative |
| Ph+ALL | 49; female | Negative |
| Ph+ALL | 19; male | Negative |
| Ph+ALL | 56; female | Negative |
| Ph+ALL | 28; male | Negative |
| Ph+ALL | 33; male | Negative |
| Ph+ALL | 65; female | Positive |
| Ph−ALL | 71; male | Positive; N |
| Ph−ALL | 79; female | Positive |
| Ph−ALL | 76; female | Positive |
| Ph−ALL | 66; male | Negative |
| Ph−ALL | 56; female | Positive; C & N |
| Ph−ALL | 61; male | Positive; C & N |
| Ph−ALL | 63; female | Negative |
| Ph−ALL | 64; female | Positive; C & N |
| Ph−ALL | 56; male | Positive; N |
| Control | 66; male; chronic anemia | Positive; N |
| Control | 73; female; thrombocytopenia, mild | Positive; N |
| Control | 58; male; non-Hodgkin Lymphoma | Positive; N |
| Control | 74; male; non-Hodgkin lymphoma | Positive; N |
FoxO3 is interpreted as positive, if >20% of cells show cytoplasmic or nuclear staining with FoxO3 antibody.
N, nuclear; C, cytoplasmic. Positive staining implies >20% cells/blasts staining positively (nuclear or cytoplasmic) for FoxO3. The 20% cut-off is similar to WHO criteria for blast counts and is a semi-arbitrary threshold for positive expression in bone marrow specimens.
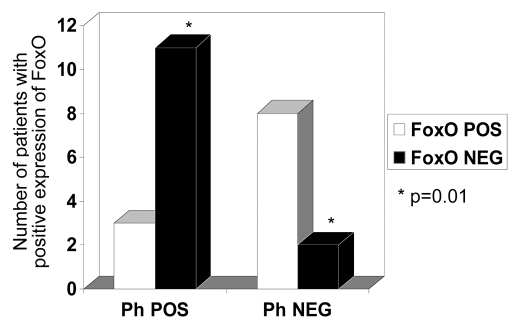
Figure 4.
Data collated from IHC expression and Table 1, showing comparison between the two groups (Philadelphia chromosome-positive ALL versus Ph-negative disease/Controls). Most cases in the Ph-negataive group showed positive expression of FoxO3 (2/10), whereas only (3/14) cases showed positive expression in the Ph+ALL group. A two-tailed Fisher's exact test revealed the difference between the groups to be significant (p = 0.01).
Table 1B.
Frequency distribution of FoxO3 expression among the Ph+ and Ph−ve cases
| Philadelphia-positive | Philadelphia-negative | |
| FoxO3 Negative | 8 | 2 |
| FoxO3 Positive | 3 | 11 |
Sensitivity, 0.73; Specificity, 0.85 (see text).
Expression of FoxO3 within normal bone marrow cells.
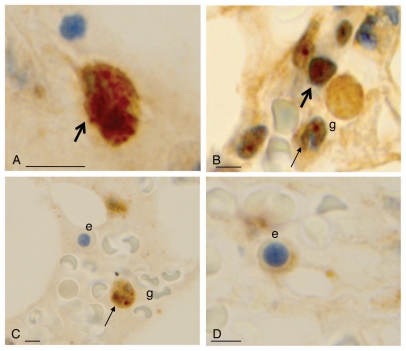
Since we propose FoxO3 to be a putative biomarker, characterizing its expression within bone marrow cells is important for histo-morphological interpretation. Our analysis reveals that within the precursor population, FoxO3 was in a biphasic pattern (nuclear and cytoplasmic; Fig. 5A). In mature granulocytic cells, there was nuclear expression of FoxO3 (Fig. 5B and C). Other marrow cells, such as erythroid precursors, show scant or no expression of FoxO (Fig. 5C, e and D, e). This indicates that FoxO3 expression is heterogeneous within the hematopoietic compartment of the bone marrow cells. Thus, analyzing FoxO3 expression within bone marrow cells should be evaluated more closely. Moreover, the expression of FoxO3 in lymphoblasts but not erythroids (negative for FoxO) nor mature granulocytes (positive for FoxO) should be scored for appropriate interpretation. While this signifies differential expression of FoxO3 within different hematopoietic lineages and its nuclear localization with maturation, the biphasic expression of FoxO3 and exact functional correlation awaits further studies.
Figure 5.
FoxO3 expression within normal hematopoietic cells. (A) FoxO3 expression is seen in the cytoplasm or nucleus of myeloid progenitor cells (thick arrows). (B and C) FoxO3 expression in more mature granulocytes (g): these mature cells show a nuclear dot-like positivity, specifically in the chromocentric regions (thin arrow of B). (C and D) FoxO3 expresion in erythroid precursors (marked as e) with very dim (D) or negative (C) expression of cytoplasmic FoxO3. The reticle bar represents ∼10 µ in each figure.
Discussion
FoxO3 proteins, belonging to the Forkhead family of transcription factors, are putative tumor suppressors and we have shown that the BCR-ABL oncogene inhibits expression of these proteins in cell culture studies and in an in vivo model of BCR-ABL-induced hematopoietic neoplasm.1,2 Additionally, suppression of FoxO3 expression has been observed in response to the expression of other oncogenes, such as FLT3-ITD and NPM-ALK, as well as in response to loss of PTEN expression.9,14–16 These studies suggest novel FoxO3-based mechanisms in tumorigenicity and indicate FoxO3 proteins as potential biomarkers in cancer. Furthermore, we have previously demonstrated that proteasomal inhibition by bortezomib can restore FoxO3 expression and cause regression of leukemia in cell culture studies and in mouse models of leukemia,9 potentially offering an alternative therapeutic strategy in leukemias where FoxO3 is downregulated and suggesting FoxO3 as a potential theranostic marker.
In order to study the relevance of these in vitro and animal studies to human disease, we employed the FDA approved drug bortezomib, as a potential therapeutic measure in a patient with Ph+ALL, who was non-responsive to other conventional therapies. The patient showed a rapid recovery and went into a long-lasting molecular remission. Encouraged by these findings, we chose to validate FoxO3 as a potential theranostic marker in human bone marrow samples. Immunohistochemical characterization is a very commonly used ancillary technique in diagnostic pathology. For a new biomarker to be clinically applicable, validation of the biomarker on a number of human bone marrow samples is necessary. We first examined the expression of FoxO3 in this Ph+ALL patient and then in multiple bone marrow archival samples of patients with acute lymphoblastic leukemia and uninvolved ‘normal’ controls. We were able to validate the expression of FoxO3 in normal and leukemic bone marrow samples and further show that FoxO3 is significantly downregulated within lymphoblasts of Ph+ALL, but not Ph-negative ALL. Even though in this limited sample analysis the sensitivity and specificity were not very high (73 and 85% respectively), most immunostain markers are not used as analytical biomarkers. With a large sample size, the analytical predictivity may substantially improve, thus needing validation in a larger clinical trial.
Philadelphia-positive ALL in adults is an aggressive disease with poor prognosis.3 While alternative therapeutic approaches remain elusive, we explored pathogenic mechanisms that can be successfully targeted downstream of BCR-ABL-mediated transduction. Since the PI3K/Akt pathway is implicated in BCR-ABL-mediated leukemogenesis, we hypothesized that FoxO3 can be an attractive marker and target in Ph+ALL and CML. We previously proposed that FoxO3 is perhaps degraded by proteasome in BCR-ABL-mediated leukemia, and effective proteasomal inhibition can restore FoxO3 and hence abrogate the disease9 in mouse models. In the current study, we demonstrate this finding in a patient who was refractive to other treatment modalities. We also show the potential theranostic value of FoxO3 using decalcified human bone marrow samples, and thus propose the clinical utility of FoxO3. A much more recent Phase I study utilizing proteasomal inhibition has shown tolerance of bortezomib in children with refractory ALL,17 with continual case accrual for further studies.
Taken together, these results show that the FoxO3 tumor suppressor is attenuated in BCR-ABL-mediated disease and may be utilized as a potential diagnostic biomarker. Furthermore, this study shows that a therapeutic regimen containing bortezomib can restore the expression of FoxO3, and induce remission. Therefore, these studies indicate FoxO3 expression is a valid theranostic biomarker in Ph+ acute lymphoblastic leukemia. A randomized prospective clinical trial to evaluate the efficacy of bortezomib in Ph+ALL, as well as to validate FoxO3 as a predictive biomarker in larger patient samples, is necessary.
Materials and Methods
Narrative case report of patient with Ph+ALL, treated with bortezomib.
A 56-year-old female presented with the sudden onset of blurred vision and diffuse bone tenderness. She was found to have a white blood count (WBC) of 178,000/µL, hematocrit of 20% and a platelet count of 85,000/µL. The differential count revealed 90% lymphoblasts, which were of precursor B-ALL phenotype by flow-cytometry (expressing CD34, CD10, CD20 and nTdT). Cytogenetic analysis confirmed that the blasts were Philadelphia chromosome-positive. She underwent leukopheresis followed by vincristine, duanorubicin, prednisone and L-asparaginase induction chemotherapy. She received three cycles of hyper-CVAD consolidation chemotherapy along with tyrosine kinase inhibitors (imatinib, switched to dasatinib due to intolerance) based on previously described reports.4–6 Her course was further complicated by the development of severe, grade 4, peripheral neuropathy, grade 3 mucositis, prolonged myelosuppression and toxic megacolon during her second cycle of hyper-CVAD. Due to these toxicities, she was unable to receive the standard treatments for Ph+ALL. She did not have a suitable HLA matched donor for a hematopoietic stem cell transplant. Due to the overall poor prognosis of Ph+ALL and the patient's intolerance of standard chemotherapy, alternative treatments were considered. She was treated with bortezomib (1.3 mg/m2 twice a week) for a month alternating with Rituximab infusions once a week, for 4 weeks. This alternating bortezomib and rituximab treatment was carried on for 1 year. She was maintained on dasatinib (70 mg BID) during this period and after.
Immunohistochemistry.
To validate FoxO3 expression in human bone marrow specimens and to show its specificity to BCR-ABL-mediated acute lymphoblastic leukemia, we characterized FoxO3 expression within bone marrow cells by immunohistochemistry (IHC). IHC was performed on a total of 24 samples obtained from adult patients for diagnostic reasons. The samples included patients with acute lymphoblastic leukemia (n = 20), including the patient who was treated with bortezomib and ‘normal’ control samples (n = 4). ‘Normal’ samples were biopsies obtained from patients for the work-up of anemia (n = 2) and uninvolved bone marrow from patients with non-Hodgkin lymphoma, performed for staging purposes (n = 2). Of the 20 patients with ALL, 11 were positive for the Philadelphia chromosome (BCR-ABL presence demonstrable by FISH and/or RT-PCR) and 9 were Ph-negative. The mean age of these patients was 56.5 years with 12 males and 8 females, a profile similar to uninvolved controls (mean age = 67.75; M/F ratio 1:1). The biopsy samples were all obtained at the time of diagnosis, before the initiation of therapy. IHC for FoxO3 protein (Millipore, Cat# 07-702) in the bone marrow biopsy specimen was performed by a modification of a manual immunostaining protocol13 for FoxO3 (1:100, citrate retrieval) adapted to an automated platform (Leica Bond™ Max). The core biopsy samples were obtained from archival bone marrow samples received at BIDMC through a protocol approved by the Institutional Review Board (2007-P-000080/6 & 2010P-000017; BIDMC).
Acknowledgements
The institutional review board approval for the human studies is based on Protocol # 2007-P-000080/6 & 2010P-000017; BIDMC. This work was supported by NIH grants CA105306 and CA131664. The authors acknowledge the contributions of Natalia Mangubat, Clarient, Inc. in optimizing and IHC validation of FoxO3 on bone marrow specimens.
Authors' Contributions
Dr. Rajan Dewar carried out the bone marrow core biopsy analysis, immunohistochemical studies and wrote the manuscript. Drs. Kenneth Miller and Yeckes-Rodin carried out the clinical study. Dr. Sing-Tsung Chen optimized immunostains for FoxO3 and participated in interpreting them. Dr. Roya Khosravi-Far conceived the study and contributed to the writing of the manuscript.
References
- 1.Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci USA. 2003;100:6523–6528. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jagani Z, Singh A, Khosravi-Far R. FoxO tumor suppressors and BCR-ABL-induced leukemia: a matter of evasion of apoptosis. Biochim Biophys Acta. 2008;1785:63–84. doi: 10.1016/j.bbcan.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 4.Ottmann O, Dombret H, Martinelli G, Simonsson B, Guilhot F, Larson RA, et al. Dasatinib induces rapid hematologic and cytogenetic responses in adult patients with Philadelphia chromosome positive acute lymphoblastic leukemia with resistance or intolerance to imatinib: interim results of a phase 2 study. Blood. 2007;110:2309–2315. doi: 10.1182/blood-2007-02-073528. [DOI] [PubMed] [Google Scholar]
- 5.Ottmann OG, Wassmann B, Pfeifer H, Giagounidis A, Stelljes M, Duhrsen U, et al. Imatinib compared with chemotherapy as front-line treatment of elderly patients with Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) Cancer. 2007;109:2068–2076. doi: 10.1002/cncr.22631. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DA, Faderl S, Cortes J, O'Brien S, Giles FJ, Kornblau SM, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 7.Vignetti M, Fazi P, Cimino G, Martinelli G, Di Raimondo F, Ferrara F, et al. Imatinib plus steroids induces complete remissions and prolonged survival in elderly Philadelphia chromosome-positive patients with acute lymphoblastic leukemia without additional chemotherapy: results of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) LAL0201-B protocol. Blood. 2007;109:3676–3678. doi: 10.1182/blood-2006-10-052746. [DOI] [PubMed] [Google Scholar]
- 8.Fisher RI, Bernstein SH, Kahl BS, Djulbegovic B, Robertson MJ, de Vos S, et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J Clin Oncol. 2006;24:4867–4874. doi: 10.1200/JCO.2006.07.9665. [DOI] [PubMed] [Google Scholar]
- 9.Jagani Z, Song K, Kutok JL, Dewar MR, Melet A, Santos T, et al. Proteasome inhibition causes regression of leukemia and abrogates BCR-ABL-induced evasion of apoptosis in part through regulation of forkhead tumor suppressors. Cancer Res. 2009;69:6546–6555. doi: 10.1158/0008-5472.CAN-09-0605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wiberg K, Carlson K, Aleskog A, Larsson R, Nygren P, Lindhagen E. In vitro activity of bortezomib in cultures of patient tumour cells—potential utility in haematological malignancies. Med Oncol. 2009;26:193–201. doi: 10.1007/s12032-008-9107-6. [DOI] [PubMed] [Google Scholar]
- 11.Yu C, Rahmani M, Conrad D, Subler M, Dent P, Grant S. The proteasome inhibitor bortezomib interacts synergistically with histone deacetylase inhibitors to induce apoptosis in Bcr/Abl+ cells sensitive and resistant to STI571. Blood. 2003;102:3765–3774. doi: 10.1182/blood-2003-03-0737. [DOI] [PubMed] [Google Scholar]
- 12.Dai Y, Rahmani M, Pei XY, Dent P, Grant S. Bortezomib and flavopiridol interact synergistically to induce apoptosis in chronic myeloid leukemia cells resistant to imatinib mesylate through both Bcr/Abl-dependent and -independent mechanisms. Blood. 2004;104:509–518. doi: 10.1182/blood-2003-12-4121. [DOI] [PubMed] [Google Scholar]
- 13.Song K, Mariappan R, Khosravi-Far R. Analysis of TNF-related apoptosis-inducing ligand in vivo through bone marrow transduction and transplantation. Methods Enzymol. 2008;446:315–331. doi: 10.1016/S0076-6879(08)01619-4. [DOI] [PubMed] [Google Scholar]
- 14.Gu TL, Tothova Z, Scheijen B, Griffin JD, Gilliland DG, Sternberg DW. NPM-ALK fusion kinase of anaplastic large-cell lymphoma regulates survival and proliferative signaling through modulation of FOXO3a. Blood. 2004;103:4622–4629. doi: 10.1182/blood-2003-03-0820. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson M, Engstrom M, Jonsson JI. FLT3 ligand regulates apoptosis through AKT-dependent inactivation of transcription factor FoxO3. Biochem Biophys Res Commun. 2004;318:899–903. doi: 10.1016/j.bbrc.2004.04.110. [DOI] [PubMed] [Google Scholar]
- 16.Modur V, Nagarajan R, Evers BM, Milbrandt J. FOXO proteins regulate tumor necrosis factor-related apoptosis inducing ligand expression. Implications for PTEN mutation in prostate cancer. J Biol Chem. 2002;277:47928–47937. doi: 10.1074/jbc.M207509200. [DOI] [PubMed] [Google Scholar]
- 17.Messinger Y, Gaynon P, Raetz E, Hutchinson R, Dubois S, Glade-Bender J, et al. Phase I study of bortezomib combined with chemotherapy in children with relapsed childhood acute lymphoblastic leukemia (ALL): a report from the therapeutic advances in childhood leukemia (TACL) consortium. Pediatr Blood Cancer. 2010;55:254–259. doi: 10.1002/pbc.22456. [DOI] [PubMed] [Google Scholar]