Abstract
Purpose
Type-2 diabetes mellitus increases risk of atherosclerotic cardiovascular disease. However, the mechanisms linking hyperglycemia and atherosclerosis remain poorly understood. One proposed mechanism involves endothelial dysfunction via activation of protein kinase C beta (PKC beta). Prior studies demonstrate beneficial effects of PKC beta inhibition on microvascular parameters, but, to date, no study has examined the effect on macrovascular atherosclerotic readouts.
Methods
The goal of this double-masked, placebo-controlled trial in type-2 diabetes was to assess the effect of the PKC beta-specific inhibitor, ruboxistaurin (32 mg/day for 6 weeks) on ultrasound assessed brachial artery flow mediated dilatation (FMD), a surrogate of macro vascular endothelial function, and urinary isoprostanes, indices of oxidant stress.
Results
Compared to placebo, ruboxistaurin tended to improve FMD (difference in 6-week change in FMD, mean±SD millimeter) at one (0.13±0.26 mm, p=0.08) and 5 min (0.12±0.21 mm, p=0.02) after cuff deflation, but had no effect on nitroglycerin-mediated dilatation or urinary isoprostanes.
Conclusions
This proof of concept trial is the first to suggest that specific inhibition of PKC beta may improve macro vascular endothelial function in type-2 diabetes. Larger trials including clinical endpoints are warranted to determine the potential efficacy of PKC beta inhibition in reducing atherosclerotic cardiovascular complications in diabetes mellitus.
Keywords: Type 2 diabetes, Protein kinase C beta, Endothelial function, Oxidant stress, Macro vascular disease
Introduction
Macrovascular atherosclerotic cardiovascular diseases (CVD) continue to be the leading cause of death in patients with type-2 diabetes mellitus [1]. This portends a significant health and societal burden, especially in an aging population with increasing obesity and type-2 diabetes. The risk of CVD in type-2 diabetes is only partly explained by traditional risk factors and current therapies fail to adequately abate CVD. Further, macrovascular, unlike microvascular complications, are only minimally ameliorated by tight glycemic control [2]. Thus, there is a great need for novel therapies that target vascular pathophysiology in the diabetic state.
Protein kinase Cs (PKCs) belong to a family of cytoplasmic serine/threonine kinases that participate in vascular cell signal transduction [3]. In particular, PKC beta (both the beta and beta2 splice variants) is present in cardiovascular tissues and is activated by circulating concentrations of glucose and fatty acids that are present in type-2 diabetes [4, 5]. Indeed, PKC beta has increased activity in both microvascular [6] and macrovascular [7, 8] diabetic tissues and is linked to vascular pathology in rodent diabetic models [9]. PKC beta mediates diverse signaling, including oxidant, inflammatory, mitogenic and angiogenic effects, in diabetic vascular tissues that may promote atherosclerotic CVD [10].
PKC beta inhibitors are a new class of drugs that are effective in attenuating the vascular complications of diabetes in animal models. In particular, PKC beta inhibition blocks hyperglycemia mediated vascular cell dysfunction in vitro while retarding retinal and renal microvascular disease in rodent models [9, 11]. The evidence so far from phase-three clinical trials of ruboxistaurin, a highly selective inhibitor of PKC beta1 and beta2 isoforms, suggests efficacy in reducing the risk of visual loss in moderately severe diabetic retinopathy [12] and attenuating peripheral neuropathy [13, 14] although findings [15] require further validation as only statistical significance for secondary endpoints was achieved. Some have suggested a role for these agents for diabetic microangiopathy [16]. From a pathophysiological standpoint [17], there is evidence that selective inhibition of PKC beta1 and beta2 may ameliorate vascular complications of diabetes [18]. Notably, PKC beta activation in diabetes is found equally in macrovascular and microvascular tissue [7, 8, 11] suggesting a potential benefit of PKC beta inhibition also in reducing diabetic macro vascular CVD. To date, however, no studies of the effect of PKC beta inhibition on CVD or it surrogates in type-2 diabetes have been reported.
The purpose of this proof of concept, placebo-controlled clinical trial of ruboxistaurin was to test the effect of PKC beta inhibition in patients with type-2 diabetes on urinary isoprostanes, indices of oxidant stress [19] and on brachial artery flow mediated dilatation (FMD), an ultrasound based measurement of macro vascular endothelial function [20–22].
Methods
This was a single-centered, double-masked, placebo-controlled, parallel, randomized clinical trial comparing ruboxistaurin (32 mg daily) with placebo in patients with type-2 diabetes. To be included, patients had to be ≥35 years of age diagnosed with type 2 diabetes mellitus (type 2 diabetes mellitus as diagnosed by their physician (criteria of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus from the American Diabetic Association [23] with fasting low-density lipoprotein (LDL) cholesterol <160 mg/dL, triglycerides <400 mg/dL, resting systolic blood pressure <160 mm Hg, and HbA1c between 7.0% and 11.0%, inclusive. Exclusions included impaired renal function (serum creatinine >2.0 mg/dl, renal transplant, or dialysis); impaired liver function (hepatic enzymes >2× upper normal limit); active use of tobacco products; use of aspirin, non-steroidal anti-inflammatory drugs or antioxidant vitamins within 14 days; use of systemic antibacterial, antifungal, or antiviral drugs or inhibitors and inducers of cytochrome P450 3A4; active infection/inflammation; major surgery (abdominal, thoracic, vascular, or cranial) within 3 months; treatment for cancer within 6 months; investigational drug use within 30 days; and pregnancy, breast feeding, or child-bearing potential without use of a reliable method of birth control. The University of Pennsylvania (Penn) institutional review board approved the study protocol, written informed consent was provided by all participants and study procedures were carried out at the Penn General Clinical Research Center (GCRC).
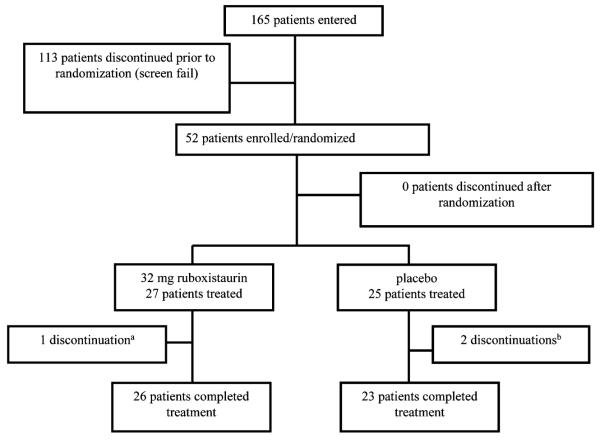
Of 165 participants undergoing screening, 52 were eligible and randomly assigned to treatment and 49 completed the study (Fig. 1). In our a priori design, participants who discontinued prior to the collection of endpoint measurements were replaced. The main outcomes were (a) urinary levels of the isoprostane, 8,12-iso-iPF2α-VI and (b) flow mediated dilation (FMD) of the brachial artery at ultrasound. Exploratory outcomes included urinary albumin excretion rate and plasma lipoprotein levels. Based on published estimates [24], a sample size of 48 patients should have provided 80% power, at two-sided alpha level of 0.05, to detect a 30% difference between groups in change in urinary isoprostanes from baseline to endpoint.
Fig. 1.
Disposition of trial participants
Protocol and procedures
If eligible at Visit 1 (screening), participants returned within 4 weeks for Visit 2 randomization to ruboxistaurin (32 mg/day) or placebo for a minimum of 6 weeks, but a maximum of 8 weeks, prior to Visit 3, study completion. Dose and time were selected based on a phase 1b trial demonstrating efficacy of this dose on vascular endpoints, including vascular function after 1 week of treatment in healthy volunteers [25] and 28 days of treatment in patients with early diabetic retinopathy [26].
At randomization, medications were recorded, urine (2× 12 h collections), for isoprostanes and albumin, and blood, for plasma lipids, routine chemistry and hematology, were collected. Brachial artery FMD was measured by ultrasound. Participants completed a daily drug diary. At study completion, similar procedures were performed, drug diaries were collected, pill counts and adverse events were recorded.
Brachial artery reactivity
Brachial artery ultrasound evaluation of endothelial function was performed as per published guidelines [20]. Participants, fasting and free from caffeine and alcohol for 12 h, were studied in a quiet, dimmed, temperature-controlled (24°C) laboratory. Female participants were studied during the luteal phase of the menstrual cycle, as FMD varies during the menstrual phase of the cycle. After baseline B mode ultrasound imaging (Acuson Sequoia 256 Cardiac ultrasound system, Siemens Medical) using a 15L8 linear-array transducer, a small blood pressure cuff was positioned proximally in order to occlude arm systolic flow. After occlusion at 200 mmHg of pressure (5 min), the cuff was released and the ultrasound diameter was again measured 1 and 5 min after hyperemia. Fifteen minutes later, a second baseline ultrasound was acquired, sublingual nitroglycerine (0.4 mg) was administered and a final scan was performed 4 min later. The images were analyzed with FDA-approved and validated commercial software (Medical Imaging Applications, Iowa City, Iowa). The diameter of the vessel was measured by a single reader (SA) who was blinded to study status. Intra-reader reliability, verified on 25% of the studies, was internally consistent (spearman r=0.95). In addition, an expert reader (EM) selected 25% of the studies at random to validate measurements, and the inter-reader correlation was high (spearman r=0.98). Brachial artery diameters as well as absolute and percent change in flow- and nitroglycerine-induced dilatation are reported. The overall coefficient of variation for FMD measurements across visits was 1.3%.
Laboratory parameters
Urinary levels (nanograms per milligram creatinine) of the isoprostane 8,12-iso-iPF2α-VI were measured by liquid chromatography (HyperClone C18-BDS, 5 μm, 150×2 mm column), tandem mass spectrometry (LC/MS/MS) as previously described [27]. Briefly, this method utilizes reverse phase solid phase extraction (SPE) followed by LC/MS/MS using negative ion electrospray, selected reaction monitoring techniques. The transitions monitored are m/z 353>115 for the endogenous iP and m/z 357>m/z 115 for the internal standard, tetradeuterated 8,12-iso-iPF2α-VI with quantitation by peak area ratio. Urine levels (nanograms per milligram creatinine) of albumin were measured on a Beckman Coulter LX 20 platform using a turbidmetric method. Plasma levels of low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low density lipoprotein (VLDL) cholesterol and triglycerides were measured enzymatically following ultracentrifugation fractionation of serum lipids in Penn’s Clinical Lipid Laboratory, which is certified by the Center for Disease Control. Serum biochemistry and blood hematology were performed in a centralized Quest laboratory.
Statistical methods
Normality of continuous variables was assessed using SAS PROC UNIVARIATE and variables with marked departures from normality were log transformed. Summary statistics (mean, standard deviation) for continuous clinical and laboratory variables and change from baseline to endpoint are presented. Categorical variables were examined between treatments using Fisher’s exact test. Analysis of endpoints was performed using a modified intention-to-treat (ITT) principle for all subjects with main outcome data (isoprostane and FMD) at visit 3 (i.e. protocol completers) using an analysis of covariance (ANCOVA) model that included a main effect term for treatment and for the baseline efficacy variable. Least-squares means were used to test the effects of ruboxistaurin versus placebo and to obtain the corresponding between treatment p-value. Statistical significance was defined as a p-value<0.05. For safety analyses, all participants who enrolled in the study were included regardless of whether they had measurements of efficacy.
Results
Baseline characteristics, discontinuation and compliance
Baseline characteristics including concomitant medications were similar both in the treatment group and the placebo group (Table 1). Participants were 31% female, 55% African American, 38% Caucasian, and had a mean age of 55 years. Two placebo-treated patients discontinued for personal reasons and one patient on ruboxistaurin was discontinued by study physician due to poor compliance. Compliance, defined as >85% of study drug taken by tablet count, was 96.3% for ruboxistaurin treatment.
Table 1.
Baseline characteristics of study participants
| Placebo (N=23a) Median (IQ range); N (%) |
RBX 32 mg (N=26a) Median (IQ range); N (%) |
|
|---|---|---|
| Age (years) | 56 (49–64) | 52 (45–60) |
| Race (Caucasian) | 12 (46%) | 7 (27%) |
| Male gender | 15 (65%) | 19 (73%) |
| Duration of diabetes (years) | 8.29 (1.82–20.22) | 7.21 (0.22–20.71) |
| HbA1c (%) | 8.30 (7.0–10.90) | 8.62 (7.00–10.80) |
| Glucose (mmol/L) | 5.17 (4.78–5.5) | 5 (4.72–5.39) |
| LDL—Cholesterol (mmol/L) | 2.49 (2.02–3.03) | 2.79 (2.10–3.41) |
| HDL—Cholesterol (mmol/L) | 1.12 (0.95–1.31) | 1.18 (1.0–1.44) |
| Triglycerides (mmol/L) | 1.43 (0.83–1.80) | 2.17 (1.67–2.40) |
| Body mass index (kg/m2) | 27.4 (25.2–30.3) | 25.6 (22.8–30.2) |
| Blood pressure (mm Hg) | ||
| Systolic | 128 (119–137) | 125 (113–136) |
| Diastolic | 79 (73–86) | 75 (68–82) |
| Medications | ||
| ACE-inhibitors or ARB | 13 (57%) | 17 (65%) |
| Statins | 14 (61%) | 10 (38%) |
| Diabetic therapy | ||
| Insulin only | 2 (9%) | 3 (11%) |
| Oral hypoglycemic agent | 17 (74%) | 22 (85%) |
| Both insulin and OHA | 4 (17%) | 1 (4%) |
RBX ruboxistaurin, IQ interquartile, HbA1c hemoglobin A1c, LDL low density lipoprotein, HDL high density lipoprotein, ACE angiotensin converting enzyme
Data are presented for subjects completing visit 3 (ie protocol completers)min 4
Effect of ruboxistaurin on endothelial function
After randomization, but prior to receiving drug, brachial artery diameters at rest, following cuff deflation and after nitroglycerine were similar in ruboxistaurin and placebo groups (Table 2). For example, in the groups randomized to receive ruboxistaurin and placebo, mean diameters were 3.89 mm vs. 3.77 mm (p=0.80) at rest, 4.29 mm vs. 4.14 mm (p=0.77) and 4.14 mm vs. 4.00 mm (p=0.80) at 1 min and 5 min post-cuff diameters respectively, and 4.62 mm vs. 4.53 mm (p=0.90) following NTG. There was high within-subject correlation between pre-cuff (baseline 1) and pre-NTG (baseline 2) diameters (spearman r=0.94). Furthermore, FMD in the ruboxistaurin group at 1 min (mean 0.39 mm±SD 0.21 mm) and 5 min (0.24±0.20) was not statistically different compared to the placebo group at 1 min (0.37±0.22, p=0.64) and 5 min (0.24±0.22, p=0.99) (Table 2 and Fig. 2a). Endothelial function, therefore, was similar in both groups prior to starting therapy.
Table 2.
Brachial artery data by visit: (a) absolute diameters and (b) absolute change with flow and nitroglycerin
| Group | Pre-therapy | Following 6 weeks of treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) of the brachial artery; mean (±standard deviation (SD)) | ||||||||||
| Placebo | BL 1 | Flow 1 min | Flow 5 min | BL 2 | NTG 4 min | BL 1 | Flow 1 min | Flow 5 min | BL 2 | NTG 4 min |
| 3.77 (±0.5) | 4.14 (±0.5) | 4.00 (±0.4) | 3.86 (±0.4) | 4.53 (±0.5) | 3.76 (±0.4) | 3.99 (±0.5) | 3.92 (±0.4) | 3.81 (±0.4) | 4.43 (±0.5) | |
| RBX | 3.89 (±0.7) | 4.29 (±0.6) | 4.14 (±0.6) | 4.05 (±0.6) | 4.62 (±0.7) | 3.93 (±0.8) | 4.33 (±0.6) | 4.22 (±0.7) | 4.13 (±0.7) | 4.68 (±0.7) |
| Absolute flow-mediated and nitroglycerin-mediated change (mm); mean (±SD) | ||||||||||
| Flow 1 min | Flow 5 min | NTG 4 min | Flow 1 min | Flow 5 min | NTG 4 min | Difference in 6-week change in FMD between treatmentsa | ||||
| 1 min | 5 min | |||||||||
| Placebo | 0.37 (±0.22) | 0.24 (±0.22) | 0.63 (±0.27) | 0.24 (±0.15) | 0.16 (±0.11) | 0.58 (±0.25) | 0.13 (±0.26) (p=0.08) | 0.12 (±0.21) (p<0.02) | ||
| RBX | 0.39 (±0.21) | 0.24 (±0.20) | 0.53 (±0.30) | 0.39 (±0.24) | 0.28 (±0.26) | 0.50 (±0.31) | ||||
RBX ruboxistaurin, NTG nitroglycerin, BL1 first baseline measurement—before cuff inflation, BL2 second baseline measurement—before nitroglycerin and 20 min after cuff deflation
p-values for RBX drug effect in repeated measures analysis of covariance (ANCOVA)
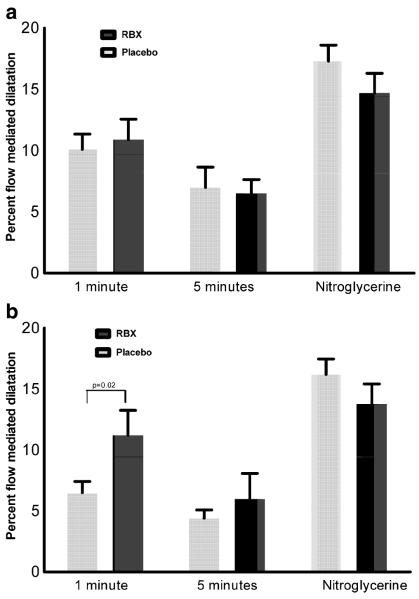
Fig. 2.
Change in flow mediated dilatation (FMD) by treatment group. Percent flow mediated dilatation (FMD) at 1 and 5 min after cuff deflation and percent nitroglycerin induced dilatation are presented a at randomization, before treatment and b following 6 weeks on treatment. After 6-weeks treatment, percent FMD in the ruboxistaurin group was greater than in placebo group at one min (11.2±9.9% vs. 6.5±4.4%; p=0.02) but at 5 min the difference between ruboxistaurin and control did not reach statistical significant (6.0±10% vs. 4.4±3.3%; p=0.23). The average change in absolute FMD over the 6-weeks of treatment tended to differ (mean±SD millimeter) between ruboxistaurin and placebo (Table 2) at one (0.13±0.26 mm, p=0.08) and 5 min (0.12±0.21, p=0.02)
Following 6 weeks of therapy, pre-cuff brachial artery diameters had excellent within-subject correlation with predrug values (spearman r=0.88). At 6 weeks, mean FMD in the ruboxistaurin group compared to the placebo group at 1 min (mean 0.39 mm±SD 0.24 mm vs. 0.24±0.15; p<0.001) and at 5 min (0.28±0.26 vs. 0.16±0.11; p<0.001) suggested improved FMD with ruboxistaurin treatment. Indeed, the average change in absolute FMD over the 6-weeks of treatment tended to differ (mean±SD mm) between ruboxistaurin and placebo (Table 2) at 1 (0.13± 0.26 mm, p=0.08) and 5 min (0.12±0.21, p=0.02). Furthermore, change in percent FMD over 6 week of treatment (Fig. 2b) at 1 min (11.2±9.9% vs. 6.5±4.4%; p=0.02) suggested improved FMD with ruboxistaurin, but not at 5 min (6.0±10% vs. 4.4±3.3%; p=0.23). In exploratory analyses, we did not find any statistically significant differences in the effect of RBX therapy on FMD across subgroups stratified by median levels of microalbuminuria and HbA1C, or by the presence or absence of statin use (data not shown). However, these exploratory analyses are underpowered and cannot exclude a true differential effect of RBX across strata. No statistically significant difference was observed between treatment groups over 6-weeks in nitroglycerin (0.4 mg) mediated dilatation, a measure of endothelium-independent vasodilatation (Table 2).
Effect of ruboxistaurin on urinary isoprostanes, urinary albumin excretion rate, laboratory parameters and adverse events
Compared to placebo, ruboxistaurin treatment produced no statistically significant effect on urinary levels of 8,12-iso-iPF2α-VI (p=0.80) or urinary albumin excretion rate (p=0.85) (Table 3). There were no differences in routine chemistries or hematology measures between treatment groups. A statistically significant difference was observed in the change in LDL-C levels (decreased in the placebo arm but not with ruboxistaurin), but not in total cholesterol, HDL-C and triglyceride levels (Table 3). No differences between treatment groups in adverse events were observed (data not shown). No deaths were reported and most adverse events were mild in severity; one participant in the placebo arm suffered a myocardial infarction.
Table 3.
Effect of ruboxistaurin on laboratory parameters
| Parameter | Treatment | Baseline | 6 weeks | Within group p-value |
Between group p-value |
|---|---|---|---|---|---|
| 8,12-iso-iPF2α-VI (ng/mg creatinine) | Placebo | 5.0 (3.2–6.9)a | 4.9 (3.4–5.9) | 0.96 | 0.91 |
| RBX 32 mg/day | 4.0 (2.6–5.2) | 4.1 (2.4–5.6) | 0.65 | ||
| Urinary albumin excretion rate (μg/mg creatinine) | Placebo | 79 (36–168) | 81 (32–344) | 0.74 | 0.85 |
| RBX 32 mg/day | 117 (48–285) | 91 (62–498) | 0.50 | ||
| Total cholesterol (mmol/L) | Placebo | 4.61 (4.0–5.1) | 4.34 (3.9–4.6) | 0.15 | 0.16 |
| RBX 32 mg/day | 4.87 (3.9–5.4) | 4.79 (3.8–5.4) | 0.45 | ||
| HDL cholesterol (mmol/L) | Placebo | 1.17 (0.9–1.3) | 1.18 (0.9–1.3) | 0.57 | 0.77 |
| RBX 32 mg/day | 1.21 (1.0–1.5) | 1.22 (1.0–1.5) | 0.16 | ||
| LDL cholesterol (mmol/L) | Placebo | 2.69 (2.1–3.1) | 2.37 (2.0–2.8) | 0.001 | 0.01 |
| RBX 32 mg/day | 2.74 (2.2–3.4) | 2.81 (2.3–3.5) | 0.92 | ||
| Triglycerides (mmol/L) | Placebo | 1.53 (1.0–2.1) | 1.66 (1.1–2.2) | 0.43 | 0.51 |
| RBX 32 mg/day | 1.60 (1.1–2.1) | 1.55 (1.0–2.1) | 0.94 |
Median (interquartile range)
Discussion
We found that specific inhibition of PKC beta appeared to improve brachial artery endothelial vasodilator function in patients with type-2 diabetes. This study provides the first suggestive evidence in patients with type-2 diabetes of a potential benefit for this class of drugs on diabetic macrovascular disease, the leading cause of death in these patients. These preliminary findings may have implications for the potential clinical utility of PKC beta inhibitors since the phase-three clinical trials of ruboxistaurin performed to date suggest that it is well tolerated and may provide efficacy in diabetic retinopathy [12] and peripheral neuropathy [13, 14].
Activation of vascular PKC beta is thought to occur preferentially in type-2 diabetes and insulin resistance through glycemic disturbance, elevation of non-esterified fatty acids and vascular oxidant stress. Each of these abnormalities increases de novo synthesis of vascular diacylglyerol (DAG) [4, 7, 8, 28, 29], and DAG directly activates most PKC isoforms (both classic and novel), including PKC beta, a major isoform in the vasculature. Oxidant signals also activate PKCs indirectly via phospholipase D and hydrolysis of phosphotidylcholine to DAG [29]. Notably, coincident increases in DAG levels and membrane associated PKC beta, a measure of in situ PKC activation, are consistently observed in the vasculature of both acute and chronic animal models of diabetes [7]. Hyperglycemia and elevated circulating free fatty acids also appear to induce vascular reactive stress in part through PKC-dependent activation of NAD(P)H oxidase [30, 31]
PKC beta activation has diverse vascular signaling effects that promote diabetes related vascular pathologies via oxidant stress [28], inflammation [32] and endothelial dysfunction [10]. In endothelial cells, PKC beta activation of NAD(P)H [33], modulation of endothelial nitric oxide synthase (eNOS) [34] and induction of adhesion molecules [32] lead to atherogenic endothelial dysfunction. In parallel in vascular smooth muscle cells, PKC beta induces mitogenic growth factors and endothelin-1 while activation of cytosolic phosphoslipase A2 generating vasoactive eicosanoids promoting vascular smooth muscle cell proliferation and hyperplasia [10, 35].
Many of these metabolic atherogenic pathophysiologies can be normalized by treatment with PKC inhibitors in animal models [11]. Indeed, PKC beta inhibition blocks hyperglycemia mediated vascular cell dysfunction in vitro [4, 36], retards retinal and renal micro vascular disease in rodent models [11, 37], and recent trials support its potential efficacy in attenuating progression of clinical microvascular complications [12, 14, 38]. Despite these experimental and microvascular effects, there is almost no data on PKC beta inhibition on atherosclerosis related readouts in humans.
PKC beta activation is found equally in large and small arteries in diabetic models [11] raising the possibility of favorable effects of PKC beta inhibition on macrovascular complications of diabetes. The degree of macrovascular endothelial vasodilator dysfunction is believed to reflect the extent of atherogenic oxidant and inflammatory pathophysiology in patients with type-2 diabetes [39]. Consistent with this concept, experimental hyperglycemia or infusion of free fatty acids can impair large vessel endothelial function both in vitro and in vivo [40, 41]. Remarkably, in a small proof of concept study, Beckman found that hyperglycemia-induced brachial artery endothelial dysfunction in healthy volunteers was completely attenuated by selective inhibition of PKC beta [25].
Ours is the first study to suggest that PKC beta inhibition might improve macrovascular endothelial function in patients with type-2 diabetes. Although, this study was of short duration and used a surrogate measure of vascular function, brachial artery FMD represents a standard for assessment of large vessel endothelial function [42, 43], is strongly correlated with atherosclerosis, and predicts poor CVD outcomes [21, 22]. Our findings should be interpreted with caution given the small proof of concept design, the small differences between treatments and the tendency for a loss of endothelial function in the placebo arm over the course of the trial. However, similar direction of ruboxistaurin effects on FMD at 1 and 5 min and the high-within subject correlations in pre-cuff diameter readings over the course of the study suggest findings may be valid. In this context, our study provides the basis for next-step trials of longer duration assessing the impact of PKC beta inhibitors on atherosclerosis readouts in type-2 diabetic patients. We did note a decrease in LDL levels in the placebo group compared to the RBX group. Because there were no changes in concomitant medication usage during the study period and other lipid parameters changed little, we believe that this difference may be due chance although dietary improvement in the placebo arm might have contributed. Regardless, a true improvement in LDL cholesterol in the placebo group should bias toward the null rather than produce a spurious finding.
PKC activation, as noted above, is closely related to vascular oxidant stress [28, 44, 45]. Therefore, we hypothesized that PKC beta inhibition would improve macrovascular endothelial function, in part, by reducing oxidant stress. However, we found no effect of ruboxistaurin on urinary excretion of isoprostanes, a validated index of oxidant stress in diabetes, vascular injury and atherosclerosis [24, 46]. Thus, oxidant stress, at least as measured by this integrated measure of non-enzymatic lipid peroxidation, may not be causal in PKC beta related endothelial dysfunction. It is possible however that alternative measures, such as indices of NO biosynthesis, superoxide or vascular NADPH, may provide more appropriate biomarkers of PKC beta related vascular oxidant stress rather than urinary isoprostanes. Alternatively, our study may have been underpowered or of too short a duration to detect effects on isoprostanes. Indeed, the lack of effect on urinary albumin excretion rate is perhaps consistent with the need for larger studies of longer duration given significant reduction in urinary albumin excretion rate by ruboxistaurin in a previous clinical trial [47]. In this context, our study is limited by short duration, relatively small sample size and focus on specific surrogate measures of macro vascular disease. In addition, the specific mechanism(s) by which PKC beta inhibition may improve human macro vascular endothelial function remains to be elucidated.
Conclusion
This is the first trial showing that specific inhibition of PKC beta may improve brachial artery FMD in type-2 diabetes, and therefore might represent a novel therapy for macrovascular complications of diabetes. Larger trials of clinical endpoints are warranted to determine the potential efficacy in diabetic CVD.
Acknowledgments
This study was supported by an unrestricted grant from Eli Lilly & Co (MPR), NCRR K23 RR15532 (MPR), the PENN General Clinical Research Center (GCRC: NIH M01-RR00040) and a Clinical and Translational Science Award (RFA-RM-06-002) from the NCRR/NIH to the University of Pennsylvania.
Dr. Mehta is the recipient of the American College of Cardiology/Merck Young Investigator Grant in Metabolic Syndrome. MPR is supported by HL RO1-073278, HL P50-083799 (SCCOR) and the W.W. Smith Charitable Trust (#H0204).
Contributor Information
Nehal N. Mehta, Cardiovascular Institute, University of Pennsylvania Medical Center, 909 BRB 2/3, 421 Curie Blvd., Philadelphia, PA 19104-6160, USA; Center for Experimental Therapeutics, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Matthew Sheetz, Eli Lilly Research Labs, Indianapolis, IN, USA.
Karen Price, Eli Lilly Research Labs, Indianapolis, IN, USA.
Lynn Comiskey, Cardiovascular Institute, University of Pennsylvania Medical Center, 909 BRB 2/3, 421 Curie Blvd., Philadelphia, PA 19104-6160, USA; Center for Experimental Therapeutics, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Shirish Amrutia, Cardiovascular Institute, University of Pennsylvania Medical Center, 909 BRB 2/3, 421 Curie Blvd., Philadelphia, PA 19104-6160, USA; Center for Experimental Therapeutics, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Nayyar Iqbal, Cardiovascular Institute, University of Pennsylvania Medical Center, 909 BRB 2/3, 421 Curie Blvd., Philadelphia, PA 19104-6160, USA; Center for Experimental Therapeutics, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA.
Emile R. Mohler, Cardiovascular Institute, University of Pennsylvania Medical Center, 909 BRB 2/3, 421 Curie Blvd., Philadelphia, PA 19104-6160, USA; Center for Experimental Therapeutics, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
Muredach P. Reilly, Cardiovascular Institute, University of Pennsylvania Medical Center, 909 BRB 2/3, 421 Curie Blvd., Philadelphia, PA 19104-6160, USA; Center for Experimental Therapeutics, Department of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA, USA
References
- 1.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–81. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Way KJ, Chou E, King GL. Identification of PKC-isoform-specific biological actions using pharmacological approaches. Trends Pharmacol Sci. 2000;21:181–7. doi: 10.1016/s0165-6147(00)01468-1. [DOI] [PubMed] [Google Scholar]
- 4.Sheetz MJ, King GL. Molecular understanding of hyperglycemia’s adverse effects for diabetic complications. JAMA. 2002;288:2579–88. doi: 10.1001/jama.288.20.2579. [DOI] [PubMed] [Google Scholar]
- 5.Koya D, King GL. Protein kinase C activation and the development of diabetic complications. Diabetes. 1998;47:859–66. doi: 10.2337/diabetes.47.6.859. [DOI] [PubMed] [Google Scholar]
- 6.Aiello LP, et al. Vascular endothelial growth factor-induced retinal permeability is mediated by protein kinase C in vivo and suppressed by an orally effective beta-isoform-selective inhibitor. Diabetes. 1997;46:1473–80. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- 7.Inoguchi T, et al. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci USA. 1992;89:11059–63. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xia P, et al. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994;43:1122–9. doi: 10.2337/diab.43.9.1122. [DOI] [PubMed] [Google Scholar]
- 9.Way KJ, Katai N, King GL. Protein kinase C and the development of diabetic vascular complications. Diabet Med. 2001;18:945–59. doi: 10.1046/j.0742-3071.2001.00638.x. [DOI] [PubMed] [Google Scholar]
- 10.Rask-Madsen C, King GL. Proatherosclerotic mechanisms involving protein kinase C in diabetes and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:487–96. doi: 10.1161/01.ATV.0000155325.41507.e0. [DOI] [PubMed] [Google Scholar]
- 11.Ishii H, et al. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–31. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- 12.The PKC-DRS Study Group The effect of ruboxistaurin on visual loss in patients with moderately severe to very severe non-proliferative diabetic retinopathy: initial results of the Protein Kinase C beta Inhibitor Diabetic Retinopathy Study (PKC-DRS) multicenter randomized clinical trial. Diabetes. 2005;54:2188–97. doi: 10.2337/diabetes.54.7.2188. [DOI] [PubMed] [Google Scholar]
- 13.Tesfaye S, et al. Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007;30:2626–32. doi: 10.2337/dc07-0608. [DOI] [PubMed] [Google Scholar]
- 14.Vinik AI, et al. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005;27:1164–80. doi: 10.1016/j.clinthera.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 15.Casellini CM, et al. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007;30:896–902. doi: 10.2337/dc06-1699. [DOI] [PubMed] [Google Scholar]
- 16.Idris I, Donnelly R. Protein kinase C beta inhibition: a novel therapeutic strategy for diabetic microangiopathy. Diab Vasc Dis Res. 2006;3:172–8. doi: 10.3132/dvdr.2006.026. [DOI] [PubMed] [Google Scholar]
- 17.Yan SF, et al. Protein kinase C beta/early growth response-1 pathway: a key player in ischemia, atherosclerosis, and restenosis. J Am Coll Cardiol. 2006;48:A47–55. doi: 10.1016/j.jacc.2006.05.063. [DOI] [PubMed] [Google Scholar]
- 18.Das Evcimen N, King GL. The role of protein kinase C activation and the vascular complications of diabetes. Pharmacol Res. 2007;55:498–510. doi: 10.1016/j.phrs.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 19.Pratico D, et al. The isoprostanes in biology and medicine. Trends Endocrinol Metab. 2001;12:243–7. doi: 10.1016/s1043-2760(01)00411-8. [DOI] [PubMed] [Google Scholar]
- 20.Corretti MC, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 21.Suwaidi JA, et al. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–54. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 22.Halcox JP, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 23.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2004;27:S5–10. doi: 10.2337/diacare.27.2007.s5. [DOI] [PubMed] [Google Scholar]
- 24.Davi G, et al. In vivo formation of 8-iso-prostaglandin f2alpha and platelet activation in diabetes mellitus: effects of improved metabolic control and vitamin E supplementation. Circulation. 1999;99:224–9. doi: 10.1161/01.cir.99.2.224. [DOI] [PubMed] [Google Scholar]
- 25.Beckman JA, et al. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:107–11. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 26.Aiello LP, et al. Inhibition of PKC beta by oral administration of ruboxistaurin is well tolerated and ameliorates diabetes-induced retinal hemodynamic abnormalities in patients. Invest Ophthalmol Vis Sci. 2006;47:86–92. doi: 10.1167/iovs.05-0757. [DOI] [PubMed] [Google Scholar]
- 27.Li H, et al. Quantitative high performance liquid chromatography/tandem mass spectrometric analysis of the four classes of F(2)-isoprostanes in human urine. Proc Natl Acad Sci USA. 1999;96:13381–6. doi: 10.1073/pnas.96.23.13381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 29.Taher MM, Garcia JG, Natarajan V. Hydroperoxide-induced diacylglycerol formation and protein kinase C activation in vascular endothelial cells. Arch Biochem Biophys. 1993;303:260–6. doi: 10.1006/abbi.1993.1281. [DOI] [PubMed] [Google Scholar]
- 30.Inoguchi T, et al. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C-dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000;49:1939–45. doi: 10.2337/diabetes.49.11.1939. [DOI] [PubMed] [Google Scholar]
- 31.Quagliaro L, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells: the role of protein kinase C and NAD(P)H-oxidase activation. Diabetes. 2003;52:2795–804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- 32.Kouroedov A, et al. Selective inhibition of protein kinase Cbeta2 prevents acute effects of high glucose on vascular cell adhesion molecule-1 expression in human endothelial cells. Circulation. 2004;110:91–6. doi: 10.1161/01.CIR.0000133384.38551.A8. [DOI] [PubMed] [Google Scholar]
- 33.Kitada M, et al. Translocation of glomerular p47phox and p67phox by protein kinase C-beta activation is required for oxidative stress in diabetic nephropathy. Diabetes. 2003;52:2603–14. doi: 10.2337/diabetes.52.10.2603. [DOI] [PubMed] [Google Scholar]
- 34.Naruse K, et al. Activation of vascular protein kinase C-beta inhibits Akt-dependent endothelial nitric oxide synthase function in obesity-associated insulin resistance. Diabetes. 2006;55:691–8. doi: 10.2337/diabetes.55.03.06.db05-0771. [DOI] [PubMed] [Google Scholar]
- 35.Yamada Y, Yokota M. Effects of protein kinase C activation and inhibition on endothelin-1 release from human aortic and pulmonary artery endothelial cells: comparison with effects on bovine endothelin-1 and human prostaglandin I2 release. Am J Hypertens. 1997;10:32–42. doi: 10.1016/s0895-7061(96)00250-6. [DOI] [PubMed] [Google Scholar]
- 36.Meier M, King GL. Protein kinase C activation and its pharmacological inhibition in vascular disease. Vasc Med. 2000;5:173–85. doi: 10.1177/1358836X0000500307. [DOI] [PubMed] [Google Scholar]
- 37.Koya D, et al. Amelioration of accelerated diabetic mesangial expansion by treatment with a PKC beta inhibitor in diabetic db/db mice, a rodent model for type 2 diabetes. FASEB J. 2000;14:439–47. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- 38.The PKC-DMES Study Group Effect of ruboxistaurin in patients with diabetic macular edema: thirty-month results of the randomized PKC-DMES clinical trial. Arch Ophthalmol. 2007;125:318–24. doi: 10.1001/archopht.125.3.318. [DOI] [PubMed] [Google Scholar]
- 39.Hink U, et al. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res. 2001;88:E14–22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 40.de Jongh RT, et al. Free fatty acid levels modulate microvascular function: relevance for obesity-associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;53:2873–82. doi: 10.2337/diabetes.53.11.2873. [DOI] [PubMed] [Google Scholar]
- 41.Steinberg HO, et al. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–9. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–95. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 43.Hamburg NM, et al. Comparison of endothelial function in young men and women with a family history of premature coronary artery disease. Am J Cardiol. 2004;94:783–5. doi: 10.1016/j.amjcard.2004.05.067. [DOI] [PubMed] [Google Scholar]
- 44.Guzik TJ, et al. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–62. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 45.Naka Y, et al. RAGE axis: animal models and novel insights into the vascular complications of diabetes. Arterioscler Thromb Vasc Biol. 2004;24:1342–9. doi: 10.1161/01.ATV.0000133191.71196.90. [DOI] [PubMed] [Google Scholar]
- 46.Reilly MP, et al. Increased formation of distinct F2 isoprostanes in hypercholesterolemia. Circulation. 1998;98:2822–8. doi: 10.1161/01.cir.98.25.2822. [DOI] [PubMed] [Google Scholar]
- 47.Tuttle KR, et al. The effect of ruboxistaurin on nephropathy in type 2 diabetes. Diabetes Care. 2005;28:2686–90. doi: 10.2337/diacare.28.11.2686. [DOI] [PubMed] [Google Scholar]