Abstract
Surgery of atrial fibrillation (AF) was first described in 1991 by James Cox in what was named the Cox-Maze procedure, and over the years it has been considered the gold-standard treatment, with best results in maintaining sinus rhythm in the long term. Nevertheless, the complexity and aggressivity of the first techniques of cut-and-sew limited the application of this procedure, and few centers were dedicated to AF surgery. In the past years, however, new devices able to ablate atrial tissue with cryotherapy, radiofrequency, or ultrasounds have facilitated this operation. In the mid-term, other energy devices with laser or microwave have been abandoned due to a lack of consistency in getting transmural lesions in a consistent and reproducible manner. Additionally, better knowledge of the physiopathology of AF, with the importance of triggering zones around the pulmonary veins, has started new minimally invasive techniques to approach paroxysmal and persistent AF patients through thoracoscopy.
1. Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia, affecting 1% of the general population and with its prevalence increasing with age [1]. Most important, AF has well-documented consequences as disabling symptoms, elevated stroke risk and major risk of congestive cardiac failure, being an independent predictor of death [2]. In summary, it represents a high cost on the health public systems of most developed countries.
Surgeons were the first ones to treat AF effectively and reverse it to sinus rhythm. James Cox described a series of surgical procedures known as Cox-Maze technique, between 1988 and 1991, that crystallized in the Cox-Maze III. This surgical approach was directed to divide both right and left atria by a series of cuts and sutures to redirect the electrical impulse to close-end paths, to finalize atrial depolarization, and be ready for the next sinus node impulse. This operation also included the exclusion of both atrial appendages and the isolation of the four pulmonary veins and the posterior wall of the left atrium. Although very effective, with over 91% patients maintaining sinus rhythm at 10 years, few surgical groups performed the Cox-Maze procedure due to the aggressiveness of it, with long suture lines and prolonged myocardial ischemic times [3, 4].
In the last decade, three factors have changed the approach of surgeons to AF: first, a better understanding of the electrophysiological basis of AF. In 1998, Haissaguerre described that most patients with paroxysmal AF have electric triggering zones localized within the antrum of the pulmonary veins [5], and that isolation of these areas was able to control AF effectively. However, later studies have demonstrated that pulmonary vein isolation alone was insufficient to control persistent or long-standing persistent AF and that a maze approach is needed to be added in these patients. Also, multiple studies have shown better results when the maze procedure was applied biatrially, compared to when only the left atrium is approached [6].
The second factor has been the development of new surgical tools able to create a similar lesion set of the Cox-Maze procedure faster and less aggressively, but maintaining a consistent, transmural, and linear lesions. Initially, these were clamps or catheters delivering heat or cold, with microwave ultrasounds, radiofrequency, laser, or liquid nitrogen, and argon, respectively. Nowadays, cryotherapy, bipolar radiofrequency, and ultrasounds are the most used energy sources and are recognized as a useful treatment by the 2010 Guidelines of the European Society of Cardiology (ESC) together with the European Association of Cardiothoracic Surgeons (EACTS) and the European Heart Rhythm Association (EHRA) [7, 8].
Finally, there has been increasing scientific evidence concerning cardiac surgeons of the deleterious effects of AF in cardiac patients and the importance of treating this arrhythmia. Recent studies relate preoperative AF with worse survival rates after valvular or coronary surgery [9]. Furthermore, patients with successful maze procedures have shown better long-term survival rates, higher freedom from stroke, and thromboembolic events, improved ventricular ejection fraction and exercise tolerance [7].
All the above factors have expanded the indications for the surgical treatment of concomitant AF to most patients with coronary or valvular surgery. For patient with long-standing persistent AF, the Cox-Maze procedure is still the gold standard treatment with the best results at 10- and 15-year followup.
2. Current Surgical Strategies
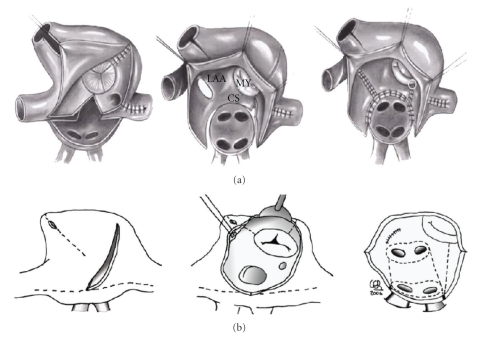
The initial Cox-Maze III operation (“Cut-and-sew technique”) described by James Cox consists in multiple biatrial lesions that interrupt the multiple reentrant circuits and redirects the sinus impulse to the atrioventricular node. This technique has shown excellent results, with over 90% sinus rhythm restoration over 15 years, although the initial series included a large percentage of patients with paroxysmal AF. When applied to long-standing AF concomitant to valvular disease, this technique has shown a 75–80% success rates. As seen in Figure 1(a), this operation includes several features. First, it isolates all pulmonary veins and the posterior wall of the left atrium. Second, it interferes in all concentric atrial structures that can facilitate atrial flutter. Those are, in the left side, the mitral annulus and the left appendage, and, in the right side, the entrance of both the superior and inferior venae cavae, the tricuspid annulus and the right appendage. Third, the procedure finishes with the excision of right and left appendages, the later to avoid the main source of atrial thrombi. The most important lines are the one connecting the pulmonary veins with the mitral annulus, to avoid left atrial flutter, and the line to the tricuspid annulus [10].
Figure 1.
(a) Cox-Maze III: Lesion pattern described by James Cox in 1991 with cut and sew technique. (b) Cox-Maze IV: Similar lesion pattern, with alternative energy lesion in dotted lines and trying to minimize incisions in both atria. Only the left appendage is excised.
As main setbacks of this operation, besides the need for long incisions that need to be sewed back prolonging myocardial ischemia and extracorporeal circulation times, between 45 minutes and 1 hour, were the need of pacemaker in 10% of patients, and a certain degree of chronic fluid retention in some patients, attributed to a lack of natriuretic peptide secretion induced by bilateral appendage amputation.
Nowadays, most surgeons apply different energy sources to perform the Cox-Maze procedure, then naming it Cox-Maze IV (Figure 1(b)). Heat-based energy sources include bipolar radiofrequency or ultrasounds. Cryotherapy can be applied using liquid nitrogen or argon. To create effective lesions that block the electrical impulse, the temperature applied to the tissue must reach 60°C or minus 65°C during two minutes to create fibrosis. Instruments have been designed in the form of long clamps to create a consistent transmural lesion line without causing injury to surrounding tissues. Other energy types initially described, as microwave or monopolar radiofrequency, were abandoned due to lack of consistent transmural lesions or by increased risk of collateral injuries. But, besides different instrumentation, the actual lesion pattern remains basically the one described by Cox in 1991, with few exceptions. First, most surgeons avoid the atrial septal lesion, making this procedure no longer associated with a higher need of pacemaker implantation than what it is associated to whatever concomitant procedure is coupled with. Second, to avoid possible deficit of atrial natriuretic peptide production, only the left appendage is excluded.
The direct vision of the heart and the rapid creation of transmural lesions with these techniques add only 15 to 30 minutes to the surgical time. Surgeons tend to minimize the cut and sew lesions to the ones needed to access the atria, trying to perform the maximum number of lines with cold or heat therapies. Several techniques have been described to avoid coronary artery injuries when approaching the mitral and tricuspid annulus.
When indicating a surgical therapy to AF, factors influencing success rate must be foreseen. These include atrial dilatation, age, years in AF, and type of AF (paroxysmal, persistent, or long-standing persistent). Probably the most important is atrial dilatation, due to chronic AF of by valvular dysfunction [11]. Scientific evidence shows that Cox-Maze procedure is less effective when left atrial postero-anterior diameter reaches 60 mm, and even when effective, atrial electrical stimuli transport usually is impaired. Type of AF is also an important condition for success rates. While pulmonary vein isolation has showed good results in patients with paroxysmal atrial fibrillation, persistent and long-standing persistent patients benefit from the complete biatrial lesion set in order to get sinus rhythm maintenance over 80% at 10 years. Therefore, surgical approach must be tailored in these patients [12].
3. Minimally Invasive Techniques for Isolated AF
With the development of new energy sources for atrial fibrillation and a better knowledge of the pathophysiology of AF, different surgical instruments have been created to allow a minimal invasive approach and effectively treat AF without big thoracic incisions. Energy is applied epicardially through direct vision with clamps that allow long transmural lesions. Moreover, epicardial ganglionic plexi around the pulmonary veins can be identified and ablated, and the left appendage excised or clipped to minimize thromboembolic events.
In 2005, Wolf published a series of 27 patients with pulmonary vein ablation with epicardially applied bipolar radiofrequency and left appendage exclusion. This procedure was named Mini-Maze IV (Figures 2 and 3). The sinus rhythm was restored in 91% of patients with previous paroxysmal AF, without mortality and a low morbidity. The procedure was performed through bilateral 5 cm thoracotomy incisions and video-thoracoscopy assistance [13]. Later on, this operation has evolved to a totally thoracoscopic technique, with three 1 cm incisions in each side. Moreover, new connecting lesions from the right to the left superior veins and to the mitral annulus have been described to treat persistent AF patients [14, 15].
Figure 2.
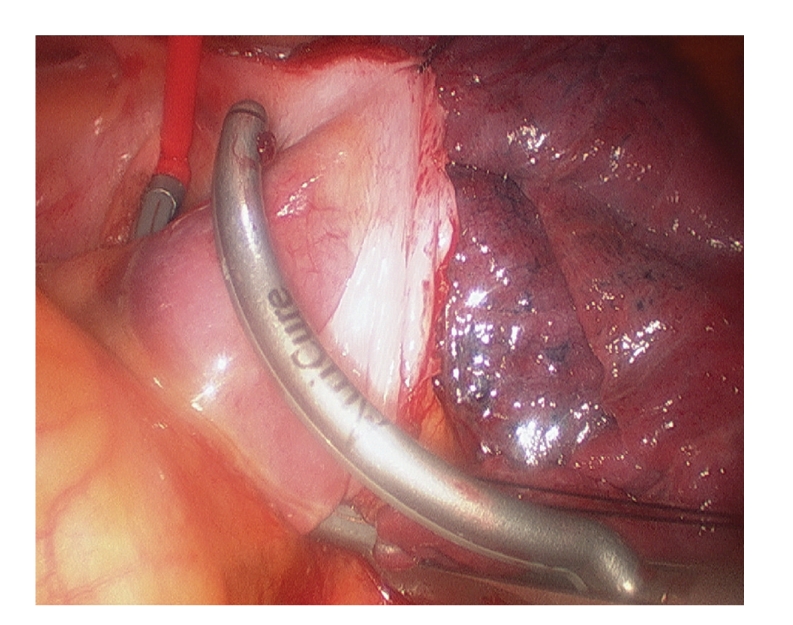
Pulmonary vein ablation by bipolar radiofrequency through a thoracoscopic approach. The ablation line can be seen in the left atrium at the antrum of the pulmonary veins.
Figure 3.
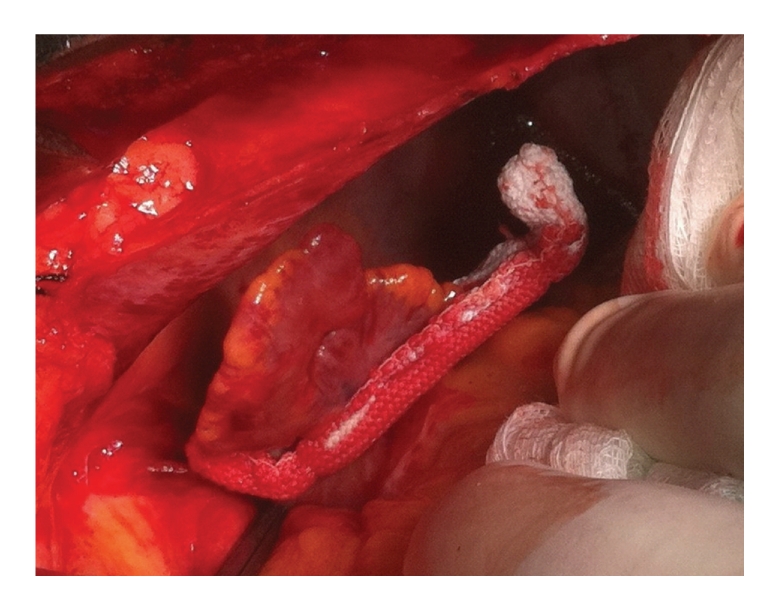
New clip device to close the left atrial appendage through sternotomy or thoracoscopic approaches.
These procedures have shown especially good results in patients with failed previous catheter ablation. In our series of 61 patients of pulmonary vein isolation with bipolar radiofrequency, postoperative sinus rhythm was maintained in 82% paroxysmal, 60% persistent, and 20% long-standing persistent AF patients at 12 months. LA size >45 mm and AF type showed to be preoperative factors that significantly influenced outcome [16]. The recent 2010 Guidelines of the ESC and EACTS recommends, minimally invasive surgical ablation of AF without concomitant cardiac surgery to patients with lone symptomatic AF after failure of catheter ablation [7, 8].
4. Conclusions
With better knowledge of the physiopathology of AF and easier access to energy sources that create consistent transmural lesions, surgery of atrial fibrillation has made a great impulse from the early days when Cox first described his Maze procedure. Concomitant surgical AF treatment has shown better survival rate, higher freedom from stroke and thromboembolic events, better ventricular function, and better exercise tolerance. Still, success rates are lower in patients with long history of AF, usually with enlarged atria, long-standing persistent AF pattern, and older age. Probably, the alteration of the atrial substrate, with more fibrotic tissue and established macro-reentrant circuits may limit maze strategies in these patients.
In addition, minimally invasive approaches have been described in the last five years with very good results for isolated paroxysmal or persistent AF. Nevertheless, prospective randomized trials are necessary to confirm their long-term results, compared to catheter ablation.
References
- 1.Kannel WB, Benjamin EJ. Current perceptions of the epidemiology of atrial fibrillation. Cardiology Clinics. 2009;27(1):13–24. doi: 10.1016/j.ccl.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geha AS, Abdelhady K. Current status of the surgical treatment of atrial fibrillation. World Journal of Surgery. 2008;32(3):346–349. doi: 10.1007/s00268-007-9380-0. [DOI] [PubMed] [Google Scholar]
- 3.Cox JL. The surgical treatment of atrial fibrillation: IV. Surgical technique. Journal of Thoracic and Cardiovascular Surgery. 1991;101(4):584–592. [PubMed] [Google Scholar]
- 4.Cox JL, Boineau JP, Schuessler RB, Jaquiss RD, Lappas DG. Modification of the maze procedure for atrial flutter and atrial fibrillation. I. Rationale and surgical results. Journal of Thoracic and Cardiovascular Surgery. 1995;110(2):473–484. doi: 10.1016/S0022-5223(95)70244-X. [DOI] [PubMed] [Google Scholar]
- 5.Haïssaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. The New England Journal of Medicine. 1998;339(10):659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 6.Khargi K, Hutten BA, Lemke B, Deneke T. Surgical treatment of atrial fibrillation; a systematic review. European Journal of Cardio-thoracic Surgery. 2005;27(2):258–265. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Calkins H, Brugada J, Packer DL, et al. HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS) Heart Rhythm. 2007;4(6):816–861. doi: 10.1016/j.hrthm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Camm AJ, Kirchhof P, Lip GYH, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) European Heart Journal. 2010;31(19):2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 9.Lim E, Barlow CW, Hosseinpour AR, et al. Influence of atrial fibrillation on outcome following mitral valve repair. Circulation. 2001;104(12, supplement 1):i59–i63. doi: 10.1161/hc37t1.094813. [DOI] [PubMed] [Google Scholar]
- 10.Cox JL. Surgical treatment of atrial fibrillation: a review. Europace. 2004;5(1):S20–S29. doi: 10.1016/j.eupc.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Gillinov AM, Bhavani S, Blackstone EH, et al. Surgery for permanent atrial fibrillation: impact of patient factors and lesion set. Annals of Thoracic Surgery. 2006;82(2):502–514. doi: 10.1016/j.athoracsur.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Barnett SD, Ad N. Surgical ablation as treatment for the elimination of atrial fibrillation: a meta-analysis. Journal of Thoracic and Cardiovascular Surgery. 2006;131(5):1029–1035. doi: 10.1016/j.jtcvs.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 13.Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. Journal of Thoracic and Cardiovascular Surgery. 2005;130(3):797–802. doi: 10.1016/j.jtcvs.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Edgerton JR, Edgerton ZJ, Weaver T, et al. Minimally invasive pulmonary vein isolation and partial autonomic denervation for surgical treatment of atrial fibrillation. Annals of Thoracic Surgery. 2008;86(1):35–39. doi: 10.1016/j.athoracsur.2008.03.071. [DOI] [PubMed] [Google Scholar]
- 15.Sirak J, Jones D, Sun B, Sai-Sudhakar C, Crestanello J, Firstenberg M. Toward a definitive, totally thoracoscopic procedure for atrial fibrillation. Annals of Thoracic Surgery. 2008;86(6):1960–1964. doi: 10.1016/j.athoracsur.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 16.Castellá M, Pereda D, Mestres CA, Gómez F, Quintana E, Mulet J. Thoracoscopic pulmonary vein isolation in patients with atrial fibrillation and failed percutaneous ablation. Journal of Thoracic and Cardiovascular Surgery. 2010;140(3):633–638. doi: 10.1016/j.jtcvs.2009.11.009. [DOI] [PubMed] [Google Scholar]