Animal germline cells ultimately produce eggs and sperm, providing an immortal link to the next generation. Establishing and maintaining the germline requires a conserved gene set (1), but traditionally classified “germline genes” appear to have a broader role in development than originally anticipated. Recent findings from well-studied animal models suggest that in some taxa, germline genes may specify a multipotent cell lineage during embryogenesis, the fates of which include both somatic cells and the germline.
During animal embryogenesis, the germline is segregated from somatic cells (2), but this lineage dichotomy is not universal in animals. Studies exploring this segregation have primarily focused on organisms from two major animal groups, the vertebrates and the ecdysozoans, including Drosophila melanogaster (fruit fly), Caenorhabditis elegans (roundworm), Mus musculus (mouse), Danio rario (zebrafish), Xenopus laevis (frog), and Ambystoma mexicanum (axolotl). In these animals, the germline segregates from the rest of the embryo prior to gastrulation, an early development stage in which cells organize into specific layers that will establish the animal’s body plan. Thus, the development of these segregated cells in vivo is absolute and limited to a germ cell fate. In the fly, worm, zebrafish, and frog, the germline is established autonomously by the inheritance of cytoplasm during cell division that contains specification factors such those encoded by the genes vasa, nanos, and piwi. Alternatively, the mouse and axolotl segregate their germline during embryogenesis through inductive cell-to-cell interactions, yet a highly similar gene set is involved.
However, in other animal taxa, germline segregation occurs after gastrulation. In these animals, long-term multipotent precursor cells are established during embryogenesis from which the germline is segregated after embryonic development is completed. For example, in the marine annelid Platynereis dumerilii (a lophotrochozoan), the embryonic 4dlineagegives rise to proliferating cells that express vasa, nanos, and piwi. However, after embryogenesis is complete, these cells contribute both to the somatic mesodermal tissues of the developing adult segments and to the germline (3). Similarly, in the snail Ilyanassa (a lophotrochozoan), the 4d lineage, which expresses vasa and nanos, gives rise to multipotent cells during embryogenesis that later contribute to the germline and to mesoderm and endoderm of the adult (4, 5). Furthermore, nanos is required to maintain the fate of the snail 4d lineage; loss of nanos function results in a loss of all 4d-derived adult structures (4). Thus, similar to the marine annelid, the snail germ line segregates from multipotent descendants after embryogenesis, and these multipotent cells are likely dependent on conserved “germline” genes such as vasa, nanos, and piwi for their establishment and maintenance.
The germline of the echinoderm Strongylocentrotus purpuratus (sea urchin) also segregates from the soma well after embryogenesis. Sea urchin embryogenesis culminates in the formation of a swimming larva and at metamorphosis, a juvenile sea urchin emerges and the larval tissues are destroyed. Cells of the small micromere lineage, which are specified at the 32-cell embryo stage, are multipotent but contribute only to adult tissues and not to tissues of the larva (6, 7). This small micromere lineage expresses vasa, nanos, and piwi and loss of nanos function disrupts formation of the juvenile and metamorphosis does not occur (6, 8, 9). Thus, similar to the snail and the marine annelid, the sea urchin germline segregates after embryogenesis, and arises from a long-term multipotent precursor cell that is established during embryogenesis through the functions of genes originally ascribed only to the germ line.
Many adult flatworms, cnidarians, and sponges contain multipotent or totipotent stem cells that give rise to multiple adult cell types, including the germline. These animals continually segregate germline from stem cells throughout adulthood. The lophotrochozoan flatworms, such as planarians, contain totipotent cells (neoblasts) that give rise to all tissue types and that express piwi, vasa, tudor, and pumilio. These genes are required for neoblast function, but were first identified as germline genes in Drosophila (10). Several members of the phylum Cnidaria, the sister group to the Bilateria, contain adult multipotent stem cells (I-cells) that give rise to both somatic cells and germ cells. In adult Hydra, for example, I-cells selectively express vasa and nanos, but their functions are not yet known (11, 12). Similar to the cnidarians, members of the phylum Porifera (sponges) are non-Bilateria that segregate their germ line continuously from an adult stem cell. Adult sponges contain a totipotent stem cell (the archeocyte) that gives rise to somatic cells and to germ cells. Piwi is selectively expressed in the archeocytes of the sponge Ephydatia uviatilis, suggesting the presence of the same conserved gene program used in the cnidarian, echinoderm, and lophotrochozoan multipotent germline precursors (13).
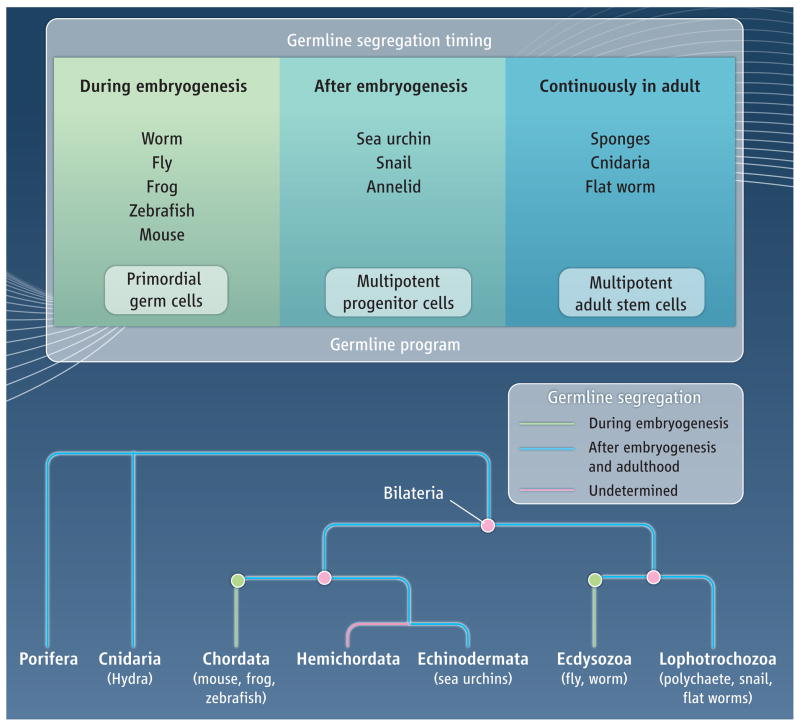
The timing of germline segregation in animals appears to occur along a continuum, at the onset of embryogenesis or continuously in the adult at the extremes (see the figure). In all cases, germline segregation requires that a population of cells, either multipotent or germline limited, be established in the embryo. Given that germline segregation from a multipotent precursor occurs after embryogenesis in the lophotrochozoans, echinoderms, cnidarians, and sponges, it is parsimonious to conclude that it is the ancestral mechanism of establishing a germline. This would predict that embryonic germline segregation evolved independently in vertebrates and ecdysozoans. It may be that the germline molecular program, which includes genes such as vasa, nanos, and piwi, originated in multipotent cells, and was subsequently co-opted by more specialized, embryonic germ cells. It will be necessary to collect more functional data from animals spanning diverse taxa to reveal the most ancient and essential portions of this proposed multipotency molecular program.
Fig. Germline segregation in animals.
(A) The timing of germline segregation from somatic cells varies, from early embryonenesis to continual specification in the adult. The same underlying molecular program may operate in all cases. In segregation during embryogenesis, primordial germ cells migrate to the somatic gonad to give rise to germ cells. In segregation after embryogenesis, a long-term multipotent precursor is established in the embryo from which the germline is segregated during larval development or adulthood. (B) Germline segregation that occurs after embryogenesis (blue line) may be the ancestral mechanism. If so, then embryonic germline segregation (green line) must have evolved independently in vertebrates and ecdysozoans.
References
- 1.Ewen-Campen B, Schwager EE, Extavour CG. Mol Reprod Dev. 2010;77:3. doi: 10.1002/mrd.21091. [DOI] [PubMed] [Google Scholar]
- 2.Saffman EE, Lasko P. Cell Mol Life Sci. 1999;55:1141. doi: 10.1007/s000180050363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rebscher N, Zelada-Gonzalez F, Banisch TU, Raible F, Arendt D. Dev Biol. 2007;306:599. doi: 10.1016/j.ydbio.2007.03.521. [DOI] [PubMed] [Google Scholar]
- 4.Rabinowitz JS, Chan XY, Kingsley EP, Duan Y, Lambert JD. Curr Biol. 2008;18:331. doi: 10.1016/j.cub.2008.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Swartz SZ, Chan XY, Lambert JD. Dev Genes Evol. 2008;218:107. doi: 10.1007/s00427-008-0203-6. [DOI] [PubMed] [Google Scholar]
- 6.Juliano CE, Yajima M, Wessel GM. Dev Biol. 2010;337:220. doi: 10.1016/j.ydbio.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka S, Dan K. Dev Growth Differ. 1990;32:145. doi: 10.1111/j.1440-169X.1990.00145.x. [DOI] [PubMed] [Google Scholar]
- 8.Juliano CE, et al. Dev Biol. 2006;300:406. doi: 10.1016/j.ydbio.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 9.Voronina E, et al. Dev Biol. 2008;314:276. doi: 10.1016/j.ydbio.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata N, Rouhana L, Agata K. Dev Growth Differ. 2010;52:27. doi: 10.1111/j.1440-169X.2009.01155.x. [DOI] [PubMed] [Google Scholar]
- 11.Mochizuki K, Nishimiya-Fujisawa C, Fujisawa T. Dev Genes Evol. 2001;211:299. doi: 10.1007/s004270100156. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki K, Sano H, Kobayashi S, Nishimiya-Fujisawa C, Fujisawa T. Dev Genes Evol. 2000;210:591. doi: 10.1007/s004270000105. [DOI] [PubMed] [Google Scholar]
- 13.Funayama N. Dev Growth Differ. 2010;52:1. doi: 10.1111/j.1440-169X.2009.01162.x. [DOI] [PubMed] [Google Scholar]