Abstract
In a continuing effort to improve the subtype selectivity and agonist potency of estrogen receptor β (ERβ) ligands, we have designed and developed a thus far unexplored structural series obtained by molecular refinements of monoaryl-substituted salicylaldoximes (Salaldox B). The most interesting compounds in this series (2c,d) show remarkably high ERβ-binding affinities, with Ki values reaching the sub-nanomolar range (Ki = 0.38 nM for 2c and 0.57 nM for 2d), and have very high levels of ERβ-subtype selectivity. Both compounds show a potent full-agonist character on ERβ (EC50 = 0.23 nM for 2c and 1.3 nM for 2d). Furthermore, 2d shows a remarkable functional subtype-selectivity, with a β/α transcription potency ratio 50-fold higher than that of estradiol.
Keywords: Estrogen, receptor binding, agonists, salicylaldoxime, docking
1. Introduction
Since the discovery of estrogen receptor beta (ERβ) in 1996 [1], much effort has been devoted to the search for molecules that are able to selectively interact with this receptor [2,3]. Such ERβ-selective agonists hold promise for the treatment of diverse diseases, such as rheumatoid arthritis and inflammatory diseases [4], certain cancers [5,6], endometriosis [7], as well as cardiovascular [8] and CNS pathologies [9]. The therapeutic potential of ERβ-selective compounds appears to be particularly favourable because the beneficial effects of stimulation through ERβ would be expected to be free from undesired proliferative effects on breast and uterus, which are mediated largely by the other receptor subtype, ERα [10,11].
ERβ, as well as ERα, belong to the superfamily of nuclear receptors that act as ligand-regulated transcription factors [12]. Their amino acid sequence shows 59% identity in the ligand binding domain (LBD), although the differences within the ligand binding cavities consist of only two, conservative amino acid substitutions. In fact, of the 23 amino acid residues that line the ligand binding cavities of the two ERs (within 4Å of the ligand), only two are different: Leu384 and Met421 of ERα are replaced by Met336 and Ile373, respectively, in ERβ [1]. These slight modifications and other minor alterations of tertiary structure make the volume of the ERβ binding pocket smaller than the one in ERα and somewhat different in shape. Due to the lack of a pronounced difference between the two receptor subtypes, the design and development of molecules that selectively bind to and activate ERβ is not a trivial task, although there are now several pharmacophore models for the kind of molecular frameworks more likely to engender ER-subtype selectivity [3].
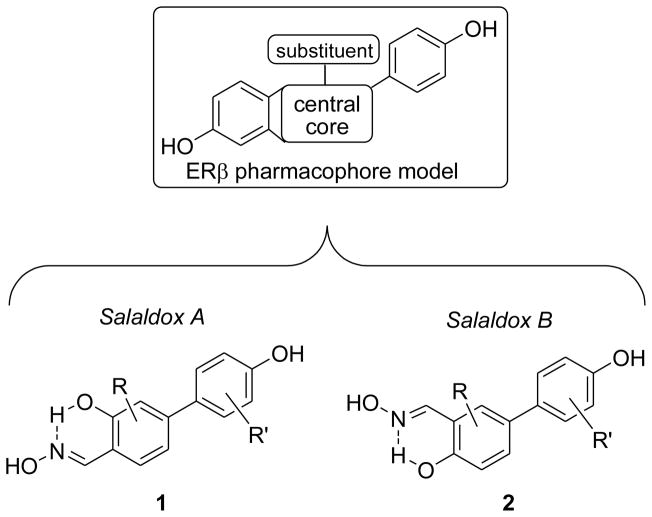
We recently reported on two restricted series of monoaryl-substituted salicylaldoximes bearing a para-hydroxylated aryl substituent at either position 4 (Salaldox A) [13,14] or 5 (Salaldox B) [14] of the central ring. These were inspired by a consolidated pharmacophore model originally developed for indazole derivatives [15] (Fig. 1). These compounds derive from our original observation that one of the two phenol groups typically present in non-steroidal ER-ligands could be isosterically replaced by a six-membered pseudocycle, formed by an intramolecular H-bond involving the phenol and the oxime nitrogen atom, which had led us to initially develop several diaryl-substituted salicyl-[16] and anthranyl-aldoximes [17] as non subtype-selective ER ligands.
Fig. 1.
Structural derivation of the two series of salicylaldoximes, Salaldox A (1) and Salaldox B (2), from the ERβ pharmacophore model.
We initially obtained highly selective ERβ-ligands belonging to the Salaldox A series (Fig. 2) by progressively refining the substitution pattern (compounds 1a,c,d) [13,14], and we confirmed the detrimental effect of a second aryl substituent on ERβ binding (1b) [16]. However, even the best Salaldox A members proved to be only partial agonists for ERβ, since their maximal activation values, compared to estradiol, were of 60% for 1c and 85% for 1d [13,14]. Moreover, the beta-selectivities shown by these two compounds in functional assays were considerably less than their ERβ-selectivities in binding assays, probably because of differences in the manner in which the ERα- and ERβ-ligand complexes interact with various cellular coregulators, which can act as modulators of ligand potency.
Fig. 2.
Reference salicylaldoximes (1a–d, 2a) and new derivatives (2b–g) designed as ERβ-selective agonists.
We later identified the simplest member of the Salaldox B class (2a) as a promising beta-selective ligand and, notably, also as the first ERβ full agonist among our oxime derivatives [14]. Nonetheless, despite its high ERβ binding preference, compound 2a also experienced a loss of beta-selectivity in transcriptional assays, most likely for the same reasons reported above for compounds 1c and 1d.
To overcome these limitations, we have continued our efforts to obtain compounds that can both bind and activate ERβ in a highly efficient and selective fashion. To this purpose, we herein report a logical extension of our structural optimization process to the Salaldox B class, using some of the same molecular interventions that proved successful in the Salaldox A series of derivatives studied previously, combined with new insights from molecular modeling. In the process, we prepared a focused series of analogs of 2a through which we have investigated the effect of introducing a 6-chloro atom in the central ring (2c) and an additional 3′-F-group in the aryl substituent (2d). We also introduced relatively small substituents (such as methyl and chlorine) in position 3 of the central ring, a place for molecular variations that has so far been unexplored within these salicylaldoxime derivatives. We first introduced a 3-methyl group (2e) and then inverted the respective 3/6-positions of the methyl and chlorine groups, such as in 2f and in its 3′-fluoro-substituted analog 2g. Finally, we obtained compound 2b as a Salaldox B analog of compound 1b, to verify whether the same structural restrictions in the Salaldox A series would also apply to this new series of ERβ-ligands.
2. Chemistry
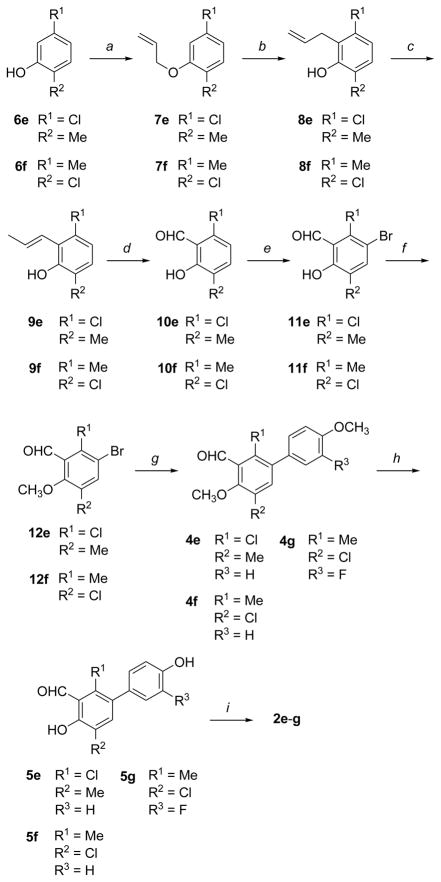
The synthesis of compounds 2b–d started from 3-bromo-2-chloro-6-methoxybenzaldehyde (3) [18], as shown in Scheme 1. When compound 3 was submitted to a single cycle of Pd-catalyzed cross-coupling reaction under classical Suzuki conditions [19] (specifically, in situ formation of Pd(PPh3)4 by reaction of palladium acetate with a 5-fold excess of triphenylphosphine with an aqueous base and conventional heating at 100 °C overnight) in the presence of 1.2 equivalent of the boronic acid, 4-methoxyphenylboronic or 3-fluoro-4-methoxyphenylboronic acid, it selectively formed the corresponding monoaryl-substituted adducts 4c,d, respectively. In fact, these conditions resulted in the chemo-selective replacement of only the bromine atom of 3, with the chloro group remaining intact. On the other hand, the repetition of two reaction cycles under the same Suzuki conditions, using a total of 3.2 equivalents of boronic acid, afforded mostly the diaryl-substituted intermediate 4b. The resulting adducts were treated with boron tribromide to obtain O-demethylated salicylaldehydes 5b–d, which were then condensed with hydroxylamine hydrochloride, thus yielding final salicylaldoximes 2b–d.
Scheme 1.
Synthesis of salicylaldoximes 2b–d. Reagents and conditions: (a) 4-MeO-C6H4B(OH)2 or 3-F-4-MeO-C6H4B(OH)2 (1.2 eq), Pd(OAc)2, PPh3, aqueous 2 M Na2CO3, 1:1 toluene/EtOH, 100 °C, 16 h; (b) 2 times: 4-MeO-C6H4B(OH)2 (1.6 eq), Pd(OAc)2, PPh3, aqueous 2 M Na2CO3, 1:1 toluene/EtOH, 100 °C, 16 h; (c) BBr3, CH2Cl2, −78 to 0 °C, 1 h; (d) NH2OH·HCl, EtOH-H2O, 50 °C, 5 h.
The synthetic route to methyl/chlorine-substituted oximes 2e–g was slightly longer, as shown in Scheme 2. Commercially available phenols 6e,f underwent O-allylation upon treatment with allyl bromide. Claisen rearrangement of the resulting ethers 7e,f at 210 °C yielded o-allyl-phenols 8e,f. The terminal double bond of 8e,f was shifted to the internal position by alkaline isomerisation with potassium tert-butoxide in DMSO at 55 °C, to give β-methylstyrene derivatives 9e,f as E-diastereomers. Oxidative cleavage of the styrene-type double bond with sodium periodate in the presence of catalytic amounts of osmium tetroxide yielded aldehydes 10e,f, which were then treated with bromine in glacial acetic acid, affording mono-brominated salicylaldehyde derivatives 11e,f.
Scheme 2.
Synthesis of salicylaldoximes 2e–g. Reagents and conditions: (a) allyl bromide, K2CO3, acetonitrile, 80 °C; (b) neat, 180 °C; (c) t-BuOK, DMSO, 55 °C; (d) OsO4, NaIO4, dioxane-H2O; (e) Br2, AcOH, RT; (f) MeI, K2CO3, acetone, RT; (g) 4-MeO-C6H4B(OH)2 or 3-F-4-MeO-C6H4B(OH)2, Pd(OAc)2, PPh3, aqueous 2 M Na2CO3, 1:1 toluene/EtOH, 100 °C, 16 h; (h) BBr3, CH2Cl2, −78 to 0 °C, 1 h; (i) NH2OH·HCl, EtOH-H2O, 50 °C, 5 h.
At this point, we initially tried to carry out a cross-coupling reaction of 11e,f with the appropriate boronic acid, to effect the replacement of the bromine atom with a suitable aryl substituent. Unfortunately, we were not able to perform this reaction efficiently, probably because of the bidentate chelating effect that the free vicinal OH/CHO groups of the salicylaldehyde portion have on both the boron atom of the boronic acid and on the palladium catalyst of the cross-coupling reaction, thus diverting the reactants from the correct reaction pathway during the Suzuki step. Therefore, we first protected the phenol hydroxyl with a methyl group, by reaction with iodomethane and potassium carbonate in acetone, and then submitted the resulting anisole derivatives 12e,f to the Suzuki coupling reaction. This time the reaction worked nicely, and monoaryl adducts 4e–g were so obtained. Final steps included O-demethylation with BBr3, to give intermediates 5e–g, and subsequent condensation with hydroxylamine hydrochloride, which afforded final oximes 2e–g.
All the oximes (2b–g) were obtained as single E-diastereoisomeric forms, presumably because the intramolecular hydrogen bond, which can only form in the E-isomer, contributes to the oxime stability. This selectivity had already been demonstrated for other oxime analogues previously reported [13,14,16,17], and it was confirmed here by the chemical shift values of the oxime protons of all the new products, which were always found downfield from 8 ppm (δ ≥ 8, see experimental section) [20].
3. Estrogen Receptor Binding Assays
ERα and ERβ binding affinities of new oximes 2b–g were determined by a radiometric competitive binding assay, using methods that have been previously described [21,22]. The relative binding affinity (RBA) values for the newly reported compounds, together with those previously obtained for compounds 1a–d and 2a [13,14,16b], are summarized in Table 1. RBA values are reported as percentages (%) of that of estradiol, which is set at 100% (Entry 1).
Table 1.
Relative Binding Affinitiesa of Compounds of the Salaldox A (1a–d) and Salaldox B (2a–g) series for the Estrogen Receptors α and β
| Entry | Ligand | hERα (%) | hERβ (%) | β/α ratio |
|---|---|---|---|---|
| 1 | estradiol | (100) | (100) | 1 |
|
Salaldox A Series
| ||||
| 2 | 1ab | 0.007 ± 0.001 | 0.55 ± 0.11 | 79 |
| 3 | 1bc | 0.92 ± 0.04 | 0.35 ± 0.01 | 0.38 |
| 4 | 1cb | 0.065 ± 0.016 | 4.21 ± 0.66 | 65 |
| 5 | 1dd | 0.11 ± 0.03 | 7.01 ± 1.00 | 62 |
|
| ||||
|
Salaldox B Series
| ||||
| 6 | 2ad | 0.064 ± 0.016 | 2.64 ± 0.62 | 41 |
| 7 | 2b | 88.4 ± 18.1 | 101 ± 2 | 1.1 |
| 8 | 2c | 4.46 ± 0.60 | 130 ± 25 | 29 |
| 9 | 2d | 1.88 ± 0.30 | 87.1 ± 15.0 | 46 |
| 10 | 2e | 0.074 ± 0.006 | 0.64 ± 0.09 | 8.6 |
| 11 | 2f | 1.47 ± 0.04 | 15.8 ± 3.5 | 11 |
| 12 | 2g | 0.39 ± 0.04 | 7.90 ± 0.40 | 20 |
Determined by a competitive radiometric binding assay with [3H]estradiol; preparations of purified, full-length human ERα and ERβ (Invitrogen, PanVera) were used; see Experimental Section. Values are reported as the mean ± the range or SD of 2 or more independent experiments; the Kd for estradiol for ERα is 0.2 nM and for ERβ is 0.5 nM. Ki values for the new compounds can be readily calculated by using the formula: Ki = (Kd[estradiol]/RBA) × 100.
See Ref. 13.
See Ref. 16b.
See Ref. 14.
We first analyzed some relevant results obtained previously with the Salaldox A series (Table 1, Entries 2–5): It turns out that the simplest member of this class (1a) is already a very ERβ-selective ligand (RBA β/α ratio = 79), although its binding affinity for the beta-receptor was rather modest (0.55%). Insertion of a chlorine into the 6 position of the central scaffold, as in compound 1c, preserved the high selectivity level (RBA β/α ratio = 65) and markedly increased the ERβ-binding affinity (4.21%) [13]. By contrast, insertion of a second para-hydroxyphenyl substituent in this scaffold produces a compound (1b) whose affinity for ERβ was dramatically reduced (0.35%) [16b], thus confirming the importance of the monoaryl-substitution motif within this class. Being inspired by a few very successful examples reported in the literature, such as biphenylcarbaldehyde oxime derivatives [23] and benzoxazole ERB-041 [24], we introduced a fluorine atom in the 3′-position of the para-hydroxyphenyl group of 1c, and the compound thus obtained (1d) possessed a higher affinity for ERβ (7.01%) than its non-fluorinated counterpart, together with a similar subtype selectivity (RBA β/α ratio = 62).
We then turned to analysis of the binding affinity of members of the Salaldox B series. Although this series was designed by a completely different structural modification of 1a, which involved an inversion of the respective positions of the hydroxy and oxime groups of the salicylaldoxime scaffold, we were surprised to find that the simplest member of Salaldox B series (2a) had a 5-fold higher ERβ-binding affinity (2.64%) compared to 1a (Table 1, Entries 2 and 6). Most importantly, the ERα binding affinity of 2a remained quite low (0.064%), so that this compound retained a notable beta-selectivity (RBA β/α ratio = 41) [14]. This promising behaviour of the “progenitor” member of the Salaldox B series (2a) indicated that this series merited further exploration.
Among the newly synthesized Salaldox B derivatives (Table 1, Entries 7–12), compound 2c, possessing a chlorine atom in the central scaffold, displays an outstanding affinity for ERβ, with a RBA value of 130% (corresponding to a Ki of 0.38 nM), and a robust beta-selectivity (RBA β/α ratio = 29). It should be noted that the affinity of 2c for ERβ is significantly higher than that of estradiol itself. Compound 2d, derived from a addition of a meta-fluorine in the para-hydroxylated aryl substituent, has a considerably higher ERβ-selectivity (RBA β/α ratio = 46) than 2c, thus confirming that this kind of structural modification often leads to an improved preference for the beta-subtype, as was seen before in the Salaldox A series [14]. The binding affinity of 2d for ERβ is also remarkably high, as shown by its 87% RBA value (corresponding to a Ki of 0.57 nM). By contrast, the addition of a methyl-group to the central ring of compound 2c results in a compound (2e) that has a dramatic drop in affinity for both receptor subtypes (RBA = 0.074% for ERα and 0.639% for ERβ). A marked recovery of binding properties is obtained, however, when the relative positions of the methyl and chlorine substituents of 2e are reversed, as shown by the good affinity values associated with compound 2f (RBA = 1.47% for ERα and 15.8% for ERβ), although the beta-selectivity is not as high as desired (RBA β/α ratio = 11). Here again, introduction of a meta-fluorine atom into the 4-hydroxyphenyl substituent of 2f, leading to 2g (RBA = 0.392% for ERα and 7.90% for ERβ), effects a 2-fold increase of ERβ-selectivity (RBA β/α ratio = 20). Finally, the single Salaldox B member bearing two para-hydroxyphenyl substituents (2b) is the only one not showing any appreciable preference for the beta-subtype (RBA β/α ratio = 1.1), although its affinities for both receptors (RBA = 88.4% for ERα and 101% for ERβ) are remarkably higher than those of its Salaldox A analog 1b, reaching values that are surprisingly close to those of estradiol. This last result further supports our original hypothesis that the monoaryl-substitution motif is a strict prerequisite for obtaining good ERβ-selectivity in this type of salicylaldoxime derivative [13,14].
4. Molecular modelling
An automated computational analysis of the newly synthesized compounds was performed to try to rationalize their binding properties. Docking of the ligands into ERα and ERβ (PDB codes 2I0J and 2I0G, respectively) was carried out using AUTODOCK 4.0 software [25]. Fig. 3A and 3B display the docking results for diaryl-substituted compound 2b into both ERs. In agreement with its similar affinity for both receptor subtypes, this compound shows the same interactions in the ERα and ERβ ligand binding pockets. The para-hydroxyl group on the aryl substituent distal to the oxime function is involved in an H-bond network, which includes (ERα residues in parentheses) E305 (E353), R346 (R394), and a water molecule. The para-hydroxyl of the aryl substituent proximal to the oxime function group forms an H-bond with T299 (T347). Finally, the pseudocycle/oxime system forms an H-bond with H475 (H524). All of these supposedly strong interactions confirm the very high binding affinities that 2b has for both ERα and ERβ.
Fig. 3.
Docking analysis into ERα and ERβ: (A) docking of 2b (green) into ERα; (B) docking of 2b (green) into ERβ; (C) docking of 2d (yellow) into ERα; (D) docking of 2d (yellow) into ERβ; (E) docking of 2e (light blue) into ERβ; (F) docking of 2f (orange) into ERβ.
The docking of monoaryl-substituted compound 2c (Fig. S1B, Supplementary data) and 2d (Fig. 3D) into ERβ produces results substantially similar to those previously obtained with their non-halogenated analog 2a [14], and highlights that: 1) the pseudocycle/oxime system is engaged in the H-bond network of the E305-R346-water system; 2) the OH of the p-hydroxyphenyl ring forms a H-bond with T299. In both compounds, the chlorine atom is inserted into a pocket delimited by A302, W335, M336, and L339. It should be noted that this orientation is completely different from that previously found in the docking analysis of Salaldox A derivatives such as 1c and 1d into ERβ-LBD, which instead place their p-hydroxyphenyl substituent in the H-bond network of the E305-R346-water system and the pseudocycle/oxime portion forming a H-bond with H475 [14]. It is interesting to note that the strong interaction between the 4′-hydroxyl of the ligands and T299 is possible only in the ERβ-LBD cavity, because only in this ER subtype is there enough space for the phenol group to reach the OH of T299 by occupying an area close to M336. The same does not happen in ERα, where the methionine (M336 in ERβ) is replaced by a bulkier and less flexible leucine (L384 in ERα) [26], causing a completely different disposition of the two compounds in this receptor subtype. In fact, compared to what happens in ERβ, in ERα 2c (Fig. S1A, Supplementary data) and 2d (Fig. 3C) are turned upside down such that their phenol 4′-OH group is involved in the H-bond network with E353, R394, and a water molecule, and their pseudocycle/oxime system forms a H-bond with H524.
The addition of a methyl group in the position para to the chloro-substituent of the central ring, as in 2e (Fig. 3E), is not tolerated in the same low energy conformation assumed by 2c and 2d in ERβ, because the binding region in the proximity of M340 is not large enough to tolerate the presence of a CH3. This causes the orientation of 2e to become rotated by 180° in the ERβ-binding pocket, resulting in a complex that is less stable than those with 2c or 2d. Thus, 2e has a dramatically reduced binding affinity for ERβ. On the other hand, if the position of the two CH3/Cl central substituents is interchanged, the resulting compounds, 2f (Fig. 3F) and 2g (Fig. S2, Supplementary data), have a preferred conformation in ERβ that is the same as the one found for compounds 2c and 2d, because the methyl substituent of 2f and 2g can now be accommodated in the pocket delimited by A302, W335, M336, and L339, while the chlorine atom, being smaller than the methyl group present in an analogous position of 2e, now fits nicely in the pocket close to M340. These factors result in the good binding affinity that both 2f and 2g have for ERβ, although their values are not better than those found for 2c and 2d.
It should be noted that in our modelling studies, the analysed ligands were simply docked into a single, rigid version of the receptor, and we did not computationally evaluate the possible fit-induced effects. This kind of approach requires the use of a flexible receptor and, hence, is much more computationally intensive than rigid receptor docking. At present, the main docking software programs are able to take into account the flexibility of only a small number of residues, making the possibility of evaluating the flexibility of a binding site very difficult. For these reasons, we used the ERβ-2d complex as a test set for a two-layer QM/MM calculation using Gaussian09 to verify the reliability of our docking results [27]. The ERβ-2d complex obtained from the docking studies was energy-minimized and then subjected to QM/MM, which has been so far used with good success by many authors in the field of drug design to find correct interactions within biological systems [28]. In these calculations, the zone of highest interest is treated quantum mechanically, while the rest of the system is treated by classical mechanics, thus reducing computational expenses. We used the ONIOM module of Gaussian09, using the B3LYP chemical model for the quantum mechanics (QM) system [29], with a 6–31G++** basis set and the Amber force field (parm96) for the molecular mechanics (MM) system. The QM system consisted of the ligand, the structural water molecule, and the side chains of T299, E305 and R346. Figure 4 shows the superimposition between the starting ERβ-2d complex and the QM/MM-optimized one. The ligand maintained the interactions with T299 and the E305-R346-water system, showing a Root Mean Square Deviation (RMSD) from the starting structure of only 0.3 Å. Also, the binding site residues showed only small movements, with an RMSD of 0.7 Å.
Fig. 4.

QM/MM simulation of the ERβ–2d complex. Superimposition between the QM/MM results (binding site and ligand coloured light blue and pink, respectively) and the structure resulting from the docking calculations (binding site and ligand coloured white and yellow, respectively).
5. Transcription assays
ERβ-selective ligands 2b–d, displaying the highest levels of binding affinity and selectivity for ERβ, as well as previously reported reference compounds 1c, 1d and 2a, were submitted to further biological testing to assess their pharmacological character. They were assayed for transcriptional activity through both receptor subtypes, together with estradiol for reference. Reporter gene transfection assays were conducted in human endometrial (HEC-1) cells, using expression plasmids for either full-length human ERα or ERβ and an estrogen-responsive luciferase reporter gene system [30].
The first representative examples of the previously reported Salaldox A series (Table 2, Entries 1–2), compound 1c, reached only partial activation of ERβ (EMAX = 60%) [13], whereas its “fluorinated” analog 1d displayed a general improvement in all functional properties, such as potency (EC50 = 4.8 nM), maximal effect (EMAX = 85%), and subtype-selectivity (β/α EC50-selectivity ratio = 4.0) [14]. In any case, these Salaldox A members could only be classified as partial agonists, because none of them reached maximal activation of transcription (normalized to that resulting from 100 nM estradiol). By contrast, the first example of the Salaldox B series, compound 2a, behaved as a full agonist on both receptor subtypes, but its functional beta-selectivity was not satisfactory (Entry 3).
Table 2.
Transcription Potencies of ERβ-ligands of the Salaldox A (1c,d) and Salaldox B (2a–d) series through Estrogen Receptors α and β.a
| Entry | Ligand | hERα activation | hERβ activation | β/α EC50 -selectivity ratio | ||
|---|---|---|---|---|---|---|
| EC50 (nM)b | EMAX (%)c | EC50 (nM)b | EMAX (%)c | |||
|
Salaldox A Series
| ||||||
| 1 | 1cd | 26 | 80 | 11 | 60 | 2.4 |
| 2 | 1de | 19 | 95 | 4.8 | 85 | 4.0 |
|
| ||||||
|
Salaldox B Series
| ||||||
| 3 | 2ae | 17 | 100 | 10 | 100 | 1.7 |
| 4 | 2b | 0.30 | 90 | 0.50 | 80 | 0.6 |
| 5 | 2c | 0.58 | 100 | 0.23 | 100 | 2.5 |
| 6 | 2d | 8.4 | 100 | 1.3 | 100 | 6.5 |
|
| ||||||
| 7 | estradiol | 0.09 | 100 | 0.72 | 100 | 0.12 |
Human endometrial cancer (HEC-1) cells, transfected with expression vectors for ERα or ERβ and an (ERE)2-pS2-luc reporter gene and were treated with the indicated compound and resulting luciferase activity was measured as expression of transcription (see Experimental Section).
Half-maximal effective concentration.
Maximal effect normalized to the activity with 100 nM estradiol, which was set at 100%.
See Ref. 13.
See Ref. 14.
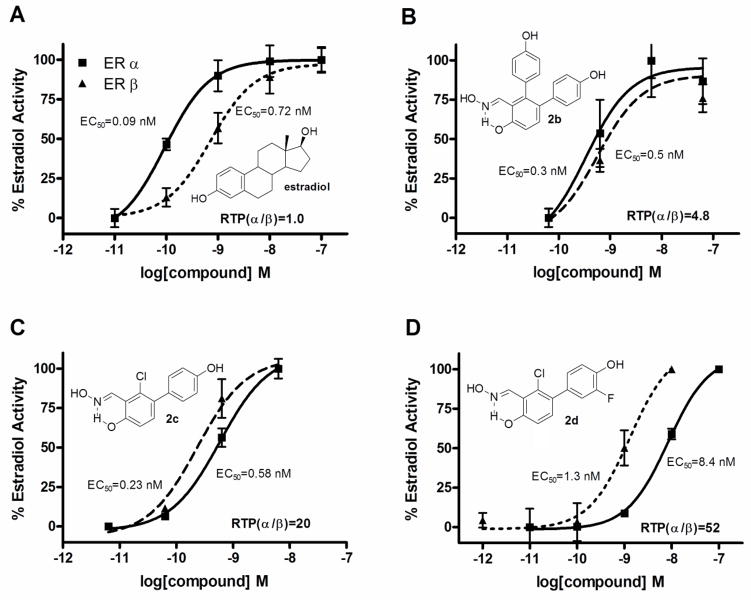
Among the newly synthesized Saladox B ligands (Table 2, Entries 4–6), diaryl-substituted derivative 2b, which proved to be non subtype-selective in binding assays, shows a similar lack of selectivity in these transcriptional assays, where it shows only a slight preference for ERα (EC50 = 0.30 nM, EMAX = 90%) versus ERβ (EC50 = 0.50 nM, EMAX = 80%). Monoaryl-substituted derivatives 2c and 2d show preferential activation of ERβ rather than ERα, consistent with their ERβ binding selectivities, and, similar to their “progenitor” 2a, both cause full activation of transcription. In particular, the single insertion of a chlorine atom in the central ring of 2a generates a full agonist (2c) having a >40-fold increased potency on ERβ and reaching a sub-nanomolar EC50 value (0.23 nM) and a slightly better beta-selectivity than 2a. The combined addition of the central chlorine group and the 3′-fluorine atom in the pendant aryl substituent, as in compound 2d, results in a jump in the selectivity of ERβ-activation (β/α EC50-selectivity ratio = 6.5), together with a substantial preservation of full-agonist potency (EC50 = 1.3 nM).
Overall, as we observed in the past [13,14], there is a general reduced ERβ-selectivity in terms of transcriptional potency versus binding affinity. This apparent discrepancy may be attributed to the fact that the receptor-ligand complex is present by itself in the binding assays, whereas in the cellular transcription assays it is engaged in various interactions with the many coregulators present in the cell; these receptor-coregulator binding interactions can act as additional modulators of ligand potency [31]. It should be noted that both 2c and 2d are by far more beta-selective full agonists than is estradiol, which has EC50 values of 0.72 nM on ERβ versus 0.09 nM on ERα (Table 2, Entry 7); one of them (2c) is even more potent on ERβ than estradiol itself.
In order to make a better comparisons of the ER subtype transcriptional potencies of our derivatives with their ER subtype binding affinities, we converted the EC50 values from the functional assays to relative transcriptional potency (RTP) values, which were calculated as RTP = EC50(estradiol)/EC50(ligand) × 100 (RTP, estradiol = 100). The RTPs give a measure of transcriptional potency relative to that of estradiol and, therefore, are suitable factors to be used in comparisons with their binding affinities, which are also measured relative to estradiol. In our present assays, estradiol has a 2.5-fold preference in favour of ERα in terms of binding (Kd [ERα] = 0.2 nM vs. [ERβ] = 0.5 nM) and a 8-fold preference in terms of transcriptional potency (EC50 [ERα] = 0.09 nM vs. [ERβ] = 0.72 nM). This seems to be caused by the fact that, when stimulated by estradiol, ERα is a more potent transcription activator than ERβ in inducing cellular responses [32]. We report herein the RTP values of our most potent ERβ-agonists 2b–d and that of estradiol, together with their dose-response curves for transcriptional activation (Fig. 5). According to these metrics, compound 2c has an RBA(β/α) ratio of 29 (Table 1) and an RTP(β/α) ratio of 20 (Fig. 5, Panel C), and compound 2d has an RBA(β/α) ratio of 46 (Table 1) and an RTP(β/α) ratio of 52 (Fig. 5, Panel D). Hence, measured relative to estradiol, the ERβ affinity preference of these compounds is, indeed, preserved in their ERβ transcriptional potency preference.
Fig. 5.
Dose-response curves for transcriptional activation by estradiol (Panel A), compound 2b (Panel B), compound 2c (Panel C) and compound 2d (Panel D) through ERβ (dashed line) and ERα (solid line). Human endometrial cancer (HEC-1) cells were transfected with expression vectors for ERα or ERβ and an (ERE)2-pS2-luc reporter gene and were treated with estradiol or compound 2b–d at the concentrations indicated. Luciferase activity was expressed relative to β-galactosidase activity from an internal control plasmid. The maximal activity with 100 nM E2 was set at 100%. Values are the mean of duplicate determinations. EC50 values give absolute potencies. The ERβ/ERα relative transcriptional potency (RTP(β/α)) ratios are calculated as explained in the text.
Although there are several examples of previously reported ERβ-agonists showing higher subtype selectivities in functional assays (i.e., DPN, SERBA-1, ERB-041) [3], compounds 2c,d are definitely the most ERβ-selective agonists of the whole salicylaldoxime class so far synthesized.
6. Conclusions
Our present studies confirm that, in contrast to our previously developed Salaldox A analogs, which never reached full activation of ERβ (partial agonists), the monoaryl-substituted Salaldox B derivatives herein reported display full agonist character on ERβ and have much higher binding affinity than their earlier counterparts. In fact, representative compounds 2c and 2d have affinities for ERβ comparable to that of estradiol itself, with Ki values in the sub-nanomolar range (Ki = 0.38 nM for 2c and 0.57 nM for 2d), together with notable levels of selectivity for ERβ over ERα. Most importantly, one of them (2d) has a remarkably improved beta-selectivity even in functional assays, which is unprecedented for any of the salicylaldoxime derivatives so far developed. This is demonstrated by ERβ/ERα selective activation by 2d, which is >50 times higher than that of estradiol, together with a comparable ERβ-agonist potency (EC50 = 1.3 nM vs 0.73 nM of estradiol). Curiously, the very same structural modifications that were previously shown to successfully improve selective ERβ-binding affinity and transcriptional activation in the Salaldox A series, such as the insertion of a Cl in the central phenyl ring and a meta-fluorine in the phenol substituent (compounds 1c and 1d), also proved to be beneficial when made at the corresponding positions of this new Salaldox B class, as shown by the behavior of compounds 2c and 2d. This could have not been easily predicted, because our docking studies indicate that the Salaldox B derivatives assume a completely different orientation in ERβ binding cavity, compared to that found for the other series. As a matter of fact, in these studies, compounds 2c and 2d have their oxime portion participating in the H-bond network with residues R346 and E305, and place their peripheral phenol OH in a position where it can establish a strong interaction with a threonine residue (T299). This last interaction is quite unusual for ERβ-agonists, since it has only been reported so far to occur when ligands having antagonist properties interact with the ERβ binding cavity [33]. Efforts to obtain X-ray structures of complexes of ERβ with these salicylaldoxime derivatives are currently underway and, if successful, should shed further light on the way these compounds interact and activate this intriguing and therapeutically exploitable nuclear receptor.
7. Experimental Section
7.1. Chemistry
7.1.1. General
Commercially available chemicals were purchased from Sigma-Aldrich or Alfa Aesar and used without further purification, with the exception of 3, which was prepared as previously reported [18]. NMR spectra were obtained with a Varian Gemini 200 MHz spectrometer. Chemical shifts (δ) are reported in parts per million downfield from tetramethylsilane and referenced from solvent references. Electron impact (EI, 70 eV) mass spectra were obtained on a ThermoQuest Finningan (TRACE GCQ plus MARCA) mass spectrometer. Melting points were measured with a Kofler apparatus. Purity was routinely measured by HPLC on a Waters SunFire RP 18 (3.0 × 150 mm, 5 μm) column (Waters, Milford, MA, www.waters.com) using a Beckmann SystemGold instrument consisting of a chromatography 125 Solvent Module and a 166 UV Detector. Mobile phases: 10 mM ammonium acetate in Millipore purified water (A) and HPLC grade acetonitrile (B). A gradient was formed from 5% to 80% of B in 10 minutes and held at 80% for 10 min; flow rate was 0.7 mL/min and injection volume was 30 μL; retention times (HPLC, tR) are given in minutes. HPLC purity of final compounds (2c,d) was determined by monitoring at 254 and 300 nm and was found in the range 96–99%. Chromatographic separations were performed on silica gel columns by flash (Kieselgel 40, 0.040–0.063 mm; Merck) or gravity column (Kieselgel 60, 0.063–0.200 mm; Merck) chromatography. Reactions were followed by thin-layer chromatography (TLC) on Merck aluminum silica gel (60 F254) sheets that were visualized under a UV lamp. Evaporation was performed in vacuo (rotating evaporator). Sodium sulfate was always used as the drying agent. Microwave assisted reaction were run in a CEM or Biotage microwave synthesizer.
7.1.2. 2-Chloro-4,4′-dimethoxybiphenyl-3-carbaldehyde (4c)
A solution of Pd(OAc)2 (2.9 mg, 0.013 mmol) and triphenylphosphine (16.9 mg, 0.064 mmol) in ethanol (1.0 mL) and toluene (1.0 mL) was stirred at RT under nitrogen for 10 min. After that period, the bromo-aryl precursor 3 [18] (0.43 mmol), an aqueous solution of Na2CO3 (1.0 mL, 2 M), and 4-methoxyphenylboronic acid (1.2 equiv) were sequentially added. The resulting mixture was heated at 100 °C in a sealed vial under nitrogen overnight. After being cooled to RT, the mixture was diluted with water and extracted with EtOAc. The combined organic phase were dried and concentrated. The crude product was purified by flash chromatography over silica gel. Elution with n-hexane/EtOAc 8:2 (Rf = 0.16) afforded 4c as a yellow solid (70% yield). 1H NMR (CDCl3) δ (ppm): 3.86 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.95–6.99 (m, 3H, H5, H3′, H5′), 7.31 (AA′XX′, 2H, JAX = 8.8 Hz, JAA′/XX′ = 2.2 Hz, H2′, H6′), 7.45 (d, 1H, J = 8.6 Hz, H6), 10.56 (s, 1H, CHO). Mp: 57–58 °C.
7.1.3. 2-Chloro-3′-fluoro-4,4′-dimethoxybiphenyl-3-carbaldehyde (4d)
Compound 4d was prepared by a cross-coupling reaction of 3 with 3-fluoro-4-methoxyphenylboronic acid (1.2 eq), following the same procedure described above for the preparation of 4c. The crude product was purified by flash chromatography over silica gel. Elution with n-hexane/EtOAc 8:2 (Rf = 0.16) afforded 4d (80% yield) as a yellow solid; 1H NMR (CDCl3) δ (ppm): 3.94 (s, 3H, OCH3), 3.95 (s, 3H, OCH3), 6.97 (d, 1H, J = 8.8 Hz, H5), 7.03 (d, 1H, J = 7.7 Hz, H5′), 7.06–7.17 (m, 2H, H2′, H6′), 7.43 (d, 1H, J = 8.8 Hz, H6), 10.55 (s, 1H, CHO). Mp: 73–74 °C.
7.1.4. 2-Chloro-4,4′-dihydroxybiphenyl-3-carbaldehyde (5c)
A solution of methoxy-substituted aldehyde 4c (0.12 mmol) in anhydrous dichloromethane (1.5 mL) was cooled to −78 °C and treated dropwise with a solution of BBr3 in dichloromethane (0.7 mL, 1M), and the resulting solution was stirred at the same temperature for 5 min and at 0 °C for 1 h. The mixture was then diluted with water and extracted with ethyl acetate. The organic phase was dried and concentrated. The crude product was purified by flash chromatography over silica gel. Elution with n-hexane/EtOAc 7:3 (Rf = 0.35) afforded pure 5c (42% yield) as a yellow solid; 1H NMR (CDCl3) δ (ppm): 6.90 (AA′XX′, 2H, JAX = 8.8 Hz, JAA′/XX′ = 2.4 Hz, H3′, H5′), 6.95 (d, 1H, J = 8.9 Hz, H5), 7.27 (AA′XX′, 2H, JAX = 8.5 Hz, JAA′/XX′ = 2.6 Hz, H2′, H6′), 7.46 (d, 1H, J = 8.8 Hz, H6), 10.53 (s, 1H, CHO), 12.11 (exchangeable s, 1H, OH). Mp: 58–59 °C.
7.1.5. 2-Chloro-3′-fluoro-4,4′-dihydroxybiphenyl-3-carbaldehyde (5d)
Compound 5d was prepared from methoxy-substituted aldehyde 4d by following the same procedure described above for the preparation of 5c. The crude product was purified by flash chromatography over silica gel. Elution with n-hexane/EtOAc 7:3 (Rf = 0.39) afforded pure 5d (81% yield) as a yellow solid; 1H NMR (CDCl3) δ (ppm): 6.95 (d, 1H, J = 8.8 Hz, H5), 7.04–7.16 (m, 3H, H2′, H5′, H6′), 7.44 (d, 1H, J = 8.8 Hz, H6), 10.52 (s, 1H, CHO), 12.12 (exchangeable s, 1H, OH). Mp: 63–64 °C.
7.1.6. (E)-2-Chloro-4,4′-dihydroxybiphenyl-3-carbaldehyde oxime (2c)
A solution of aldehyde 5c (1.0 mmol) in ethanol (15 mL) was treated with a solution of hydroxylamine hydrochloride (140 mg, 2.02 mmol) in water (3.5 mL), and the mixture was heated to 50 °C for 5h. After being cooled to RT, part of the solvent was removed under vacuum, and the mixture was diluted with water and extracted with EtOAc. The organic phase was dried and evaporated to afford a crude residue that was purified by column chromatography over silica gel. Elution with n-hexane/EtOAc 6:4 (Rf = 0.48) afforded pure 2c (99% yield) as a white solid; 1H NMR (CD3OD) δ (ppm): 6.81 (AA′XX′, 2H, JAX = 8.6 Hz, JAA′/XX′ = 2.5 Hz, H3′, H5′), 6.89 (d, 1H, J = 8.6 Hz, H5), 7.17 (AA′XX′, 2H, JAX = 8.6 Hz, JAA′/XX′ = 2.5 Hz, H2′, H6′), 7.20 (d, 1H, J = 8.6 Hz, H6), 8.79 (s, 1H, –CH=N–). 13C NMR (CD3OD) δ (ppm): 115.73, 115.86, 116.22, 131.50, 131.72, 131.98, 132.83, 133.87, 150.27, 157.83, 158.85. MS m/z 263 (M+, 15), 155 (M+H + –OH –C6H4O, 100). Mp: 144–146 °C. HPLC, tR 10.3 min.
7.1.7. (E)-2-Chloro-3′-fluoro-4,4′-dihydroxybiphenyl-3-carbaldehyde oxime (2d)
Compound 2d was prepared from aldehyde 5d by following the same procedure described above for the preparation of 2c. The crude product was purified by flash chromatography over silica gel. Elution with n-hexane/EtOAc 7:3 (Rf = 0.35) afforded pure 2d (93% yield) as a white solid; 1H NMR (acetone-d6) δ (ppm): 6.96 (d, 1H, J = 8.6 Hz, H5), 7.04–7.19 (m, 3H, H2′, H5′, H6′), 7.30 (d, 1H, J = 8.4 Hz, H6), 8.82 (exchangeable bd, 1H, J = 1.3 Hz, 4′-OH), 8.84 (s, 1H, –CH=N–), 10.91 (exchangeable s, 1H, OH), 11.14 (exchangeable s, 1H, OH). 13C NMR (acetone-d6) δ (ppm): 115.55, 116.38, 118.12 (d, J = 22.8 Hz), 118.28, 126.78 (d, J = 3.7 Hz), 132.20 (d, J = 6.4 Hz), 132.35, 132.61, 133.92, 145.17 (d, J = 12.8 Hz), 150.36, 151.73 (d, J = 238.6 Hz), 159.06. MS m/z 281 (M+, 100), 263 (M+ –H2O, 64). Mp: 125–127 °C. HPLC, tR 10.7 min.
7.2. Biological Methods
7.2.1. General
Full-length human ERα and ERβ were obtained from PanVera/Invitrogen (Carlsbad, CA). [3H]Estradiol ([3H]E2) ([2,4,6,7-3H]estra-1,3,5(10)-triene-3,17β-diol) was obtained from GE Healthcare (Piscataway, NJ) and had a specific activity of 70–120 Ci/mmol. Cell culture media were purchased from Gibco BRL (Grand Island, NY). Calf serum was obtained from Hyclone Laboratories, Inc. (Logan, UT), and fetal calf serum was purchased from Atlanta Biologicals (Atlanta, GA). The expression vectors for human ERα (pCMV5-hERα) and human ERβ (pCMV5-ERβ) were as described previously [34,35]. The estrogen responsive reporter plasmid, (ERE)2-pS2-Luc, was constructed by inserting the (ERE)2-pS2 fragment from (ERE)2-pS2-CAT into the MluI/BglII sites of pGL3-Basic vector (Promega, Madison, WI). The luciferase assay system was from Promega (Madison, WI). The plasmid pCMVβ-gal (Clontech, Palo Alto, CA), which contains the β-galactosidase gene, was used as an internal control for transfection efficiency.
7.2.2. Estrogen receptor binding assays
Relative binding affinities were determined by competitive radiometric binding assays with 2 nM [3H]E2 as tracer, as a modification of methods previously described [21,22]. The source of ER was purified full-length human ERα and ERβ purchased from Pan Vera/Invitrogen (Carlsbad, CA). Incubations were done at 0 °C for 18–24 h, and hydroxyapatite was used to absorb the purified receptor-ligand complexes (human ERs) [22]. The binding affinities are expressed as relative binding affinity (RBA) values, where the RBA of estradiol is 100%; under these conditions, the Kd of estradiol for ERα is ca. 0.2 nM, and for ERβ 0.5 nM. The determination of these RBA values is reproducible in separate experiments with a CV of 0.3, and the values shown represent the average ± range or SD of 2 or more separate determinations.
7.2.3. Cell culture and transient transfections
Human endometrial cancer (HEC-1) cells were maintained in culture as described [30]. Transfection of HEC-1 cells in 24-well plates used a mixture of 0.35 mL of serum-free MEM medium and 0.15 mL of HBSS containing 5 μL of lipofectin (Life Technologies, Rockville, MD), 20 μL of transferrin (Sigma, St. Louis, MO), 0.2 μg of pCMVβ-galactosidase as internal control, 0.5 μg of the reporter gene plasmid, 50 ng of ER expression vector. The cells were incubated at 37 °C in a 5% CO2 containing incubator for 4 h. The medium was then replaced with fresh medium containing 5% charcoal-dextran treated calf serum and the desired concentrations of ligands. Reporter gene activity was assayed at 24 h after ligand addition. Luciferase activity, normalized for the internal control β-galactosidase activity, was assayed as described [30].
7.3 Docking Methods
The crystal structure of ERα (pdb code 2I0J) [36] and ERβ (pdb code 2I0G) [36] was taken from the Protein Data Bank. After adding hydrogen atoms the two proteins complexed with their reference inhibitor were minimized using Amber 9 software [37] and parm03 force field at 300 K. The complexes were placed in a rectangular parallelepiped water box, an explicit solvent model for water, TIP3P, was used and the complexes were solvated with a 10 Å water cap. Sodium ions were added as counterions to neutralize the system. Two steps of minimization were then carried out; in the first stage, we kept the protein fixed with a position restraint of 500 kcal/mol/Å2 and we solely minimized the positions of the water molecules. In the second stage, we minimized the entire system through 5000 steps of steepest descent followed by conjugate gradient (CG) until a convergence of 0.05 kcal/A•mol.
The ligands were built using Maestro [38] and were minimized by means of Macromodel [38] in a water environment using the CG method until a convergence value of 0.05 kcal/A•mol, using the MMFFs force field and a distance-dependent dielectric constant of 1.0.
Automated docking was carried out by means of the AUTODOCK 4.0 program [25]; Autodock Tools was used in order to identify the torsion angles in the ligands, add the solvent model and assign the Kollman atomic charges to the protein. The ligand charge was calculated using the Gasteiger method. In order to prevent the loss of the intramolecular H-bond of the pseudocycle/oxime system, during the docking we blocked the torsions involved in this intramolecular bond. The regions of interest used by Autodock were defined by considering SERBA-1 into both receptors as the central group; in particular, a grid of 50, 40, and 46 points in the x, y, and z directions was constructed centered on the center of the mass of this compound. A grid spacing of 0.375 Å and a distance-dependent function of the dielectric constant were used for the energetic map calculations.
Using the Lamarckian Genetic Algorithm, the docked compounds were subjected to 100 runs of the Autodock search, using 500000 steps of energy evaluation and the default values of the other parameters. Cluster analysis was performed on the results using an RMS tolerance of 1.0 Å and the best docked conformation was used for the analysis.
All graphic manipulations and visualizations were performed by means of Chimera [39].
7.3.2 QM/MM calculations
Geometry optimization was performed by means of quantum mechanical calculations based on the Gaussian 09 software [27]. Prior to QM/MM the ERβ–2d complex was energy-minimized using AMBER 11 and the parm96 force field [40]. The complex was placed into a rectangular parallelepiped water box; an explicit solvent model for water, TIP3P, was used, and the complex was solvated with a 10 Å water cap. Sodium ions were added as counterions to neutralize the system. Two steps of minimization were then carried out. In the first stage, we kept the complex fixed with a position restraint of 500 kcal/(mol•Å2) and we solely minimized the positions of the water molecules. In the second stage, we minimized the entire system through 20000 steps of steepest descent followed by conjugate gradient until a convergence of 0.05 kcal/(mol•Å) was attained. All the α carbons of the protein were blocked with a harmonic force constant of 10 kcal/(mol•Å2). The minimized structure was used as starting structure for the QM/MM calculations. The QM region was described by the B3LYP chemical model with a 6–31G++** basis set and contained the ligand, the structural water molecule and the side chains of T299, E305 and R346. The B3LYP model is a combination of the Becke three-parameter hybrid functional [41] with the Lee–Yang–Parr correlation functional (which also includes density gradient terms [42]. On the MM region, the parm96 force field was applied using no constraints.
Supplementary Material
Acknowledgments
One of the authors (CG) is grateful to the Italian Ministry for University and Research (MIUR), Rome, Italy, for a triennial (2008–2010) PhD fellowship (“grandi progetti strategici”). Dr. Giorgio Placanica and Dr. Caterina Orlando are gratefully acknowledged for technical assistance in the analysis of the chemical products. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR001081). Support from the National Institutes of Health is gratefully acknowledged (PHS R37 DK015556, JAK; NRSA F30 ES016484-01 and T32 GM070421, JRG).
ABBREVIATIONS
- ER
estrogen receptor
- ERα
estrogen receptor subtype alpha
- ERβ
estrogen receptor subtype beta
- LBD
ligand binding domain
- RBA
relative binding affinity
- RTP
relative transcriptional potency
- TLC
thin-layer chromatography
- CG
conjugated gradient
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuiper GGJM, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson J-Å. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohler ML, Narayanan R, Coss CC, Hu K, He Y, Wu Z, Hong S-S, Hwang DJ, Miller DD, Dalton JT. Expert Opin Ther Patents. 2010;20:507–534. doi: 10.1517/13543771003657164. [DOI] [PubMed] [Google Scholar]
- 3.Minutolo F, Macchia M, Katzenellenbogen BS, Katzenellenbogen JA. Med Res Rev. doi: 10.1002/med.20186. in press. published online: 4 Dec 2009, and references therein. [DOI] [Google Scholar]
- 4.Harris HA, Albert LM, Leathurby YL, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr Endocrinol. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 5.Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Curr Top Med Chem. 2006;6:181–202. [PubMed] [Google Scholar]
- 6.Prins GS, Korach KS. Steroids. 2008;73:233–244. doi: 10.1016/j.steroids.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. Hum Reprod. 2005;20:936–941. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- 8.Harris HA. Mol Endocrinol. 2007;21:1–13. doi: 10.1210/me.2005-0459. [DOI] [PubMed] [Google Scholar]
- 9.Weiser MJ, Foradori CD, Handa RJ. Brain Res Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang EC, Frasor J, Komm B, Katzenellenbogen BS. Endocrinology. 2006;147:4831–4842. doi: 10.1210/en.2006-0563. [DOI] [PubMed] [Google Scholar]
- 11.Lindberg MK, Movérare S, Skrtic S, Gao H, Dahlman-Wright K, Gustafsson J-Å, Ohlsson C. Mol Endocrinol. 2003;17:203–208. doi: 10.1210/me.2002-0206. [DOI] [PubMed] [Google Scholar]
- 12.Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, Gustafsson J-Å. Pharmacol Rev. 2006;58:773–781. doi: 10.1124/pr.58.4.8. [DOI] [PubMed] [Google Scholar]
- 13.Minutolo F, Bellini R, Bertini S, Carboni I, Lapucci A, Pistolesi L, Prota G, Rapposelli S, Solati F, Tuccinardi T, Martinelli A, Stossi F, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA, Macchia M. J Med Chem. 2008;51:1344–1351. doi: 10.1021/jm701396g. [DOI] [PubMed] [Google Scholar]
- 14.Minutolo F, Bertini S, Granchi C, Marchitiello T, Prota G, Rapposelli S, Tuccinardi T, Martinelli A, Gunther JR, Carlson KE, Katzenellenbogen JA, Macchia M. J Med Chem. 2009;52:858–867. doi: 10.1021/jm801458t. [DOI] [PubMed] [Google Scholar]
- 15.De Angelis M, Stossi F, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA. J Med Chem. 2005;48:1132–1144. doi: 10.1021/jm049223g. [DOI] [PubMed] [Google Scholar]
- 16.(a) Minutolo F, Bertini S, Papi C, Carlson KE, Katzenellenbogen JA, Macchia M. J Med Chem. 2001;44:4288–4291. doi: 10.1021/jm010948j. [DOI] [PubMed] [Google Scholar]; (b) Minutolo F, Antonello M, Bertini S, Rapposelli S, Rossello A, Sheng S, Carlson KE, Katzenellenbogen JA, Macchia M. Bioorg Med Chem. 2003;11:1247–1257. doi: 10.1016/s0968-0896(02)00640-5. [DOI] [PubMed] [Google Scholar]
- 17.(a) Minutolo F, Antonello M, Bertini S, Ortore G, Placanica G, Rapposelli S, Sheng S, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA, Macchia M. J Med Chem. 2003;46:4032–4042. doi: 10.1021/jm0308390. [DOI] [PubMed] [Google Scholar]; (b) Tuccinardi T, Bertini S, Martinelli A, Minutolo F, Ortore G, Placanica G, Prota G, Rapposelli S, Carlson KE, Katzenellenbogen JA, Macchia M. J Med Chem. 2006;49:5001–5012. doi: 10.1021/jm060560u. [DOI] [PubMed] [Google Scholar]
- 18.Akermark B. Acta Chem Scand. 1970;24:1459–1460. [PubMed] [Google Scholar]
- 19.Miyaura N, Suzuki A. Chem Rev. 1995;95:2457–2483. [Google Scholar]
- 20.Karabatsos GJ, Hsi N. Tetrahedron. 1967;23:1079–1095. [Google Scholar]
- 21.Katzenellenbogen JA, Johnson HJ, Jr, Myers HN. Biochemistry. 1973;12:4085–4092. doi: 10.1021/bi00745a010. [DOI] [PubMed] [Google Scholar]
- 22.Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Biochemistry. 1997;36:14897–14905. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- 23.Mewshaw RE, Bowen SM, Harris HA, Xu ZB, Manas ES, Cohn ST. Bioorg Med Chem Lett. 2007;17:902–906. doi: 10.1016/j.bmcl.2006.11.066. [DOI] [PubMed] [Google Scholar]
- 24.Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith JC, Jr, Harris HA. J Med Chem. 2004;47:5021–5040. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- 25.Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 26.Gellman SH. Biochemistry. 1991;30:6633–6636. doi: 10.1021/bi00241a001. [DOI] [PubMed] [Google Scholar]
- 27.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision. Gaussian, Inc; Wallingford CT: 2009. p. A.1. [Google Scholar]
- 28.(a) Topf M, Várnai P, Richards WG. J Am Chem Soc. 2002;124:14780–14788. doi: 10.1021/ja026219q. [DOI] [PubMed] [Google Scholar]; (b) Friesner RA. Drug Discovery Today: Technologies. 2004;1:253–260. doi: 10.1016/j.ddtec.2004.11.008. [DOI] [PubMed] [Google Scholar]; (c) Yonezawa Y, Nakata K, Sakakura K, Takada T, Nakamura H. J Am Chem Soc. 2009;131:4535–4540. doi: 10.1021/ja807814x. [DOI] [PubMed] [Google Scholar]
- 29.Bauschlicher CW. Chem Phys Lett. 1995;246:40–44. [Google Scholar]
- 30.Sun J, Meyers MJ, Fink BE, Rajendran R, Katzenellenbogen JA, Katzenellenbogen BS. Endocrinology. 1999;140:800–804. doi: 10.1210/endo.140.2.6480. [DOI] [PubMed] [Google Scholar]
- 31.(a) Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. J A J Steroid Biochem Mol Biol. 2000;74:279–285. doi: 10.1016/s0960-0760(00)00104-7. [DOI] [PubMed] [Google Scholar]; (b) Gee AC, Carlson KE, Martini PG, Katzenellenbogen BS, Katzenellenbogen JA. Mol Endocrinol. 1999;13:1912–1923. doi: 10.1210/mend.13.11.0373. [DOI] [PubMed] [Google Scholar]
- 32.Huang Y, Li X, Muyan M. Endocrine. 2010 doi: 10.1007/s12020-010-9411-8. published online: 11 November 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(a) Zhou H-B, Sheng S, Compton DR, Kim Y, Joachimiak A, Sharma S, Carlson KE, Katzenellenbogen BS, Nettles KW, Greene GL, Katzenellenbogen JA. J Med Chem. 2007;50:399–403. doi: 10.1021/jm061035y. [DOI] [PubMed] [Google Scholar]; (b) Muthyala RS, Sheng S, Carlson KE, Katzenellenbogen BS, Katzenellenbogen JA. J Med Chem. 2003;46:1589–1602. doi: 10.1021/jm0204800. [DOI] [PubMed] [Google Scholar]
- 34.Wrenn CK, Katzenellenbogen BS. J Biol Chem. 1993;268:24089–24098. [PubMed] [Google Scholar]
- 35.McInerney EM, Weis KE, Sun J, Mosselman S, Katzenellenbogen BS. Endocrinology. 1998;139:4513–4522. doi: 10.1210/endo.139.11.6298. [DOI] [PubMed] [Google Scholar]
- 36.Norman BH, Dodge JA, Richardson I, Borromeo PS, Lugar CW, Jones SA, Chen K, Wang Y, Durst GL, Barr RJ, Montrose-Rafizadeh C, Osborne HE, Amos RM, Guo S, Boodhoo A, Krishnan V. J Med Chem. 2006;49:6155–6157. doi: 10.1021/jm060491j. [DOI] [PubMed] [Google Scholar]
- 37.Case DA, Darden TA, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Pearlman DA, Crowley M, Walker RC, Zhang W, Wang B, Hayik S, Roitberg A, Seabra G, Wong KF, Paesani F, Wu X, Brozell S, Tsui V, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Beroza P, Mathews DH, Schafmeister C, Ross WS, Kollman PA. AMBER. Vol. 9. University of California; San Francisco: 2006. [Google Scholar]
- 38.Schrödinger Inc. Portland, OR: 1999. [Google Scholar]
- 39.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 40.Case DA, Darden TA, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Cai Q, Ye X, Wang J, Hsieh M-J, Cui G, Roe DR, Mathews DH, Seetin MG, Sagui C, Babin V, Luchko T, Gusarov S, Kovalenko A, Kollman PA. AMBER. Vol. 11. University of California; San Francisco: 2010. [Google Scholar]
- 41.Becke A. Phys Rev A. 1988;38:3098–3100. doi: 10.1103/physreva.38.3098. [DOI] [PubMed] [Google Scholar]
- 42.(a) Lee C, Yang W, Parr RG. Phys Rev B. 1988;37:785–789. doi: 10.1103/physrevb.37.785. [DOI] [PubMed] [Google Scholar]; (b) Miehlich B, Savin A, Stoll H, Preuss H. Chem Phys Lett. 1989;157:200–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.