Abstract
Previous studies have shown that certain 1,2,3,4-tetrahydroisoquinoline derivatives (TIQs) are neurotoxins inducing Parkinsonism. Further, individual enantiomers of these toxins such as (R/S)-N-methylsalsolinol ((R/S)-NMSal) possess distinct neurotoxicological properties. In this work, a chiral capillary electrophoresis (CE) method with electrospray ionization-tandem mass spectrometric (ESI-MS/MS) detection was developed for the quantification of TIQ enantiomers. Enantioseparation was achieved with sulfated β-cyclodextrin (sulfated β-CD) as chiral selector. To avoid any potential contamination of MS ionization source by the non-volatile chiral selector, partial filling technique was deployed in the CE separation. TIQ derivatives, including (R/S)-6,7-dihydroxy-1-methy-TIQ (salsolinol, Sal), (R/S)-1-benzyl-TIQ (BTIQ), and (R/S)-NMSal, were base-line resolved with resolution values (R) ranging from 3 (for Sal) to 4.5 (for BTIQ), which were much better than those reported previously by HPLC methods. ESI-MS/MS detection of the resolved TIQ enantiomers was specific and sensitive (LOD = 1.2 μM for Sal enantiomers). The proposed chiral CE-MS/MS method was used to study in vitro formation of (R/S)-NMSal. It was found that NMSal was formed from the incubation of epinine (a dopamine metabolite) with acetaldehyde (a metabolite of alcohol). More interestingly, four isomers of NMSal were separated and detected in the incubation solution. They were identified as (R)-e.e-NMSal, (R)-e.a-NMSal, (S)-e.e-NMSal, and (S)-e.a-NMSal. This was the first lab evidence that this parkinsonian neurotoxin exists in multiple isomeric forms.
Keywords: Chiral capillary electrophoresis, mass spectrometry, neurotoxin, tetrahydroisoquinoline, N-methylsalsolinol, stereoisomer
1. Introduction
It is well documented that some tetrahydroisoquinoline derivatives such as N-methylsalsolinol (NMSal) cause neurotoxicological damages similar to those caused by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [1-3]. MPTP is a well known synthetic neurotoxin that causes Parkinsonism in humans, monkeys, and various animals [4]. Therefore, study on TIQs' neurotoxicity has been intensive [5-7]. Very importantly, it has been found that many chiral TIQ compounds exhibit enantioselective neurotoxicity, that is the two enantiomers possess distinct neurotoxicological properties [8-10]. For example, (R)-enantiomer of NMSal was found 1000 times more potent to induce Parkinsonism in rat than the (S)-enantiomer [2, 11]. Study on TIQs' enantioselective neurotoxicity requires sensitive quantification of these toxic compounds with stereochemical selectivity.
Analytical methods based on high-performance liquid chromatography (HPLC) [12-17], capillary electrophoresis (CE) [18-21], gas chromatography-mass spectrometry (GC-MS) [22-226], and HPLC-MS [27-29] have been developed for enantiomeric quantification of TIQs. Since many TIQs such as Sal and NMSal are highly hydrophilic and easily oxidized in basic solutions, the cumbersome sample pretreatment and pre-column derivatization procedures required in GC-MS analysis can be problematic causing a significant loss of the analytes. The chiral HPLC-MS methods reported previously allowed a facile determination of Sal enantiomers without pre-column derivatization. However, efforts to achieve a chiral separation of other TIQ neurotoxins including MNSal and BTIQ on the β-cyclodextrin bonded silica column failed in our previous studies [29].
Compared with HPLC, CE offers advantages including high separation efficiency, short separation time, and the compatibility with small sample volume/mass. In recent decade much interest has been given to coupling of CE with MS for chiral analysis. Extensive reviews on this topic were given [30 - 31]. A major challenge remains the potential contamination of MS ionization source by the non-volatile chiral selector and other additives in CE running buffer. Several chiral selectors including chiral crown ethers [32 - 33], proteins [34], chiral micelles [35], and cyclodextrins [36-37] were used in chiral CE-MS analysis. In search for an effective assay of TIQ enantiomers, a CE-MS method with high separation efficiency, peak identification capability, and assay sensitivity was developed. In this work, sulfated β-cyclodextrin (β-CD) was selected as the chiral selector since it was the most effective chiral selector for resolving Sal enantiomers by using HPLC based on our previous investigations. In addition, since sulfated β-CD is negatively charged, it migrates away from the MS ionization source in a CE-MS separation. To avoid any potential contamination by the non-volatile chiral selector, partial filling technique was deployed. Three most extensively studied TIQ neurotoxins, i.e. Sal, NMSal, and BTIQ were selected as the model analytes. By using the chiral analytical method, in vitro formation of NMSal from incubation of epinine (a dopamine metabolite) with acetaldehyde (an alcohol metabolite) was investigated. Taking advantage of the mass-specific detection, stable isotope labeled chemicals could be used to facilitate the CE peak identification in the study of this important neurotoxin.
2. Materials and Methods
2.1. Materials
Racemic Sal, racemic 1-BTIQ, dopamine (DA), epinine, acetaldehyde, acetaldehyde-2,2,2-d3, ammonium acetate, acetic acid and sulfated sodium salt of β-Cyclodextrin (sulfated β-CD) were purchased from Sigma–Aldrich (St. Louis, MO, USA). (+)-(R)- /(-)-(S)-Sal enantiomers were prepared from racemic Sal as described in our previous work [19]. Milli-Q water (Millipore) was used through out the work. Prior to CE analysis, all samples and the running buffer were filtered through a nylon 0.22 μm syringe filter.
2.2. CE-MS Apparatus
The CE-MS system consisted of an Agilent 7100 capillary electrophoresis system and a ThermoFinnigan mass spectrometer (LCQ DECA). A CE-MS adapter kit from Agilent Technologies was used for the coupling. All CE operations including capillary flush, chiral selector loading, sample injection, and separation were automated. The mass spectrometer was equipped with an ESI source and a syringe pump. It was operated in a positive ion mode. Multiple stage mass spectrometry (MS/MS) experiments were performed to isolate and fragment the targeted ions. The operating conditions of the MS detector were optimized with a solution of Sal (1.0 μM) infused into the ESI-MS system with a syringe pump at a flow rate of 2μL /mL. Parameters were optimized using the Autotune Program. Data were collected and analyzed by using Xcalibur.
2.3. Chiral CE-MS Assay
Capillary was flushed with the CE running buffer for 3 min, and then the chiral selector solution was introduced into the capillary by pressure injection at 100 mbar for 50 s. A sample solution was injected at 50 mbar for 12 s. The capillary inlet end was placed in the CE running buffer vial and separation was started by applying a positive voltage. At the same time MS detection began (sheath liquid was automatically turned on by the mass spectrometer).
CE conditions: column, 50μm ID /360μm OD × 75 cm long fused-silica capillary; CE running buffer, 20 mM acetic acid /ammonium acetate buffer at pH 5.5; chiral selector solution, 1.0 mM sulfated β-CD in CE running buffer; CE voltage, positive 25.0 kV; column temperature, 20 °C.
MS conditions: sheath liquid, 50% methanol in water containing 0.1% acetic acid at 2μL /min; spray voltage, 4 kV; capillary temperature, 220°C; sheath gas, 20 arbitrary units (au); auxiliary gas, 0 au. For SRM experiments, normalized collision energy was set at 30 with an isolation width of 2.0 u, and the activation time was set at 30 ms.
2.4. In vitro study of NMSal formation
Epinine was dissolved in 50mM phosphate buffered saline (PBS) (pH 7.4). The solution was mixed with acetaldehyde. The final concentrations were 10 mM and 30 mM for epinine and acetaldehyde, respectively. The mixture was incubated at 37°C for 2h and then centrifuged at 10000×g for 10min. Supernatant was collected for NMSal quantification. Incubations of epinine with acetaldehyde-d3 were carried out similarly.
3. Results and Discussion
3.1. Chiral CE-MS of TIQs
In our previous work on chiral CE separation of TIQs, a very complex running buffer containing β-CD as the chiral selector had to be used, which did not allow its coupling with MS detection [18]. Ammonium acetate buffer was selected as the background electrolyte in this work because it was volatile and would not contaminate the MS ionization source. Volatile chiral selectors such as certain chiral crown ethers are ideal for chiral CE-MS analysis. However, our efforts to resolve Sal enantiomers by using chiral crown ethers (e.g. 2-hydroxymethyl-18-crown-6) came out with no success. From our computational studies, cyclodextrins form inclusion complexes with Sal, NMSal, and BTIQ with stereochemical preferences in terms of the stabilization energy [38]. Sulfated β-CD, although non-volatile, is negatively charged, and thus migrates against the EOF or away from the MS detector in the proposed CE-MS set-up. Therefore, it was selected as the chiral selector. Further, partial filling technique [32, 39-40] was deployed to ensure that sulfated β-CD would not get into the MS ionization source. Results from studying various separation conditions with Sal enantiomers as the model solutes are described as following.
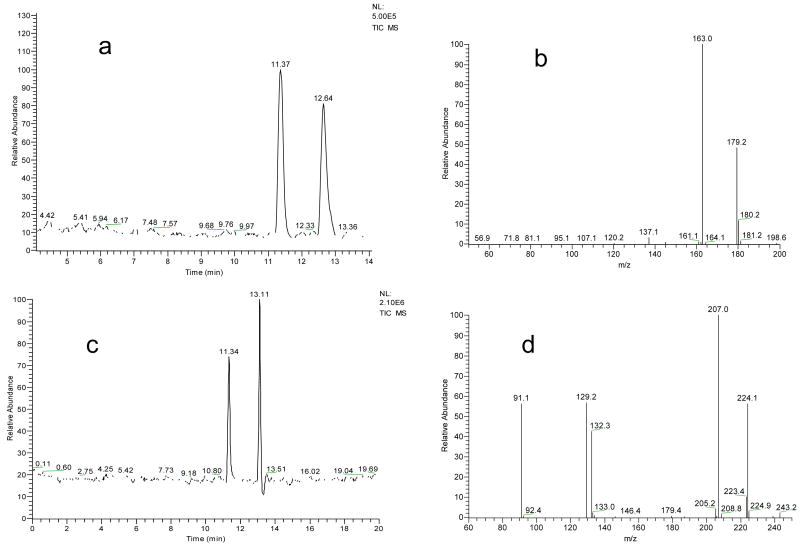
The pH of CE running buffer affects the magnitude of EOF and the apparent charge numbers on the chiral selector and the analytes. It, therefore, affects the separation results. In the tested pH range from 3 to 7, the best resolution of Sal enantiomers was achieved at pH 5.5. The migration time of Sal increased as the running buffer pH decreased. When it was < 3, Sal would not be eluted out. Concentration of sulfated β-CD in the range of 0.1 -10.0 mM was investigated. Sal enantiomers were base-line resolved with sulfated β-CD concentration at 1.0 mM or above. At high sulfated β-CD concentrations (e.g. 5mM), better resolutions were obtained, but the migration times increased significantly (to about 30mins). To obtain both an acceptable separation resolution and good separation efficiency, sulfated β-CD at 1mM was used for further studies. Sheath liquid was used for the coupling. The composition of sheath liquid was investigated to obtain the best MS detection sensitivity. Mixtures of water and methanol or acetonitrile at various ratios containing acetic acid were tested. It was found that better MS signals were obtained with water /methanol (50:50 v/v) with 0.1% acetic acid. Acetic acid was added to enhance the formation of positive ions in ESI. The flow rate of sheath liquid was set at 2μl /min. An unstable ESI spray was observed at a flow rate of 1μl /min. Under the selected experimental conditions, enantiomers of Sal, BTIQ, and NMSal were all base-line separated and sensitively detected. Typical electropherograms from the separations and the MS2 spectra are shown in Figure 1. The resolution values (R) were 3 for Sal enantiomers and 4.5 for BTIQ enantiomers, which were much better than those previously reported by using HPLC-MS methods [27-29]. Moreover, the chiral CE separation was completed within 15 min.
Fig 1.
Separation of Sal and BTIQ enantiomers by the proposed chiral CE-MS/MS method: (a) TIC of m/z 180 from Sal separation; (b) MS2 of m/z 180 from (a); (c) TIC of m/z 224 from BTIQ separation; and (d) MS2 of m/z 224 from (c). Separation conditions: running buffer, 20 mM ammonium acetate buffer (pH 5.5) containing 1 mM sulfated β-CD with the use of partial filling technique; capillary, 50 μm id × 75 cm length; and voltage applied, positive 25 kV. ESI-MS detection conditions: sheath liquid, 50% methanol (v/v) with 0.1% acetic acid at 2 μL /min; ESI spray voltage, 4 kV; detection mode, positive. Ananlyte concentration: 25 μM for each enantiomer.
3.2. Analytical figures of merit
The present chiral CE-MS method was evaluated for enantiomeric quantification of Sal in terms of the response linearity, limit of detection, and reproducibility. Five point calibration curves were prepared by analyzing authentic Sal racemate solutions at concentrations of 5.0, 10.0, 25.0, 50.0, and 100 μM. Peak heights were used for the quantification. Linear regression analysis on the results of peak heights versus concentrations yielded the following calibration equations:
where H is peak height and C the concentration (μM). A good linearity was obtained for both calibration curves with correlation coefficients > 0.985. From the calibration curves, limits of detection (LOD) were estimated to be 1.2 μM for (R)-Sal and 1.5μM for (S)-Sal (signal / noise ratio =3). The reproducibility of analyte response (RSD %) in terms of peak height and migration time were studied by separating a Sal racemate solution (10.0 μM each enantiomer) for 6 times. The RSDs of peak height and migration time for both enantiomers were found <1.7% and 2.4% (n= 6), respectively.
3.3. In vitro formation of NMSal
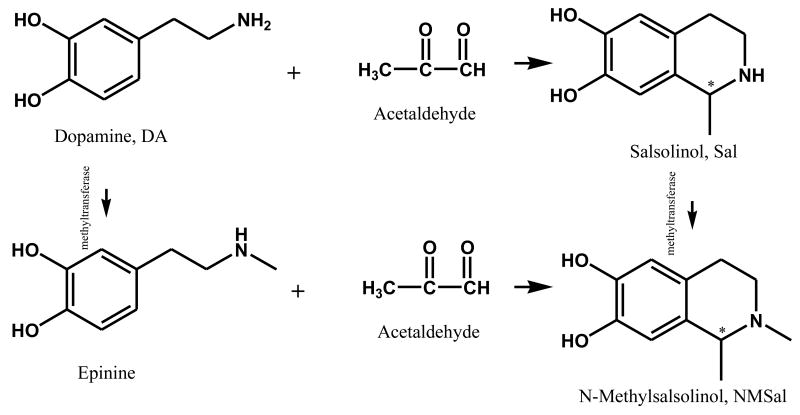
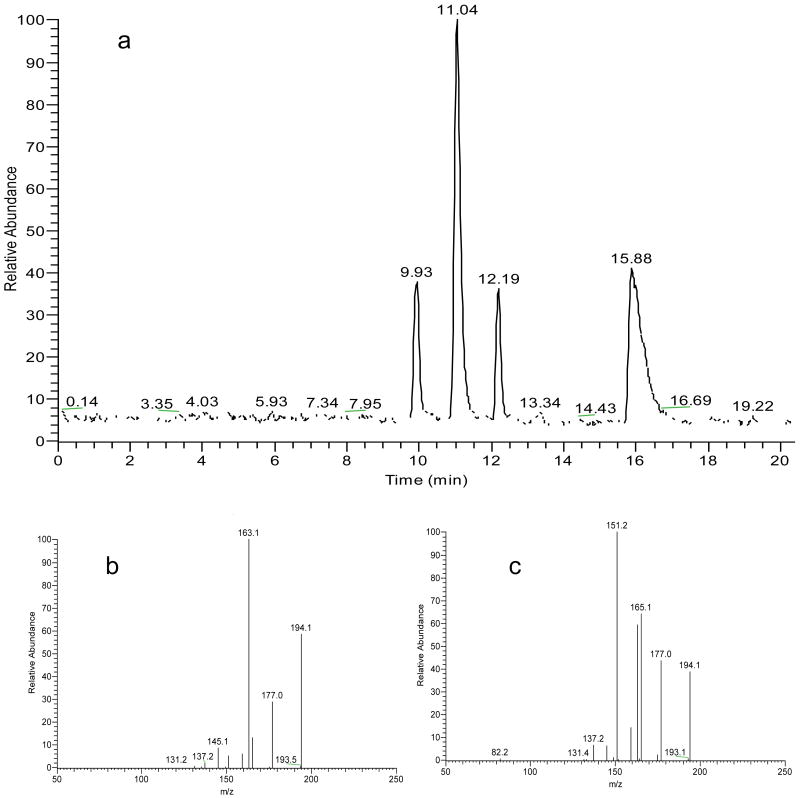
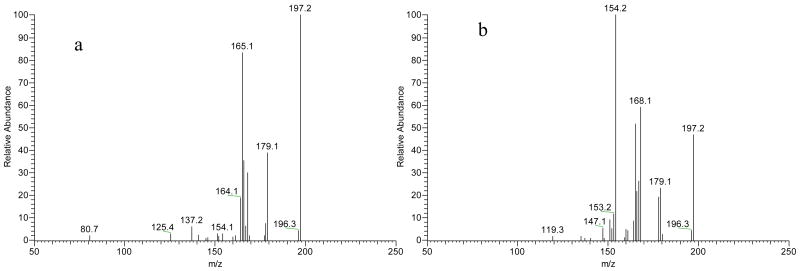
The proposed chiral CE-MS/MS method was used to study in vitro formation of NMSal, a parkinsonian neurotoxin. There are several possible synthetic pathways of NMSal as outlined in Fig. 2. Dopamine is an important neurotransmitter. Loss of dopaminergic neurons is a key pathological feature of Parkinson's disease. Condensation of dopamine with acetaldehyde (a metabolite of alcohol) forms Sal that can be converted to NMSal by N-methyltransferase [1]. Another possible synthetic pathway of NMSal is the condensation of epinine and acetaldehyde. Epinine can be formed from dopamine via N-methylation [41].To the best of our knowledge, little study of NMSal formation from epinine and acetaldehyde has been done so far. In this work, epinine was incubated with acetaldehyde in mimic physiological conditions for 2 hrs. The resultant incubation solutions were analyzed to quantify NMSal enantiomers by the chiral CE-MS method. Although detection of two enantiomers of NMSal was expected, four CE peaks were actually observed in the TIC electropherogram of m/z 194 ion, protonated molecular ion [M+H]+ of NMSal, as shown in Fig. 3. Further MS/MS investigations revealed that the MS2 spectra for the first and the third peaks were identical, and those for the second and the fourth peaks were identical. The two different MS2 spectra are shown in Figure 3b & 3c. Although the two spectra were not identical they contained almost the same product ions with only differences in relative abundances. This indicated that the four species detected were likely four isomers of NMSal. Its chemical structure was determined from the MS2 spectrum. Ions m/z 177, 163, 165, 151, 145, and 137 in the spectrum were all assigned as the product ions of [NMSal+H]+ m/z 194. The proposed fragmentation pathways are shown in Fig. 4. The structure elucidation was further verified by using NMSal-d3 obtained from incubation of epinine and acetaldehyde-d3 (CD3COCOH). Again, four CE peaks were obtained from the separation with MS detection set for m/z 197, [M+H]+ of NMSal-d3. The MS2 spectra for the first and the third peaks were identical (Fig. 5a), and those for the second and the fourth peaks (Fig. b5) were identical. Most product ions appearing in the spectrum were in consistent with the fragmentation pathways of NMSal shown in Fig. 4: C11H13D2NO+ m/z 179 corresponding to C11H15NO+ m/z 177; C10H9D2O2+ m/z 168 to C10H11O2+ m/z 163; C9H8D3NO2+ m/z 168 to C9H11NO2+ m/z 165; C10H8D3O2+ m/z 154 to C10H11O2+ m/z 151; and finally C8H9O2+ m/z 137 was observed from both NMSal and NMSal-d3.
Fig 2.
Potential biosynthetic pathways of NMSal, a parkinsonian neurotoxin: the condensation of dopamine (a neurotransmitter) with acetaldehyde (an alcohol metabolite) produces Sal that can be converted to NMSal by N-methytransferase; and the condensation of epinine that can be formed from dopamine via N-methylation with acetaldehyde produces NMSal.
Fig 3.
Chiral CE-MS analysis of a sample solution from incubating epinine with acetaldehyde: (a) TIC electropherogram of m/z 194; (b) MS2 of m/z 194 at 9.93 min (peak 1); and (c) MS2 of m/z 194 at 11.04 min (peak 2). CE and MS detection condition were as in Figure 1. Incubation conditions: [Epinine] = 10 mM; [acetaldehyde] = 30 mM; medium, PBS pH 7.4; temperature, 37 °C; time, 3 hours. Incubation solution was diluted 10 times before analysis. MS2 spectra for peak 1 and peak 3 were identical, and those for peak 2 and peak 4 were identical.
Fig 4.
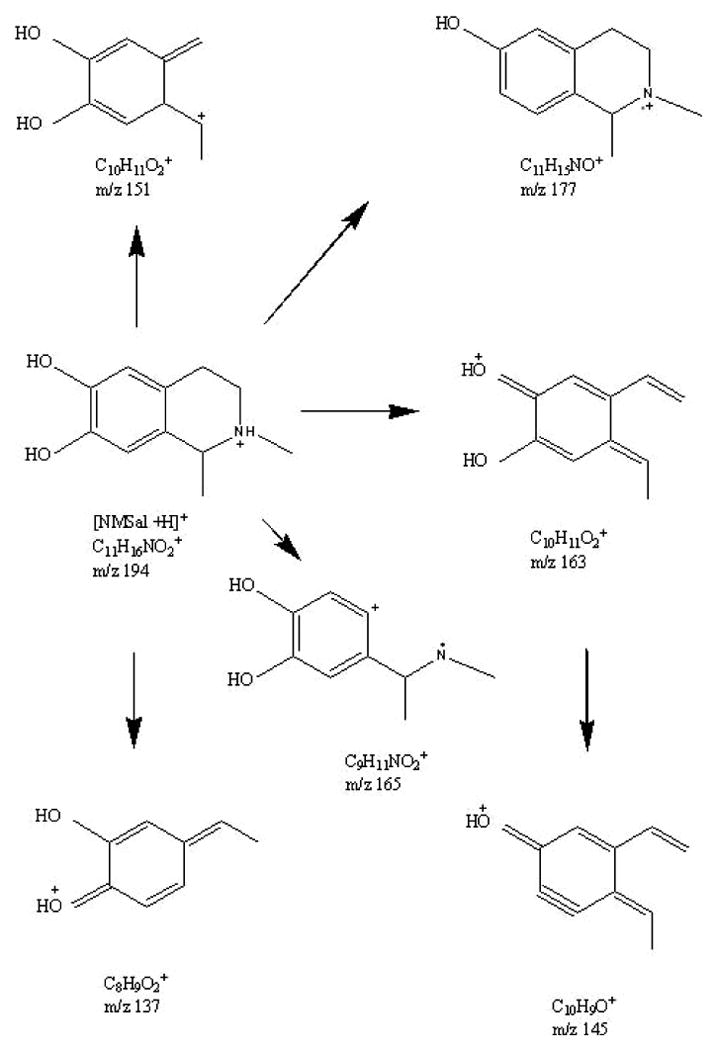
Proposed CID fragmentation pathways of NMSal.
Fig 5.
MS2 spectra of NMSal-d3 obtained from separating an incubation solution of epinine and acetaldehyde-d3: (a) for the first and third peaks, and (b) for the second and fourth peaks. Refer to Fig 3a for the TIC electropherogram.
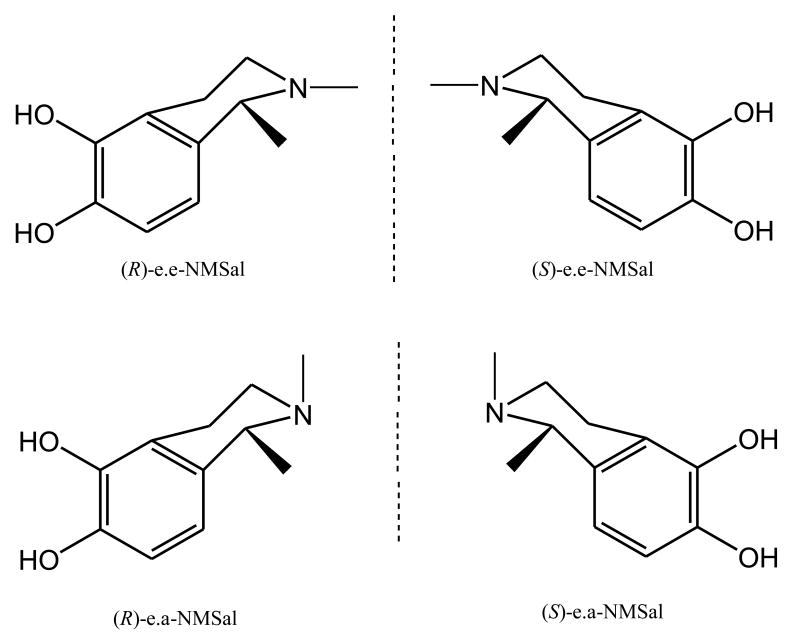
Above results indicate that NMSal exists in for isomeric forms. It should be pointed out that only two isomers (enantiomers) of Sal were detected in the incubation solution from dopamine and acetaldehyde (see Fig 2 for the reaction), and the enantiomeric ratio was found to be 1, i.e. racemic Sal was produced from the incubation. We believe that the methyl group substitution at the nitrogen atom in the piperidine structure of NMSal leads to the complex stereoisomerism. The four NMSal isomers detected are two pairs of enantiomers whose MS spectra are identical. The two conformational isomeric forms result from the two different orientations the methyl group assumes (either axial or equatorial). When the methyl group occupies an equatorial position, the isomer is more stable than that when it occupies an axial position. This is why two small peaks (axial conformation) and two large peaks (equatorial conformation) were observed in the electropherogram (Fig. 3a). By comparing the two MS2 spectra (Fig 3b & 3c), it can be seen that the relative abundance of ion m/z 165 from equatorial isomer is much larger than that from axial isomer. These results support the proposed chemical structure of this ion (Fig. 4). Based on the above analysis, the separated four NMSal isomers were identified as (R)-e.e-NMSal, (R)-e.a-NMSal, (S)-e.e-NMSal, and (S)-e.a-NMSal. Their chemical structures are shown in Fig 6. This is the first demonstration that NMSal exists in four isomeric forms. The complex stereoisomerism should be taken into consideration when studying the neurotoxicity of this important neurotoxin.
Fig 6.
Chemical structures of NMSal isomers formed from incubation of epinine with acetaldehyde. They were eluted into 4 peaks in the chiral CE-MS separation and identified by MS/MS.
4 Conclusions
A chiral CE-MS/MS method was developed for enantiomeric quantification of tetrahydroisoquinoline-derived neurotoxins. The method had much better chiral separation efficiency compared with the HPLC-based methods previously reported. Analysis operations, including capillary flush, chiral selector loading, sample injection, separation, and MS detection were all automated. The assay was proved to be sensitive, selective, reproducible, and easy to carry out. Study of in vitro formation of NMSal, a parkinsonian neurotoxin, showed that NMSal was formed from incubation of epinine (a dopamine metabolite) with acetaldehyde (a metabolite of alcohol). More interestingly, four NMSal isomers were separated and identified in the incubation solution by using the proposed highly selective chiral CE-MS/MS method. This was the first lab evidence that NMSal exists in multiple isomeric forms.
Acknowledgments
Support from US National Institutes of Health (SC1 GM089557) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nagatsu T. Neurosci Res. 1997;29:99. doi: 10.1016/s0168-0102(97)00083-7. [DOI] [PubMed] [Google Scholar]
- 2.Naoi M, Maruyama W, Akao Y, Yi H. Neurotoxicology and Teratology. 2002;24:579. doi: 10.1016/s0892-0362(02)00211-8. [DOI] [PubMed] [Google Scholar]
- 3.Lorenc-Koci E, Antkiewicz-Michaluk L, Kamińska A, Lenda T, Zieba B, Wierońska J, Smiałowska M, Schulze G, Rommelspacher H. Neurosci. 2008;156:973. doi: 10.1016/j.neuroscience.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 4.Langston JW, Ballard P, Tetrud JW, Irwin I. Science. 1983;219:979. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- 5.Yi H, Akao Y, Maruyama W, Chen K, Shih J, Naoi M. J Neurochem. 2006;96:541. doi: 10.1111/j.1471-4159.2005.03573.x. [DOI] [PubMed] [Google Scholar]
- 6.DeCuypere M, Lu Y, Miller DD, LeDoux MS. J Neurochem. 2008;107:1398. doi: 10.1111/j.1471-4159.2008.05709.x. [DOI] [PubMed] [Google Scholar]
- 7.Lee J, Ramchandani VA, Hamazaki K, Engleman EA, McBride WJ, Li TK, Kim HY. Alcohol Clin Exp Res. 2010;34:242. doi: 10.1111/j.1530-0277.2009.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storch A, Ott S, Hwang YI, Ortmann R, Hein A, Frenzel S, Matsubara K, Ohta S, Wolf HU, Schwarz J. Biochem Pharmacol. 2002;63:909. doi: 10.1016/s0006-2952(01)00922-4. [DOI] [PubMed] [Google Scholar]
- 9.Maruyama W, Narabayashi H, Dostert P, Naoi M. J Neural Transm. 1996;103:1069. doi: 10.1007/BF01291791. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama W, Naoi M, Kasamatsu T, Hashizume Y, Takahashi T, Kohda K, Dostert P. J Neurochem. 1997;69:322. doi: 10.1046/j.1471-4159.1997.69010322.x. [DOI] [PubMed] [Google Scholar]
- 11.Naoi M, Maruyama W, Dostert P, Hashizume Y, Nakahara D, Takahashi T. M Ota Brain Res. 1996;709:285. doi: 10.1016/0006-8993(95)01325-3. [DOI] [PubMed] [Google Scholar]
- 12.Stammel W, Thomas H. Anal l Lett. 1993;26:2513. [Google Scholar]
- 13.Baum SS, Rommelspacher H. J Chromatogr B. 1994;660:235. doi: 10.1016/0378-4347(94)00300-9. [DOI] [PubMed] [Google Scholar]
- 14.Stammel W, Woesle B, Thomas H. Chirality. 1995;7:10. [Google Scholar]
- 15.Deng Y, Maruyama W, Yamamura H, Kawai M, Dostert P, Naoi M. Anal Chem. 1996;68:2826. doi: 10.1021/ac960185l. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, Maruyama W, Kawai M, Dostert P, Yamamura H, Takahashi T, Naoi M. J Chromatogr B. 1997;689:313. doi: 10.1016/s0378-4347(96)00359-3. [DOI] [PubMed] [Google Scholar]
- 17.Kenneth M, Chris S, John M. Enantiomer. 2000;5:377. [Google Scholar]
- 18.Quan Z, Song YR, Peters G, Wu MS, Sheng YH, Hwang HM, Liu YM. Anal Sci. 2005;21:115. doi: 10.2116/analsci.21.115. [DOI] [PubMed] [Google Scholar]
- 19.Quan Z, Song Y, Saulsberry A, Sheng Y, Liu YM. J Chromatogr Sci. 2005;43:121. doi: 10.1093/chromsci/43.3.121. [DOI] [PubMed] [Google Scholar]
- 20.Ilisz I, Fodor G, Iványi R, Szente L, Tóth G, Péter A. J Chromatogr B. 2008;875:273. doi: 10.1016/j.jchromb.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Jiang CX, Tong MY, Breitbach S Zachary, Armstrong DW. Electrophoresis. 2009;30:3897. doi: 10.1002/elps.200900215. [DOI] [PubMed] [Google Scholar]
- 22.Haber H, Henklein P, Georgi M, Melzig MF. J Chromatogr B. 1995;672:179. doi: 10.1016/0378-4347(95)00213-3. [DOI] [PubMed] [Google Scholar]
- 23.Haber H, Stender N, Mangholz A, Ehrenreich H, Melzig MF. J Chromatogr B. 1999;735:299. doi: 10.1016/s0378-4347(99)00431-4. [DOI] [PubMed] [Google Scholar]
- 24.Musshoff F, Schmidt P, Dettmeyer R, Priemer F, Jachau K, Madea B. Forensic Sci Intl. 2000;113:359. doi: 10.1016/s0379-0738(00)00225-5. [DOI] [PubMed] [Google Scholar]
- 25.Liu YM, Gordon P, Green S, Sweedler JV. Anal Chim Acta. 2000;420:81. [Google Scholar]
- 26.Musshoff F, Lachenmeier DW, Kroener L, Schmidt P, Dettmeyer R, Madea B. Cell Mol Biol (Paris, France, Print) 2003;49:837. [PubMed] [Google Scholar]
- 27.Lee J, Huang B, Yuan Z, Kim HY. Anal Chem. 2007;79:9166. doi: 10.1021/ac0715827. [DOI] [PubMed] [Google Scholar]
- 28.Rojkovicova T, Mechref Y, Starkey JA, Wu G, Bell RL, McBride WJ, Novotny MV. J Chromatogr B. 2008;863:206. doi: 10.1016/j.jchromb.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Cai M, Liu YM. Rapid Comm Mass Spectrom. 2008;22:4171. doi: 10.1002/rcm.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simó C, García-Cañas V, Cifuentes A. Electrophoresis. 2010;3:1442. doi: 10.1002/elps.200900673. [DOI] [PubMed] [Google Scholar]
- 31.Rudaz S, Veuthey JL, Schappler J. Chromatogr Sci Series. 2010;100:363. [Google Scholar]
- 32.Tanaka Y, Otsuka K, Terabe S. J Chromatogr A. 2000;875:323. doi: 10.1016/s0021-9673(99)01334-5. [DOI] [PubMed] [Google Scholar]
- 33.Moini M, Schultz CL, Mahmood H. Anal Chem. 2003;75:6282. doi: 10.1021/ac034708i. [DOI] [PubMed] [Google Scholar]
- 34.Fanali S, Desiderio C, Schulte G, Heitmeier S, Strickmann D, Chankvetadze B, Blaschke G. J Chromatogr A. 1998;800:69. [Google Scholar]
- 35.Akbay C, Rizvi SAA, Shamsi SA. Anal Chem. 2005;77:1672. doi: 10.1021/ac0401422. [DOI] [PubMed] [Google Scholar]
- 36.Schulte G, Heitmeier S, Chankvetadze B, Blaschke G. J Chromatogr A. 1998;800:77. [Google Scholar]
- 37.Giuffrida A, León C, García-Cañas V, Cucinotta V, Cifuentes A. Electrophoresis. 2009;30:1734. doi: 10.1002/elps.200800333. [DOI] [PubMed] [Google Scholar]
- 38.Hwang HM, Quan Z, Liu YM. Intl J Quan Chem. 2009;109:81. doi: 10.1002/qua.21852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grard S, Morin PH, Dreux M, Ribet JP. J Chromatogr A. 2001;926:3. doi: 10.1016/s0021-9673(01)01005-6. [DOI] [PubMed] [Google Scholar]
- 40.Shamsi SA. Electrophoresis. 2002;23:4036. doi: 10.1002/elps.200290017. [DOI] [PubMed] [Google Scholar]
- 41.Laduron P. Nat New Biol. 1972;238:212. doi: 10.1038/newbio238212a0. [DOI] [PubMed] [Google Scholar]