Abstract
Thioredoxin reductase 1 (TR1) is a major antioxidant and redox regulator in mammalian cells and appears to function as a double-edged sword in that it has roles in preventing and promoting/sustaining cancer. TR1 is over-expressed in many cancer cells and targeting its removal often leads to a reversal in numerous malignant characteristics which has marked this selenoenzyme as a prime target for cancer therapy. Since alterations in TR1 activity may lead to a better understanding of the etiology of cancer and new avenues for providing better therapeutic procedures, we have described herein techniques for removing and re-expressing TR1 employing RNAi technology and for assessing the catalytic activity of this enzyme.
1. Introduction
Selenium has long been known to have a role in decreasing the incidence of certain forms of cancer (reviewed in Lu et al., 2009; Selenius et al., 2009; Zeng and Combs, 2008). More recently, it has been shown that selenium-containing proteins (selenoproteins) play a role in preventing colon (Irons et al., 2006) and prostate cancers (Diwadkar-Navsariwaka et al., 2006), and have roles in protecting against other forms of cancer (Hatfield and Gladyshev, 2009; Rayman et al., 2009; Selenius et al., 2009; Steinbrenner et al., 2009). It has also become apparent that selenium has a role in promoting and/or sustaining malignancy. For example, thioredoxin reductase 1 (TR1) is a selenium-containing enzyme that appears to be a double-edged sword in having roles in preventing as well as promoting/sustaining cancer (e.g, see Arnér, 2009; Hatfield, 2007; Hatfield et al., 2009; Yoo et al., 2006 and 2007a). This essential selenoenzyme has an important role in embryogenesis (Jakupoglu et al., 2005), is expressed in all cell types and organs and is one of the major antioxidant and redox regulators in mammalian cells (Gromer et al,. 2005; Holmgren, 2006). It also has significant roles in transcription, DNA repair, cell proliferation and angiogenesis (see Arnér, 2009 for review; and Arnér and Holmgren, 2006; Bigalow and Miller, 2005; Fujino et al., 2006; Rundlöf and Arnér, 2004 for earlier studies). TR1 maintains thioredoxin1 (Trx1) in the reduced state and therefore has an essential role in the Trx system (Arnér and Holmgren, 2000). The selenium-containing moiety, selenocysteine (Sec), is the C-terminal penultimate amino acid in TR1 (Gladyshev et al., 1996) and this Sec residue is required for TR1’s catalytic activity (Nordberg et al., 1998; Zhong et al., 1998; Zhong and Holmgren, 2000). TR1 has also been reported to have a role in activating the p53 tumor suppressor and other tumor suppressor activities (Merrill et al., 1999), and is targeted by carcinogenic electrophilic compounds (Moos et al., 2003). These observations, the fact that one of the foremost characteristics of most, if not all, cancer cells is that they suffer from oxidative stress (Arnér, 2009; Arnér and Holmgren 2006; Biaglow and Miller, 2005; Fujino et al., 2006; Rundlöf and Arnér, 2004; Steinbrenner and Sies, 2009), and the findings that TR1 acts by reducing Trx1 and other redox regulators in cells (see Arnér, 2009 for review; and Arnér and Holmgren, 2000; Gromer et al., 2005; Holmgren, 2006 for earlier studies), provide strong evidence that this selenoenzyme plays a major role in cancer prevention.
There is also strong evidence that TR1 has an important role in cancer promotion and/or cancer maintenance. For example, TR1 is over-expressed in many cancers and cancer cell lines (Arnér, 2009; Arnér and Holmgren 2006; Biaglow and Miller, 2005; Fujino et al., 2006; Gundimeda et al., 2009; Hedström et al., 2009; Rundlöf et al., 2004; Yoo et al., 2007a), and this selenoenzyme may have similar roles in malignant and normal cells in preventing or reducing oxidative stress. Numerous inhibitors of TR1 and other potent anti-cancer drugs that target TR1 activity and alter cancer-related properties also suggest that this protein has a major role in promoting and/or sustaining cancer (see Arnér, 2009; Chew et al., 2009, Gandin et al., 2009; Honeggar et al., 2009; Lam et al., 2009; Li et al., 2009; Selenius et al., 2009; Urig and Becker, 2006; Yan et al., 2009; Yoo et al., 2007a and references therein). Furthermore, comparing the expression of TR1 to that of other selenoproteins in a variety of cancer cell lines showed that TR1 was uniquely over-expressed (Yoo et al., 2007a). In addition, knockdown of TR1 in a mouse lung carcinoma cell line (LLC1) using RNAi technology reversed several malignant characteristics of these cells including manifesting a dramatic reduction in tumor progression and metastasis in mice injected with the TR1-deficient cell line compared with mice injected with the corresponding cell line expressing normal levels of TR1 (Yoo et al., 2006). The targeted removal of TR1 in a mouse cell line driven by oncogenic k-ras (DT) resulted in this mouse line losing self-sufficiency of growth, exhibiting a defective progression in the S-phase and a decreased expression of DNA polymerase a (Yoo et al., 2007a). Each of the above studies demonstrating that the impediment of TR1 activity in cancer cells upsets the malignancy process strongly suggest that this selenoenzyme is an excellent target for cancer therapy.
Since alteration of TR1 activity and function may provide insights into the etiology of cancer, the techniques used for targeting the knockdown of TR1 and its re-expression in cancer cells, the assays used for monitoring TR1 expression and the means of assessing reduction of tumor formation and metastasis related to TR1 deficiency should provide important avenues for exploring the underlying mechanisms of this disease. We, therefore, describe herein these techniques.
2. Materials and Methods
2.1. Mammalian cell lines and mice
Mouse Lewis lung carcinoma (LLC1) cells (obtained from ATCC) were used for targeting TR1 removal and examining the resulting effects of TR1 deficiency on various cancer-related properties including tumor formation and metastasis. LLC1 cells were selected for these studies as they are immunologically compatible with C57BL/6 mice and are known to form solid tumors when injected into the flank of these mice and to metastasize to lungs when injected into their tail vein. Mouse kidney (TCMK-1 obtained from ATCC) cells were used to compare the efficiency of TR1 knockdown constructs by transient transfection and the relative effects of the four siTR1 targeting constructs on the efficiency of removal of this selenoprotein. DT cells (provided by Dr. Yoon Sang Cho-Chung) which had been transformed with k-ras and are malignant k-ras expressing cells, were derived from NIH 3T3 cells (Noda et al., 1983) and used for stable transfection with a siTR1/siGPx1 double knockdown vector (Xu et al., 2009) as described below. C57BL/6 mice were used for a tumor formation assay by injecting malignant cells into their flanks and for a metastasis assay by injecting malignant cells into their tail veins and analyzing tumor formation in the lungs.
2.2. Targeted removal and re-expression of TR1
The knockdown vector used for this study, pU6-m3, was modified from pSilencer 2.1-U6 Hygro (Ambion, Inc.) by introducing the following changes: 1) the G and C bases at positions 468 and 469 were changed to a single A that made the U6 promoter closer in sequence to the corresponding wild type gene; 2) the EcoRI site at position 4110 was deleted; and 3) a new XhoI site was inserted at position 384. Introduction of the two cloning sites resulted in a vector encoding multiple siRNA target sequences. Candidate siRNA target regions were chosen in the 3′-UTR of mouse TR1 and glutathione peroxidase 1 (GPx1) mRNA (accession number: NM_015762, NM_008160) by using the online siDESIGN program (Dharmacon, Inc.). Four of each sense-antisense oligonucleotides for TR1 and GPx1 knockdown candidate sequences were cloned into the pU6-m3 knockdown construct by using the BamH1-HindIII cloning site (Xu et al., 2009; Yoo et al., 2007b). TR1 candidate target regions are shown in Fig. 1 along with their targeting sequences. Each candidate knockdown construct was confirmed by sequencing. Knockdown efficiencies were examined by analyzing the levels of TR1 or GPx1 protein expression by 75Se-labeling after transient transfection into mouse kidney (TCMK-1) cells. The mouse TR1 and GPx1 genes, including the 5′- and 3′-UTRs, were amplified by PCR using cDNA from TCMK-1 cells and cloned into a pcDNA3.1 expression vector (Invitrogen). To circumvent the knockdown site for knock-in (re-expression) of TR1 and GPx1, the genes were modified by introducing mutations in the siRNA target region by PCR using mutant primers, siTR1-3m, 5′-gtcttagtctcaaggtacctatgtctaatgtc-3′, and GPx1-3m 5′-gcgagagatgggttcaata-3′ where the bolded letters indicate mutated regions. TR1 has an AU-rich mRNA instability element (designated ARE) located in the 3′-UTR that affects the stability of the mRNA. To increase TR1 mRNA stability, the ARE was deleted (bases 2407 to 3310) using BlpI and XhoI restriction sites. Each of the resulting constructs generated for knock-in of TR1 and GPx1 were designated as TR1 ki and GPx1 ki as discussed below and in Fig. 6.
Figure 1.
Relative positions of the siTR1 targeted regions within the TR1 gene. The overlapping reading frame (ORF) of TR1 is shown along with the TGA Sec codon that occurs as the penultimate codon in the gene. The relative positions of the SECIS element, the ARE region and the four siTR1 RNAs, which are designated 1–4 (see text), along with their targeting sequences, are shown.
Figure 6. Simultaneous knockdown of TR1 and GPx1 and their individual or simultaneous re-expression.
DT cells were stably transfected initially with either the pU6-m3 control construct or the siTR1/siGPx1 double knockdown construct. The resulting stably transfected siTR1/siGPx1 double knockdown cells were subsequently transiently transfected with either the control pcDNA3.1 expression vector or with one of the following expression vectors encoding either the TR1 wild type gene (designated TR1 wt in the figure), TR1 knock-in gene (designated TR1 ki), GPx1 wild-type gene (designated GPx1 wt), GPx1 knock-in gene (designated GPx1 ki) or TR1 and GPx1 knock-in genes (designated TR1 ki and GPx1 ki). All cell lines were labeled with 75Se, cell extracts prepared and electrophoresed. In lane 1, the cells that were stably transfected with the pU6-m3 control construct were transiently transfected with pcDNA3 expression control vector, and in lanes 2–7, the cells that were stably transfected with the double siTR1-siGPx1 construct were transiently transfected as follows: lane 2, the pcDNA3.1 expression control vector; lane 3, TR1 wt; lane 4, TR1 ki; lane 5, GPx1 wt; lane 6, GPx1 ki; and lane 7, TR1 ki-GPx1 ki. (The figure was reproduced from Xu et al. (2009) Nature Protocols 4, 1338–1349).
Knockdown of TR1 or double knockdown of TR1 and GPx1 was carried out by transfecting LLC1 or DT cells as follows: 3×105 cells/well were seeded in a 6-well plate containing 4 μg of each knockdown construct in the presence of Lipofectamine 2000 (Invitrogen) and incubated overnight. Cells were grown in the presence of 500 μg/ml of hygromycin B (Mediatech, Inc.) until the untransfected cells died and the transfected cells formed colonies. The resulting TR1 and TR1/GPx1 knockdown cells were confirmed by 75Se-labeling, western and northern blot analyses and catalytic activity assays.
2.3. Monitoring TR1 expression
2.3.1. Northern blot analysis
Northern blot analysis of TR1 was carried out by isolating total RNA using TRIzol (Invitrogen) according to the manufacturer’s instructions. The resulting RNA preparation was electrophoresed in 1X MOPS buffer (3-(N-morpholino)propanesulfonic acid) on a 1% agarose gel containing 1X MOPS buffer and 6% formaldehyde and transblotted in 10X SSC (saline-sodium citrate) to a Hybond N+ nylon membrane (GE Healthcare). Membranes were hybridized with a 32P-labeled TR1 cDNA probe prepared by PCR of the entire coding region of the TR1 gene from genomic DNA of TCMK-1 cells, washed twice with 2X SSC, 0.1% SDS and once with 0.1X SSC, 0.1% SDS, exposed to a PhosphorImager (GE Healthcare) and quantified using ImageQuant software (GE Healthcare). Ribosomal 18S and 28S RNA were used as loading controls.
2.3.2. 75Se-Labeling
To examine the expression of TR1 and other selenoproteins, cells were seeded onto a 6-well plate (3×105 cells/well) and incubated for 18 h. 40 μCi of 75Se (20 nM; specific activity 1000 Ci/mmol; University of Missouri Research Reactor) were added to each well and the cells incubated for 24 h. Labeled cells were washed with ice-cold PBS twice, lysed with lysis buffer (20 mM Tris-Cl, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 10 mM NaF, 5 mM EDTA, and protease inhibitor cocktail), harvested using a cell scraper, incubated on ice with intermittent vortexing and prepared by centrifugation at 13,000 × g for 10 min. Protein concentrations of lysates were measured using BCA Protein Assay Reagent (Thermo Fisher Scientific Inc.). 30 μg of each protein sample were separated by electrophoresis on a 4–12% Bis-Tris NuPAGE gel. The gel, following electrophoresis, was stained with Coomassie Blue, dried, exposed to a PhosphorImager and quantified using ImageQuant software.
2.3.3. Western blot analysis
Cells were washed with ice-cold PBS and whole cell lysates prepared as above. 30 μg of each protein sample were electrophoresed on 4–12% Bis-Tris NuPAGE gels, the separated proteins transferred to a PVDF membrane, and then incubated initially with primary antibody according to the manufacturer’s recommendation and finally with HRP-conjugated secondary antibody (Cell Signaling Technology). Membranes were reacted with SuperSignal West Dura Extended Duration Substrate (Pierce) and exposed to X-ray film.
2.2.4. Thioredoxin reductase activity assay
Activity of TR was determined spectrophotometrically by the method of Holmgren and Bjornstedt (1995) and Hill et al. (1997) as modified by Hintze et al. (2003) and Smith and Levander (2002). The assay is based on the reduction of 5,5′-dithio-bis(2-nitrobenzoic acid (DTNB) by TR1. The time-dependent increase in maximal absorbance was measured at 412 nm (extinction coefficient 27.2 or 13,600 M−1cm−1 for TNB) using a microplate reader (Molecular Devices, Spectramax Plus 384 with Softmax Pro software). Since the assay is not completely specific for TR activity, specific activity was determined by subtracting the measured activity in the presence of the thioredoxin TR inhibitor aurothioglucose (ATG; Sigma) from the total activity. A unit of activity was defined as 1.0 μmol 5-thio-2-nitrobenzoic acid formed per min/mg protein. Protein concentrations were measured using the BCA reagent.
Materials
Phosphate buffer
0.25 M Na2HPO4
Inhibition Buffer
0.25mM ATG in cold phosphate buffer (protected from light at 4°C).
Reaction Mix (for six microwells (one reaction ± inhibitor in triplicate))
75 μL 0.035 M β-NADPH,
75 μL 0.2 M EDTA,
600 μL 0.25 M phosphate buffer
117 μL 0.063 M DTNB (in Ethanol),
15 μL bovine serum albumin (BSA, 20 mg/ml),
685 μL dH2O
Cells were harvested with cold phosphate buffer (300 μl/well), sonicated on ice, centrifuged, and the supernatant was kept on ice until measurement. The reaction mix minus the NADPH was kept at 37°C with the NADPH added immediately prior to adding the sample at measurement.
Blank controls consisted of a) a buffer-blank in triplicate, containing 70 μL phosphate buffer and 220 μL complete reaction mix; and b) a buffer + ATG-blank in triplicate, containing 50 μl phosphate buffer, 220 μl complete reaction mix and 20 μL inhibition buffer.
-
For each sample, two reactions were set up in triplicate in a 96-well plate. Reaction 1 (sample): 25 μl cell lysate; 45 μl phosphate buffer; 220 μl complete reaction mix (amount of cell lysate may be doubled, adjusting the amount of phosphate buffer in reaction mix).
Reaction 2 (sample + ATG): 25 μl cell lysate; 25 μL phosphate buffer; 220 μl complete reaction mix; 20 μl inhibition buffer.
Once the complete reaction mix (37°C) was added, the plate was measured immediately with a Spectramax Plus384 microplate reader. Automix was initiated before first read. Using the kinetic assay setup with path length check, and wavelength set at 412 nm, absorption was measured for 2 min in four 15-sec intervals. The appropriate negative controls were subtracted from the results obtained for the samples.
To calculate the specific TR activity in the samples, the change in absorbance/min (slope) from samples with ATG was subtracted from the slope calculated for samples without ATG. The resulting value was divided by the extinction coefficient 27.2, adjusted for the amount of protein per sample, and subsequently expressed as μmol TNB/min/mg protein.
2.4. Tumor formation activity of TR1 knockdown cells assessed by in vitro and in vivo assays
2.4.1. Anchorage independent growth assay
To assess the anchorage independent growth of cells, 1000 control or TR1 knockdown (siTR1) cells were suspended in noble agar (0.35%) in growth medium (3.5 ml) containing 10% fetal bovine serum (FBS) and the cells were evenly spread onto 60 mm plates that had been previously covered with a 4 ml basal layer of noble agar (0.7%) containing Dulbecco’s modified Eagle’s medium without FBS. Plates were placed into a humidified CO2 incubator at 37°C for 14 days and 500 μl of fresh growth medium was added onto agar plates every 5 days to replenish media and prevent the plates from drying out. At the end of the incubation period, colonies were visualized by staining the plates overnight with 500 μl of ρ-iodonitrotetrazolium violet (0.5 mg/ml, INT) at 37°C in the above incubator and the plates photographed.
2.4.2. Tumor formation assays
To assess tumor formation or metastatic capabilities of TR1-deficient (siTR1) and control cell lines, 2×105 control (pU6-m3) or siTR1 cells that had been maintained in a linear growth phase were either subcutaneously injected into the flanks of mice or into the tail veins of mice, respectively. Mice that were injected subcutaneously into their flanks were monitored every 2 days for 2 weeks, and after the two week period, mice were euthanized, tumor tissue excised, tumors fixed with 10% neutral-buffered formalin and stored at −80°C for further analysis as described below. Mice that were injected into tail veins were euthanized after four weeks, autopsied for examining the extent of tumor formation in all tissues and the lungs removed, photographed, fixed with 10% neutral-buffered formalin and stored at −80°C for further analysis. All mice used for assessing tumor formation and metastatic capabilities of the two cell lines were 5-week-old, C57BL/6 females. Animal care was in accordance with the National Institutes of Health institutional guidelines under the expert direction of Dr. Kyle Stump (NCI, NIH, Bethesda, MD).
2.4.3. In vitro chemotaxis and invasion assays
To further examine the effect of TR1 knockdown in cancer cell lines, 4×104 of either control or TR1 knockdown cells were seeded in the upper chamber of a 24-well transwell migration and invasion assay plate (BD Biosciences) containing a matrigel-coated membrane (8.0 μm pore size) and 500 μl of serum deficient media to trigger serum gradient-induced chemotaxis. The cells were incubated in the upper chamber of the plate for 18 h to allow cells to migrate through the membrane to the serum-containing media (750 μl) in the lower well. The membrane was washed with PBS twice and fixed with 100% methanol. Cells were visualized by hematoxylin staining and those cells that did not move across the membrane were removed with swap. Cells that migrated through the membrane were photographed.
3. Results and Discussion
We targeted the knockdown of TR1 in various malignant cells and examined the resulting effects of TR1 deficiency on cancer development (Yoo et al., 2006 and 2007a). The 3′-UTRs in eukaryotic selenoprotein mRNAs contain the selenocysteine insertion sequence (SECIS) element that governs the incorporation of Sec in response to UGA and therefore is essential for the expression of this class of proteins (Low et al., 2000). This information afforded us an opportunity to target the removal of selenoproteins and their subsequent re-expression without disrupting the protein internal coding sequence of the mRNA (Yoo et al., 2007b). Other investigators have also used RNAi technology to target the removal of TR1 in various normal or cancer cells (e.g., see Eriksson et al., 2009; Honeggar et al., 2009; Liu and Shen, 2009; Watson et al., 2008) and the reader is referred to these studies to see additional approaches of targeting TR1 knockdown that vary from those described herein. One advantage of using mouse cancer cell lines in RNAi technology studies is that the resulting protein-deficient cells can be injected into mice that are not immuno-compromised to further examine the effect of the loss of the targeted protein on, for example, tumorigenicity and metastasis. It should also be noted that RNAi technology has some pitfalls that must be taken into consideration to fully assess the effects of altering the expression of the targeted protein. For example, off-targeting is one of the major concerns to rule out in order to ensure that any observed phenotypic or functional changes are due totally to alteration in activity of the specifically intended protein and not to an unintended target. The subject of off-targeting has been addressed by us in considerable detail elsewhere (Xu et al., 2009), and the simplest and easiest means of overcoming this potential problem is to initially target more than one site in the intended protein and determine the effects of each siRNA to ensure that those siRNAs acting efficiently to remove the target give similar phenotypic results (see section 3.1). It should also be noted that the level of mRNA knockdown may be quite substantial in some cases and may not coincide with the level of the loss of the corresponding protein wherein the protein expression may remain quite high. The level of protein, therefore, should be analyzed following the knockdown of the corresponding mRNA target by western blot analysis and, in case of selenoproteins, also by 75Se-labeling. In addition, in assessing levels of TR1, another potential problem is that the Sec moiety occurs at the C-terminal penultimate position and the UGA codon can serve as a stop codon resulting in a truncated protein which migrates at the same place on gels as the normal protein following electrophoresis. Although 75Se-labeling can be used to address these latter potential problems in accurately assessing TR1 levels, it is often best to additionally measure the catalytic activity of TR1 that explicitly demonstrates the active level of this selenoenzyme relative to that found in the corresponding control cell line or tissue.
3.1. Knockdown of TR1
The positions of the four siTR1 targeting sites, designated siTR1-1, siTR1-2, siTR1-3 and siTR1-4, relative to each other and to the SECIS element and the ARE within the 3′-UTR of TR1 are shown in Fig. 1. The corresponding constructs were generated and TCMK-1 cells were transiently transfected with each construct and the control vector, pU6-m3, as described in Materials and Methods. The transfected cell lines were labeled with 75Se and the resulting labeled selenoproteins from each are shown in Fig. 2A. TCMK-1 cells that were not transfected were also labeled and the resulting selenoproteins examined as a control along with the other control cells (pU6-m3 transfected cells). Cells transfected with pU6-m3, siTR1-1 and siTR1-2, and the untransfected cells expressed similar selenoprotein levels including the level of TR1. Since siTR1-1 and siTR1-2 did not target TR1 reduction, they were not further examined. Cells transiently transfected with the siTR1-3 and siTR1-4, however, manifested dramatically reduced levels of TR1. LLC1 cells were then stably transfected with these two constructs and the control construct. The knockdown of TR1 relative to untransfected LLC1 cells and pU6-m3 transfected control cells was examined following 75Se-labeling, western blotting (Fig. 2B) and northern blotting (Fig. 2C). Both sites that were targeted in TR1 mRNA resulted in a dramatic reduction in the expression of this selenoprotein relative to that observed with the control cells as assessed by these three criteria. However, the knockdown of TR1 appeared to be slightly more efficient with siTR1-3 than with siTR1-4. We therefore examined the catalytic activity of this selenoenzyme with only siTR1-3 which also demonstrated the virtual loss of activity (Fig. 2D).
Figure 2.
Knockdown of TR1. In A, DT cells were transiently transfected with the control construct, pU6-m3, or with the siRNA constructs, siTR1-1, siTR1-2, siTR1-3 or siTR1-4. The untransfected cells (lane 1) and the resulting transfected cells (lanes 2–5 as indicated) were labeled with 75Se, proteins extracted, 30 μg of each sample applied to a gel, the gel electrophoresed and exposed to a PhosphorImager as described in the Materials and Methods. In B–D, LLC1 cells were stably transfected with pU6-m3, siTR-1-3 or siTR1-4, the untransfected and transfected cells examined by (B) labeling each cell line with 75Se as indicated in the figure, proteins samples prepared, electrophoresed and exposed a PhosphorImager as given in A, (C) preparing each cell line for northern blot analysis and blotting using 28S and 18S ribosomal RNAs as loading controls and (D) preparing only the untransfected cells, pU6-m3, and siTR1-3 cell lines for catalytic analysis and analyzing TR activity as described in Materials and Methods. (Figures in panels B and D were reproduced from Yoo et al. (2006) J. Biol. Chem. 281, 13005–13008).
3.2. Phenotypic changes in siTR1 transfected cells
It was important to determine whether the two TR1 targeting constructs acted similarly in causing phenotypic changes in transfected cells to rule out the effect of off-targeting (see Xu et al., 2009 and references therein). A characteristic of many cancer cells, unlike most normal cells, is that they can grow unanchored in soft agar. The ability of the two cell lines stably transfected with siTR1-3 and siTR1-4 to grow in soft agar was compared to the two control cell lines, LLC1 and LLC/pU6-m3 (Fig. 3). The anchorage-independent growth of siTR1-3 and siTR1-4 transfected cells was inhibited suggesting that reduction in TR1 expression altered this malignant characteristic. siTR1-4 appeared to grow slightly better than siTR1-3 which is consistent with the observations above showing that the former targeting construct was not as efficient in removing TR1 expression by western blotting and TR1 mRNA expression by northern blotting. However, it should also be noted that the LLC/pU6-m3 transfected cells appeared to grow slightly more efficiently than the corresponding untransfected cells. The LLC/siTR1-3 transfected cells manifested other phenotypic changes that included morphology, a slight reduction in growth rate and a dramatic reduction in the levels of two cancer-related proteins, Hgf and Opn1 (data not shown; see Yoo et al., 2006), suggesting that additional cancer-related properties are altered making the TR1 deficient cells more like normal cells.
Figure 3.
Phenotypic changes of TR1-deficient cells. LLC1 cells stably transfected with the pU6-m3 control, siTR1-3 or siTR1-4 constructs were suspended in noble agar/growth medium and evenly spread on plates covered with 0.7% noble agar and grown for 14 days as described in Materials and Methods. At the end of the growth period, colonies were stained overnight with ρ-iodonitrotetrazolium violet, visualized and photographed.
3.3. Tumorigenesis of TR1-deficient cells
Further studies were carried out only with the siTR1-3 construct. Mice were injected in the flank with LLC1 cells stably transfected with the siTR1-3 construct or the pU6-m3 construct to assess the ability of these two cell lines to form a solid tumor (Fig. 4A). Progression of tumor formation was determined after two weeks by euthanizing the mice, and removing and examining the tumors. Mice injected with the control, pU6-m3 cells had much larger tumors with an average weight of 0.341 g compared to the mice injected with the siTR1-3 transfected cells that had tumors with an average weight of 0.063 g (see Fig. 4A and figure legend). The tumors that formed in mice injected with the TR1-deficient cells were due to the loss of the siTR1-3 targeting vector as shown by western blotting and PCR analysis (Fig. 4B). The reason that the siTR1-3 vector was lost is that all the constructs described herein were retained in stably transfected cells with hygromycin B, whereas mice injected with cells encoding the construct could not be treated with this drug. PCR analysis of genomic DNA from both the pU6-m3 and siTR1 tumors demonstrated that the siTR1-3 construct was lost in the tumor cells (Fig. 4B, lower panel). Western blot analysis showed higher levels of TR1 in tumors developed from siTR1-3 transfected cells than siTR1-3 cells suggesting that TR1 was required for tumor growth and that the tumors arose due to reversal of the TR1 knockdown in this cancer model (Fig. 4B, upper panel). The western blot analysis study is consistent with the finding in the PCR experiment.
Figure 4.
Tumorigenicity of TR1-deficient cells. Mice were injected in the flank with LLC1 cells transfected with the pU6-m3 or siTR1-3 constructs, tumor formation monitored every 2 days, and after 2 weeks, mice were euthanized, tumors removed, weighed and the weights averaged from three separate mice (average weight 0.341 g in mice injected with the pU6-m3 control construct and 0.063 g in mice injected with the siTR1-3 construct) and photographed. In B, TR1 western blot analysis of tumor extracts (shown in the upper panel) and PCR analysis of genomic DNA extracted from tumors of the pU6-m3 and siTR1-3 constructs (shown in the lower panel). (The figures were reproduced from Yoo et al. (2006) J. Biol. Chem. 281, 13005–13008).
3.4. In vitro and in vivo metastatic analysis of TR1-deficient cells
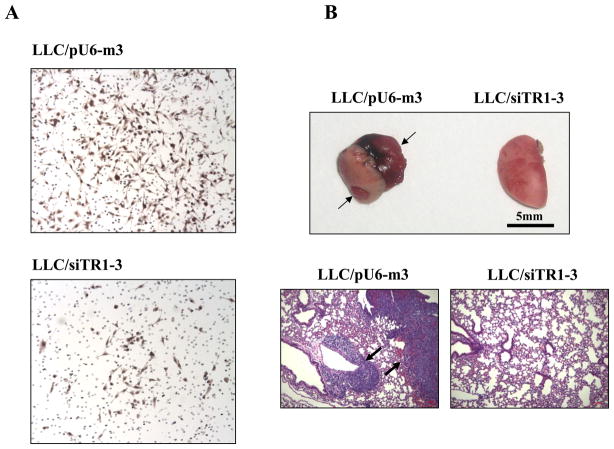
The metastatic potential of malignant cells can be examined in vitro prior to an in vivo analysis by assessing their invasiveness and chemostatic potential. The effect of TR1 deficiency on the metastatic potential of malignant cells was examined by using an in vitro invasive and chemotactic assay and comparing the efficiencies over an 18 h period of pU6-m3 control and siTR1-3 transfected LLC1 cells to migrate through the serum gradient environment and the basement membrane (Fig. 5A). The TR1-deficient cells manifested a significantly decreased migration compared to the control cells suggesting that this selenoenzyme is involved with the process of migration in malignant cells (Figure 5A). The involvement of TR1 in chemotaxis such as a role in sensing the serum gradient, invasion of the TR1-deficient cells through the extracellular matrix, and the intracellular migratory pathway in the TR1-deficient cells need to be further examined to elucidate the molecular mechanism(s) of TR1 and its probable substrate(s) in the metastatic potential of malignant cells. However, the metastatic potential of the LLC/siTR1-3 cells as assessed by an in vitro assay was severely altered suggesting that metastasis in an in vivo assay would also likely be altered.
Figure 5.
Effects of TR1 knockdown on chemotaxis and metastasis. In A, LLC/pU6-m3 or siTR1-3 transfected cells (4×104cells/chamber) were incubated in a transwell migration and invasion assay plate containing a matrigel-coated membrane and migrated cells were stained with hematoxylin and photographed as described in Materials and Methods. In B, metastasis was assessed by injecting LLC/pU6-m3 or siTR1-3 transfected cells into the tail veins of mice, and after four weeks, lungs were removed photographed (upper panel) and the slides shown in the lower panel were prepared from lung samples from both animals, H&E stained and photographed as described in Materials and Methods. (The figures shown in B were reproduced from Yoo et al. (2006) J. Biol. Chem. 281, 13005–13008).
To assess the in vivo metastatic ability of pU6-m3 control and siTR1-3 cells, lungs of mice injected in the tail vein with either of these cell lines were examined four weeks post-injection for the presence of tumors (Fig. 5B). No tumors were found in the mice injected with the TR1 knockdown cells, whereas tumors were present in the lungs of mice injected with the pU6-m3 control cells. Lung tissue from mice with apparent normal lungs that had been injected with the siTR1-deficient cells and from mice with cancerous lungs that had been injected with the pU6-m3 control cells were examined for pathological changes (Fig. 5B, lower panel). Extensive malignancy was observed in mice injected with the pU6-m3 control cells, but only normal tissue was found in mice injected with the siTR1 knockdown cells.
3.5. Re-expression of TR1 in TR1 deficient cells
In assessing the role of TR1 in the etiology of cancer by means of using RNAi technology, it is important to have a means of re-expressing the knocked down protein to further examine its role in the malignancy process. For example, the reintroduction of a protein can be done wherein the exogenous protein contains a His-tag and is expressed in the absence of the corresponding endogenous protein for examining alterations that may occur in the targeted protein that might otherwise be undetected. The His-tagged protein can then be isolated and purified in the absence of endogenous protein for further study. Since we have also developed techniques for simultaneous removal of multiple selenoproteins and their subsequent re-expression, either individually or simultaneously (Xu et al., 2009; Yoo et al., 2007b), and such procedures can be used to further explore the role of selenoproteins in cancer etiology, the technology of multiple knockdown and knock-in is further considered. DT cells were used for stable transfection with a siTR1/siGPx1 double knockdown construct for their simultaneous removal and subsequent knocking back-in by transiently transfecting with the construct that either individually or simultaneously circumvented the targeting vector resulting in the re-expression of one or both of the removed selenoproteins. Transfected cells were labeled with 75Se to monitor the expression of selenoproteins in transfected DT cells (Fig. 6).
Two of the control cell lines stably transfected with either pU6-m3 or the double siTR1/siGPx1 knockdown vector are shown, respectively, in lanes 1 and 2 of the figure. The double targeting vector effectively removed TR1 and GPx1 expression. Replacement of the TR1 and GPx1 wild type genes did not result in expression of the corresponding selenoproteins due to the presence of the siTR1 and siGPx1 RNAs generated from the stably transfected vector (Fig. 6, lanes 3 and 5, respectively). Circumventing the targeting regions with transiently transfected constructs (designated in the figure as “ki” for knock-in) that either individually or collectively bypassed the siRNAs by encoding TR1 and/or GPx1 genes with mutations corresponding to the siRNAs resulted in re-expression of the corresponding selenoprotein or selenoproteins (Fig 6, lanes 4, 6 and 7).
The only variation in the above procedure in order to isolate the reintroduced exogenous protein is to prepare the circumvention construct with an N-terminal or a C-terminal His-tag on one or the other of the reintroduced proteins.
4. Conclusions and future perspectives
Reducing the expression of TR1 in cancer cells using RNAi technology has provided new insights into the underlying roles of this selenoenzyme in the malignant process. For example, TR1 deficiency in LLC1 cells resulted in a reversal of its ability to grow unanchored in soft agar and altered its tumorigenicity and metastatic properties making each of these characteristics more similar to those of normal cells. In fact, it appeared that TR1 activity was required in tumor formation following injection of the TR1 knockdown cells as the resulting tumors that formed had lost their TR1 knockdown construct (Fig. 4B).
The means by which levels of TR1 are measured in cells and tissues is also an important factor to consider as partially or fully inactive TR1 can also arise. For example, inactive TR1 can arise by the cessation of translation at the UGA codon and partially active TR1 can result from replacement of Sec with Cys. Labeling cells in culture or tumors (i.e., by labeling the mouse) and examining the resulting levels of TR1 following electrophoresis on gels and/or measuring catalytic activity in cells and tissues can overcome such possible problems.
Re-expression of TR1 in TR1 knockdown cells provides a means of expressing exogenous protein in the absence of the corresponding endogenous protein. By inserting a His-tag at the N- or C-terminus for isolating the protein, this technique can open new opportunities of exploring the function of TR1 in malignant and normal cells.
Other investigators have compared the effects of reducing TR1 expression by siRNA knockdown and specific inhibitors and have found that TR1 and Trx1 may act independently of each other in some situations in the malignancy process (Eriksson et al., 2009; Watson et al., 2008). These studies also provide insights into other possible pathways for exploring the underlying roles of TR1 and Trx1 in malignancy.
It should also be noted that TR1-deficient DT cells lost their self-sufficiency in growth and were found to have a defective progression in their S-phase and a reduced expression of DNA polymerase a (Yoo et al., 2007a). These studies suggested that TR1 is essential for self-sufficiency in growth and provide further evidence that this selenoenzyme acts as a pro-cancer protein in DT cells and a prime target in cancer therapy. Interestingly, self-sufficiency in growth is one of the six hallmarks of cancer as defined by Hanahan and Weinstein (2000). Knockdown of TR1 in LLC1 cells demonstrated that TR1 has a role in tissue invasion and metastasis (Yoo et al., 2006) that is another of the Hanahan and Weinstein cancer hallmarks. Thus, RNAi technology has been used to show that TR1 plays a major role in two of the six cancer hallmarks and can likely be used to demonstrate whether this selenoenzyme has roles in the other four cancer hallmarks which are insensitivity to anti-growth signals, evading apoptosis, sustained angiogenesis and limitless replicative potential (Hanahan and Weinstein, 2000). In fact, we have observed that TR1 deficiency in a breast cancer cell line, EMT6, altered the ability of these cells to evade apoptosis and we are examining the role of TR1 in the other cancer hallmarks (M.-H. Yoo, B.A. Carlson, V.N. Gladyshev, D.L. Hatfield, unpublished data).
Unquestionably, RNAi technology has proven to be a powerful tool in the study of protein function, and now, in the study of selenoprotein function in cancer. The various approaches employing this technology by others (Eriksson et al., 2009; Honeggar et al., 2009; Liu and Shen, 2009; Watson et al., 2008) and us (Yoo et al., 2006, 2007a,b; Xu et al., 2009) have yielded many new insights into the underlying roles of TR1 in the malignancy process, and undoubtedly, will shed much more light on the molecular mechanisms driving cancer.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health, NCI, Center for Cancer Research to DLH and NIH grants to VNG.
References
- Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Arnér ESJ, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Arnér ES. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim Biophys Acta. 2009;1790:495–526. doi: 10.1016/j.bbagen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Biaglow JE, Miller RA. The thioredoxin reductase/thioredoxin system: novel redox targets for cancer therapy. Cancer Biol Ther. 2005;4:6–13. doi: 10.4161/cbt.4.1.1434. [DOI] [PubMed] [Google Scholar]
- Chew EH, Nagle AA, Zhang Y, Scarmagnani S, Palaniappan P, Bradshaw TD, Holmgren A, Westwell AD. Cinnamaldehydes inhibit thioredoxin reductase and induce Nrf2: potential candidates for cancer therapy and chemoprevention. Free Radic Biol Med. 2009 doi: 10.1016/j.freeradbiomed.2009.10.028. (In Press) [DOI] [PubMed] [Google Scholar]
- Diwadkar-Navsariwala V, Prins GS, Swanson SM, Birch LA, Ray VH, Hedayat S, Lantvit DL, Diamond AM. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci U S A. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson SE, Prast-Nielsen S, Flaberg E, Szekely L, Arnér ES. High levels of thioredoxin reductase 1 modulate drug-specific cytotoxic efficacy. Free Radic Biol Med. 2009;47:1661–1671. doi: 10.1016/j.freeradbiomed.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Fujino G, Noguchi T, Takeda K, Ichijo H. Thioredoxin and protein kinases in redox signaling. Semin Cancer Biol. 2006;16:427–435. doi: 10.1016/j.semcancer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Gandin V, Fernandes AP, Rigobello MP, Dani B, Sorrentino F, Tisato F, Bjornstedt M, Bindoli A, Sturaro A, Rella R, Marzano C. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem Pharmacol. 2009 doi: 10.1016/j.bcp.2009.07.023. (In Press) [DOI] [PubMed] [Google Scholar]
- Gladyshev VN, Jeang KT, Stadtman TC. Selenocysteine, identified as the penultimate C-terminal residue in human T-cell thioredoxin reductase, corresponds to TGA in the human placental gene. Proc Natl Acad Sci U S A. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gromer S, Eubel JK, Lee BL, Jacob J. Human selenoproteins at a glance. Cell Mol Life Sci. 2005;62:2414–2437. doi: 10.1007/s00018-005-5143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundimeda U, Schiffman JE, Gottlieb SN, Roth BI, Gopalakrishna R. Negation of the cancer-preventive actions of selenium by over-expression of protein kinase Cepsilon and selenoprotein thioredoxin reductase. Carcinogenesis. 2009;30:1553–1561. doi: 10.1093/carcin/bgp164. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hatfield DL. Thioredoxin reductase 1: A double-edged sword in cancer prevention and promotion. CCR Frontiers in Science. 2007;6:8–10. [Google Scholar]
- Hatfield DL, Gladyshev VN. The Outcome of Selenium and Vitamin E Cancer Prevention Trial (SELECT) reveals the need for better understanding of selenium biology. Mol Interv. 2009;9:18–21. doi: 10.1124/mi.9.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield DL, Yoo MH, Carlson BA, Gladyshev VN. Selenoproteins that function in cancer prevention and promotion. Biochim Biophys Acta. 2009;1790:1541–1545. doi: 10.1016/j.bbagen.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedstrom E, Eriksson S, Zawacka-Pankau J, Arnér ES, Selivanova G. p53-dependent inhibition of TrxR1 contributes to the tumor-specific induction of apoptosis by RITA. Cell Cycle. 2009;8:3576–3583. doi: 10.4161/cc.8.21.9977. [DOI] [PubMed] [Google Scholar]
- Hill KE, McCollum GW, Burk RF. Determination of thioredoxin reductase activity in rat liver supernatant. Anal Biochem. 1997;253:123–125. doi: 10.1006/abio.1997.2373. [DOI] [PubMed] [Google Scholar]
- Hintze KJ, Wald KA, Zeng H, Jeffery EH, Finley JW. Thioredoxin reductase in human hepatoma cells is transcriptionally regulated by sulforaphane and other electrophiles via an antioxidant response element. J Nutr. 2003;133:2721–2727. doi: 10.1093/jn/133.9.2721. [DOI] [PubMed] [Google Scholar]
- Holmgren A. In: Selenium: Its Molecular Biology and Role in Human Health. 2. Hatfield DL, Berry MJ, Gladyshev VN, editors. Springer Science+Business Media; New York: 2006. pp. 183–194. [Google Scholar]
- Holmgren A, Bjornstedt M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995;252:199–208. doi: 10.1016/0076-6879(95)52023-6. [DOI] [PubMed] [Google Scholar]
- Honeggar M, Beck R, Moos PJ. Thioredoxin reductase 1 ablation sensitizes colon cancer cells to methylseleninate-mediated cytotoxicity. Toxicol Appl Pharmacol. 2009;241:348–355. doi: 10.1016/j.taap.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons R, Carlson BA, Hatfield DL, Davis CD. Both selenoproteins and low molecular weight selenocompounds reduce colon cancer risk in mice with genetically impaired selenoprotein expression. J Nutr. 2006;136:1311–1317. doi: 10.1093/jn/136.5.1311. [DOI] [PubMed] [Google Scholar]
- Jakupoglu C, Przemeck GK, Schneider M, Moreno SG, Mayr N, Hatzopoulos AK, de Angelis MH, Wurst W, Bornkamm GW, Brielmeier M, Conrad M. Cytoplasmic thioredoxin reductase is essential for embryogenesis but dispensable for cardiac development. Mol Cell Biol. 2005;25:1980–1988. doi: 10.1128/MCB.25.5.1980-1988.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam JB, Chow KH, Xu A, Lam KS, Liu J, Wong NS, Moon RT, Shepherd PR, Cooper GJ, Wang Y. Adiponectin haploinsufficiency promotes mammary tumor development in MMTV-PyVT mice by modulation of phosphatase and tensin homolog activities. PLoS One. 2009;4:e4968. doi: 10.1371/journal.pone.0004968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang J, Li J, Chen D, Matteucci M, Curd J, Duan JX. Inhibition of Both Thioredoxin Reductase and Glutathione Reductase may Contribute to the Anticancer Mechanism of TH-302. Biol Trace Elem Res. 2009 doi: 10.1007/s12011-009-8544-1. (In Press) [DOI] [PubMed] [Google Scholar]
- Liu ZB, Shen X. Thioredoxin reductase 1 upregulates MCP-1 release in human endothelial cells. Biochem Biophys Res Commun. 2009;386:703–708. doi: 10.1016/j.bbrc.2009.06.100. [DOI] [PubMed] [Google Scholar]
- Low SC, Grundner-Culemann E, Harney JW, Berry MJ. SECIS-SBP2 interactions dictate selenocysteine incorporation efficiency and selenoprotein hierarchy. EMBO J. 2000;19:6882–6890. doi: 10.1093/emboj/19.24.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Berndt C, Holmgren A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim Biophys Acta. 2009;1790:1513–1519. doi: 10.1016/j.bbagen.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Merrill GF, Dowell P, Pearson GD. The human p53 negative regulatory domain mediates inhibition of reporter gene transactivation in yeast lacking thioredoxin reductase. Cancer Res. 1999;59:3175–3179. [PubMed] [Google Scholar]
- Moos PJ, Edes K, Cassidy P, Massuda E, Fitzpatrick FA. Electrophilic prostaglandins and lipid aldehydes repress redox-sensitive transcription factors p53 and hypoxia-inducible factor by impairing the selenoprotein thioredoxin reductase. J Biol Chem. 2003;278:745–750. doi: 10.1074/jbc.M211134200. [DOI] [PubMed] [Google Scholar]
- Noda M, Selinger Z, Scolnick EM, Bassin RH. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc Natl Acad Sci U S A. 1983;80:5602–5606. doi: 10.1073/pnas.80.18.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg J, Zhong L, Holmgren A, Arnér ES. Mammalian thioredoxin reductase is irreversibly inhibited by dinitrohalobenzenes by alkylation of both the redox active selenocysteine and its neighboring cysteine residue. J Biol Chem. 1998;273:10835–10842. doi: 10.1074/jbc.273.18.10835. [DOI] [PubMed] [Google Scholar]
- Rayman MP, Combs GF, Jr, Waters DJ. Selenium and vitamin E supplementation for cancer prevention. JAMA. 2009;301:1876. doi: 10.1001/jama.2009.625. [DOI] [PubMed] [Google Scholar]
- Rundlöf AK, Arnér ESJ. Regulation of the Mammalian Selenoprotein Thioredoxin Reductase 1 in Relation to Cellular Phenotype, Growth, and Signaling Events. Antioxid Redox Signal. 2004;6:41–52. doi: 10.1089/152308604771978336. [DOI] [PubMed] [Google Scholar]
- Rundlöf AK, Janard M, Miranda-Vizuete A, Arnér ES. Evidence for intriguingly complex transcription of human thioredoxin reductase 1. Free Radic Biol Med. 2004;36:641–656. doi: 10.1016/j.freeradbiomed.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Selenius M, Rundlof AK, Olm E, Fernandes AP, Bjornstedt M. Selenium and selenoproteins in the treatment and diagnostics of cancer. Antioxid Redox Signal. 2009 doi: 10.1089/ars.2009.2884. (In Press) [DOI] [PubMed] [Google Scholar]
- Smith AD, Levander OA. High-throughput 96-well microplate assays for determining specific activities of glutathione peroxidase and thioredoxin reductase. Methods Enzymol. 2002;347:113–121. doi: 10.1016/s0076-6879(02)47012-7. [DOI] [PubMed] [Google Scholar]
- Steinbrenner H, Sies H. Protection against reactive oxygen species by selenoproteins. Biochim Biophys Acta. 2009;1790:1478–1485. doi: 10.1016/j.bbagen.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Urig S, Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol. 2006;16:452–465. doi: 10.1016/j.semcancer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Watson WH, Heilman JM, Hughes LL, Spielberger JC. Thioredoxin reductase-1 knock down does not result in thioredoxin-1 oxidation. Biochem Biophys Res Commun. 2008;368:832–836. doi: 10.1016/j.bbrc.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Yoo MH, Carlson BA, Gladyshev VN, Hatfield DL. Simultaneous knockdown of the expression of two genes using multiple shRNAs and subsequent knock-in of their expression. Nat Protoc. 2009;4:1338–1348. doi: 10.1038/nprot.2009.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Shieh B, Reigan P, Zhang Z, Colucci MA, Chilloux A, Newsome JJ, Siegel D, Chan D, Moody CJ, Ross D. Potent activity of indolequinones against human pancreatic cancer: identification of thioredoxin reductase as a potential target. Mol Pharmacol. 2009;76:163–172. doi: 10.1124/mol.109.055855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Xu XM, Carlson BA, Gladyshev VN, Hatfield DL. Thioredoxin reductase 1 deficiency reverses tumor phenotype and tumorigenicity of lung carcinoma cells. J Biol Chem. 2006;281:13005–13008. doi: 10.1074/jbc.C600012200. [DOI] [PubMed] [Google Scholar]
- Yoo MH, Xu XM, Carlson BA, Patterson AD, Gladyshev VN, Hatfield DL. Targeting thioredoxin reductase 1 reduction in cancer cells inhibits self-sufficient growth and DNA replication. PLoS ONE. 2007a;2:e1112. doi: 10.1371/journal.pone.0001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo MH, Xu XM, Turanov AA, Carlson BA, Gladyshev VN, Hatfield DL. A new strategy for assessing selenoprotein function: siRNA knockdown/knock-in targeting the 3′-UTR. RNA. 2007b;13:921–929. doi: 10.1261/rna.533007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H, Combs GF., Jr Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem. 2008;19:1–7. doi: 10.1016/j.jnutbio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Zhong L, Arnér ES, Ljung J, Aslund F, Holmgren A. Rat and calf thioredoxin reductase are homologous to glutathione reductase with a carboxyl-terminal elongation containing a conserved catalytically active penultimate selenocysteine residue. J Biol Chem. 1998;273:8581–8591. doi: 10.1074/jbc.273.15.8581. [DOI] [PubMed] [Google Scholar]
- Zhong L, Holmgren A. Essential role of selenium in the catalytic activities of mammalian thioredoxin reductase revealed by characterization of recombinant enzymes with selenocysteine mutations. J Biol Chem. 2000;275:18121–18128. doi: 10.1074/jbc.M000690200. [DOI] [PubMed] [Google Scholar]