Abstract
Adiposity-associated inflammation and insulin resistance are strongly implicated in the development of type 2 diabetes and atherosclerotic cardiovascular disease. This article reviews the mechanisms of adipose inflammation, because these may represent therapeutic targets for insulin resistance and for prevention of metabolic and cardiovascular consequences of obesity. The initial insult in adipose inflammation and insulin resistance, mediated by macrophage recruitment and endogenous ligand activation of Toll-like receptors, is perpetuated through chemokine secretion, adipose retention of macrophages, and elaboration of pro-inflammatory adipocytokines. Activation of various kinases modulates adipocyte transcription factors, including peroxisome proliferator-activated receptor-γ and NFκB, attenuating insulin signaling and increasing adipocytokine and free fatty acid secretion. Inflammation retards adipocyte differentiation and further exacerbates adipose dysfunction and inflammation. Paracrine and endocrine adipose inflammatory events induce a local and systemic inflammatory, insulin-resistant state promoting meta-bolic dyslipidemia, type 2 diabetes, and cardiovascular disease. Developing therapeutic strategies that target both adipose inflammation and insulin resistance may help to prevent type 2 diabetes and cardiovascular disease in the emerging epidemic of obesity.
Keywords: type 2 diabetes, insulin resistance, adiposity-associated inflammation, adipose tissue, insulin, fatty acids
Obesity, particularly visceral obesity, is closely linked to the development of the metabolic syndrome, type 2 diabetes mellitus, and atherosclerotic cardiovascular disease (CVD).1 Activation of innate immune pathways in adipose tissue has been proposed to link obesity to insulin resistance (IR) and atherosclerosis. Recruitment and infiltration of adipose tissue macrophages (ATMs) lead to adipocyte inflammation.2,3 In this environment, a variety of endogenous and exogenous innate Toll-like receptor (TLR) antigens may promote a local metabolic endotoxemia and maintain adipocyte dysfunction and IR.4 Inflammatory adipose tissue is also a critical player in systemic IR through secretion of various adipocytokines5 and free fatty acids (FFAs)6 that regulate hepatic, skeletal muscle, and vascular insulin signaling. Finally, several chemokines, cytokines, kinases, and transcription factors have been implicated in adipose inflammation, systemic IR, and a chronic inflammatory atherogenic state7 that contributes to type 2 diabetes and atherosclerosis.
Inflammation Modulates Adipose Functions
Preadipocytes
Preadipocytes have the capacity for phagocytosis and cytokine secretion.8 Lipopolysaccharide (LPS) induces chemokine secretion from and TLR expression in preadipocytes, demonstrating their capacity to recruit macrophages.9 This inflammatory response also contributes to IR in adipocytes.9 As preadipocytes undergo differentiation, their inflammatory capacity diminishes.9 Thus, inflammatory modulation of adipocyte differentiation plays an important role in local and systemic inflammation and IR (Table 1).
Table 1.
Adipose Inflammatory Functions in Insulin Resistance and Cardiovascular Disease
| Component | Function | Key References |
|---|---|---|
| Preadipocytes | Macrophage-like, pro-inflammatory, interfere with adipocyte insulin signaling. |
9 |
| Innate immunity | Innate antigens and TLRs transduce adipocyte inflammation; mediate diet-induced obesity and insulin resistance. |
4, 10, 11 |
| ATMs | Resident ATMs shift toward pro-inflammatory phenotype in obesity, whereas high-fat diet recruits ATMs (CCR2+) via chemokines; ATMs contribute to adipose inflammation and insulin resistance. |
15–18 |
| Chemokines | CCL2 (MCP-1) and its receptor, CCR2, play a key role in diet-induced recruitment and retention of ATMs, obesity, and insulin resistance; RANTES promotes T-cell chemotaxis and is elevated in obesity. |
19, 20, 22 |
| Adipokines | Adipose inflammation regulates adiponectin, resistin, leptin, RBP-4, visfatin, and lipocalin-2 production; adipokines modulate systemic insulin signaling, hepatic lipoprotein production, innate immunity, and vascular inflammation. |
5, 29 |
| FFAs | Adipose inflammation induces higher adipocyte FFA production; FFAs induce insulin resistance in skeletal muscle and liver, modulate lipoprotein production, and impair endothelial functions. |
6 |
| Cytokines | Adipose inflammation increases local and systemic cytokine production, which induces adipocyte insulin resistance, systemic insulin resistance, endothelial dysfunction, and vascular inflammation. |
45, 52, 53, 57 |
TLR, Toll-like receptor; ATM, adipose tissue macrophage; MCP, monocyte chemotactic protein; RANTES; regulated on activation, normal T cell expressed and secreted; RBP, retinol binding protein; FFA, free-fatty acid.
Innate Immunity and TLR4
TLR4 is an LPS receptor, also activated by long-chain fatty acids (FAs), that transduces cytokine expression.4 TLR4 can signal via MyD88 to activate NFκB signaling or via an MyD88-independent pathway to induce interferon-regulatory genes.10 Activation of TLR4 in adipocytes induces NFκB target genes and decreases Akt and GSK3β phosphorylation, key mediators of insulin signaling and glucose uptake.10 Tsukumo et al11 showed that a loss-of-function mutation in TLR4 protects mice from diet-induced obesity and IR. Compared with control conditions, a high-fat diet induced smaller fat depots, less adipose macrophage infiltration, lower FFAs, and normal insulin pathway phosphorylation as well as lower cytokines, blood glucose, and hepatic triglycerides in TLR-deficient mice.11 TLR4 and its adaptor proteins, MyD88 and interleukin-1 receptor–associated kinase (IRAK-1), are also important in vascular inflammation and IR.12 Not surprisingly, endotoxemia induces IR in vivo.4,13 Indeed, our group reported that experimental human endotoxemia promotes adipose inflammation and alters adipokine function coincident with systemic IR.14 Thus, TLR4 is a critical player in inflammation, IR, and vascular injury.
Recruitment and Actions of ATMs
ATMs play an important role in adipose inflammation and IR as well as in adiposity and type 2 diabetes.2 There are 2 different populations of ATMs—the constitutive or resident ATMs and the short-lived or recruited ATMs.15,16 Resident ATMs assist with homeostasis and tissue remodeling, but in obese mice, they shift toward the classical, pro-inflammatory M1 (CCR2+) phenotype.17 A high-fat diet increases circulating M1 (CCR2+) monocytes15,17 and promotes their recruitment and retention in adipose tissue.15,16 Recruited ATMs induce adipocyte inflammation, promote adipose neovascularization, and interfere with insulin signaling.18 When mixed with macrophage media, adipocytes demonstrate increased inflammatory genes, adhesion to monocytes, increased NFκB activity, and decreased insulin-stimulated glucose uptake.3 Thus, ATM-secreted factors cause inflammation in adipocytes, and adipocyte/macrophage cross-talk contributes to IR.3
Chemokines in Adipose Inflammation, Insulin Resistance, and Atherosclerosis
Chemokines and their receptors play a critical role in ATM recruitment to adipose tissue. Monocyte chemotactic protein-1 (MCP-1) is strongly implicated in ATM recruitment, adipose expansion and remodeling, and angiogenesis.19,20 MCP-1 is overexpressed in obese rodents and obese diabetic humans, and it is associated with macrophage infiltration and IR.16,20 Furthermore, MCP-1 deficiency or inhibition of MCP-1 expression in obese mice ameliorates IR and reduces ATMs.19 CCR2, the MCP-1 receptor, also plays a role in adipose inflammation. In fact, on a high-fat diet, CCR2 knockout mice had reduced ATMs coincident with less obesity, increased insulin sensitivity and circulating adiponectin, and lower inflammatory cytokines and hepatic triglycerides.21 RANTES (which stands for “regulated on activation, normal T cell expressed and secreted”), a recently discovered chemokine, plays a role in T-cell chemotaxis and is found to be elevated in the adipose tissue of obese mice and humans.22 Both MCP-1 and RANTES are up-regulated in atherosclerotic lesions,23 and attenuation of their signaling inhibits macrophage foam-cell formation and lesion development.24,25 Overall, several chemokines26 play critical roles in obesity, IR, and atherosclerosis.
Adipokines in Inflammation, Insulin Resistance, and Atherosclerosis
Adipokines provide an important link between obesity and IR. Adiponectin is a unique adipokine that is inversely related to the metabolic syndrome, type 2 diabetes, and atherosclerotic CVD.27 Adiponectin increases FA oxidation while reducing glucose production in liver, and ablation of the gene in mice induces IR, type 2 diabetes, and atherosclerosis.28 Adiponectin is also anti-inflammatory; it suppresses tumor necrosis factor (TNF) actions in nonalcoholic fatty liver disease and inhibits NFκB in and monocyte adhesion to endothelial cells.29 Resistin, another adipokine, is secreted by adipocytes in rodents but is restricted to immune cells in humans.30 Human resistin is generated by infiltrating inflammatory cells in human adiposity and can stimulate synthesis and secretion of cytokines in adipocytes and endothelial cells.31 Leptin, a well-known adipokine, normally functions centrally to suppress appetite, but most obese patients are leptin resistant with increased circulating leptin.32 In obesity, hyperleptinemia contributes to inflammation through modulation of T-cell and monocyte functions.33,34 A role for retinol-binding protein 4 (RBP-4; a more recently described adipokine) in IR has been proposed, and RBP-4 expression in adipose is linked to inflammation.35
Visfatin is a novel adipokine that is increased during the development of obesity, is pro-inflammatory,36 and has an insulin-mimetic effect via binding to the insulin receptor.37 A member of the lipocalin family, lipocalin-2, also known as neutrophil gelatinase–associated lipocalin,38 modulates inflammation and is another adipokine that is elevated in the adipose tissue of obese mouse models39 and in the plasma of obese and insulin-resistant humans.40 In vitro studies suggest that lipocalin-2 induces IR in adipocytes and hepatocytes.39 The plasma level of another member of the lipocalin family, lipocalin-type prostaglandin D synthase, serves as a biomarker of coronary atherosclerosis.41 Thus, multiple adipose-secreted factors that modulate systemic IR are regulated by inflammatory signals and in turn have a significant impact on innate immune and vascular inflammation.
Free Fatty Acids
Nutritional FFAs modulate the inflammatory response, particularly via NFκB activity, and promote IR.6,42 Further-more, inflammatory modulation of adipocyte differentiation increases FFA release. Mechanisms of FFA-associated IR include protein kinase C (PKC) activation, endoplasmic reticulum stress, and increased oxidative burden.43 FFAs inhibit insulin receptor substrates (IRSs) and induce IR in skeletal muscle and liver.6 Increased FFA flux from adipose tissue to liver causes hepatic IR by increasing gluconeogenesis, glycogenolysis, and glucose-6-phosphatase expression and activity6 and by enhancing lipogenesis and triglyceride synthesis attributable to activation of the transcription factor sterol-CoA regulatory element binding protein.6 Finally, FFAs cause endothelial IR and damage by impairing insulin and nitric oxide–dependent signaling,44 thus contributing to the vascular injury observed in adiposity.
Adipose Cytokines Promote Insulin Resistance, Atherosclerosis, and Thrombosis
TNF-α is increased in adipose tissue and in the circulation in obese, insulin-resistant, and atherogenic states.45,46 In adipocytes and skeletal muscle, TNF-α inhibits tyrosine phosphorylation of IRS-1, which decreases insulin signaling.47,48 TNF-α receptor deficiency protects against IR.47,49 In humans, TNF-α infusion decreases insulin sensitivity and increases phosphorylation of extracellular signal-regulated kinase-1/2 (ERK-1/2), c-Jun N-terminal kinase (JNK), and serine 312 on IRS-1.50,51 Interleukins (ILs) have also been implicated in obesity and IR. IL-6 is elevated in obesity and is increased in the portal circulation, thereby stimulating hepatic production of acute-phase reactants such as C-reactive protein.52 However, the role of IL-6 in IR remains controversial, and it may in fact be protective.50 IL-18 is an inflammatory cytokine in the IL-1 family53 that is elevated in obesity and is an independent predictor of CVD.54,55
Several adipose-secreted factors not only are associated with inflammation but also promote hypercoagulability and thrombosis. Plasminogen activator inhibitor-1 (PAI-1) is a regulatory serine-protease inhibitor that decreases fibrinolysis56 and correlates well with visceral adiposity and hyperinsulinemia.57 PAI-1 knockout mice are protected against obesity and IR, likely secondary to maintenance of peroxisome proliferator-activated receptor-γ (PPAR-γ) and adiponectin expression.58 Activation of the rennin–angiotensin system (RAS) in adipocytes up-regulates PAI-1 expression through the angiotensin type I receptor,59 and hence blockade of the RAS may help to ameliorate PAI-1-related obesity and IR.
Molecular Mechanisms of Inflammatory Adipose Dysfunction and Insulin Resistance
The Insulin Signaling Pathway
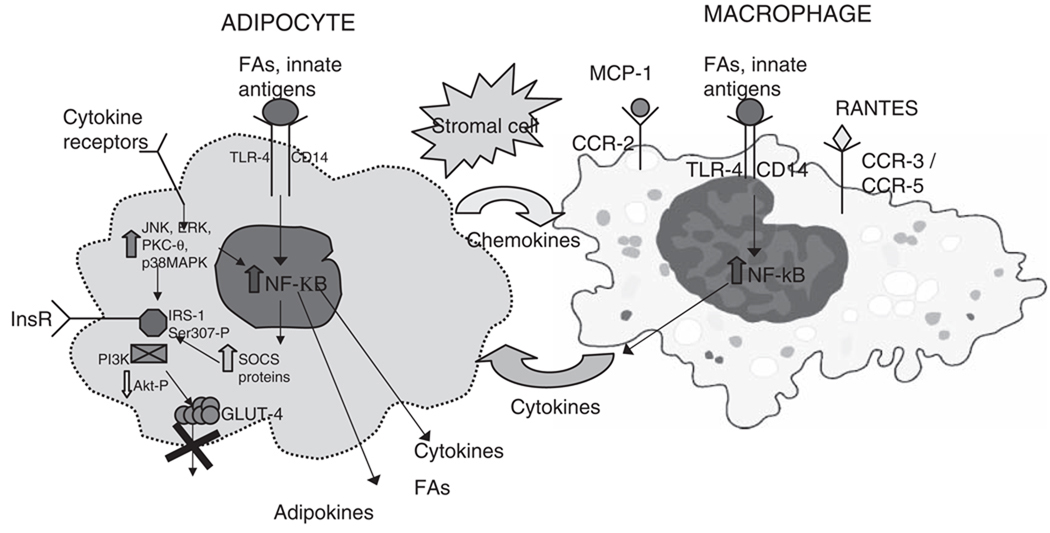
Inflammation and adiposity cause IR by interfering with insulin signaling. Insulin receptor substrates are central molecular targets for inflammatory effects. Tyrosine phosphorylation of IRSs is important for normal insulin signal transduction. Sustained endotoxemia in a rat model decreases tyrosine phosphorylation of IRSs and decreases activation of PI3 kinase.60 In mice, inflammatory serine 307 phosphorylation of IRS-1 interferes with insulin signaling61 (Figure 1).
Figure 1.
Macrophage–adipocyte interplay in adipose tissue. Adipocytes secrete chemokines, which attract macrophages to adipose tissue. Binding of fatty acids (FAs) and innate antigens to Toll-like receptors (TLRs) on macrophages and adipocytes activates NFκB, modulating cytokine and adipokine biosynthesis and secretion. Adipocytokines can then bind to receptors on adipocytes and, via kinases, activate NFκB to stimulate suppression of cytokine signaling (SOCS) protein transcription. Kinases, via direct phosphorylation, and SOCS proteins, via insulin receptor substrate (IRS) binding and degradation, interfere with insulin signaling and prevent GLUT-4 translocation, leading to insulin resistance. Adipose inflammation also retards adipocyte differentiation, promoting a pro-inflammatory, insulin-resistant preadipocyte phenotype. Inflammatory modulation of adipocytokine and FA production and secretion results in hepatic, skeletal muscle, and vascular insulin resistance; metabolic dyslipidemia; and a systemic pro-inflammatory, atherogenic state. Adipokines (ie, leptin, resistin, lipocalin-2, etc); cytokines (ie, tumor necrosis factor [TNF]-α, interleukin-6 [IL-6], interleukin-18 [IL-18], plasminogen activator inhibitor-1 [PAI-1], etc); chemokines (ie, monocyte chemotactic protein-1 [MCP-1]; regulated on activation, normal T cell expressed and secreted [RANTES]; fractalkine) and chemokine receptors (CCRs); kinases (ie, c-Jun N-terminal kinase [JNK], extracellular signal-regulated kinase [ERK], protein kinase C-θ [PKC-θ], p38 mitogen activated protein kinase [MAPK], phosphoinositide 3-kinase [PI3K], v-akt murine thymoma viral oncogene homolog 1 [AKT]). InsR, insulin receptors.
Kinases and Regulatory Phosphorylation
Pro-inflammatory cytokines induce phosphorylation of diverse kinases. NFκB drives transcription of inflammatory cytokines and contributes to IR in the setting of obesity and a high-fat diet.62 Normally, NFκB is inhibited by IκBα and therefore remains in the cytoplasm in an inactive state.62 With the appropriate stimuli, IκKβ (a serine kinase) is activated, which phosphorylates and degrades IκBα, and this frees up NFκB to enter the nucleus.62 IκKβ also interferes with insulin signaling by phosphorylating IRS-1.62 IκKβ overexpression increases NFκB activity and decreases insulin signaling, whereas IκKβ deficiency increases insulin sensitivity.62
The mitogen-activated protein kinase (MAPK) family comprises another set of kinases including JNK, p38 MAPK, and ERK. JNK isoforms couple inflammatory and metabolic signals, are activated through TNF-α signaling, and can phosphorylate serine 307 on IRS-1.63 Another MAP kinase family member, p38 MAPK, contributes to adipocyte IR by interfering with genes involved in insulin signaling, including GLUT-4 and phosphoinositide phosphatase.64 ERKs phosphorylate IRS-1 at a couple of serine residues.43 In obesity, JNK-1, p38 MAPK, and ERK2 levels and activation are induced in visceral adipose, suggesting depot-specific roles in IR.65
PKC-θ is another pro-inflammatory kinase implicated in IR. FFA metabolites activate PKC-θ, which increases serine 307 phosphorylation of IRS-1 and decreases insulin signaling.7,43 Human IRAK-1, homologous to mouse pelle-like kinase, is a serine/threonine kinase activated by cytokines that phosphorylates JNK, IκKβ, and NFκB and reduces insulin action.66
Negative Feedback Regulator—Suppressor of Cytokine Signaling Proteins
Suppressor of cytokine signaling (SOCS) family proteins are increased by inflammatory cytokines.67 SOCS proteins target cytokine tyrosine kinase receptor signaling in a negative feedback loop.67 However, SOCS proteins are elevated in insulin-resistant tissues and attenuate signaling via the insulin receptor (InsR), also a tyrosine kinase.67 SOCS proteins impair InsR signaling in 2 ways: by binding directly to IRSs, thus blocking InsR-mediated tyrosine phosphorylation,67 and by promoting ubiquitination and degradation of IRSs.68 Thus, SOCS proteins are key mediators of inflammatory IR and adipose dysfunction, although limited data are available in human disease.
Inflammation Modulates Transcription Factors That Regulate Adipocyte Differentiation
Transcription factors are crucial regulators of adipose differentiation and insulin sensitivity; they are the common final integration of many of the inflammatory pathways outlined above. PPAR-γ, the master regulator of adipogenesis, is regulated by serine phosphorylation and is attenuated in insulin-resistant states and by activation of inflammatory pathways.69,70 GATA2, another transcription factor regulated by serine phosphorylation, inhibits adipogenesis.71 Adipocyte IR prevents insulin-induced GATA2 phosphorylation, facilitating GATA2 inhibition of adipogenesis.71 FOXO1 is another transcription factor that also inhibits adipogenesis.72 Insulin negatively regulates FOXO1 activity via phosphorylation,73 whereas Sirt2 plays a role in inhibiting adipocyte differentiation by deacetylating and activating FOXO1.74 Recently, several additional transcription factor families that are regulated by inflammation, including bone morphogenic proteins75,76 and interferon regulatory factors,77 have been identified as important regulators of adipocyte differentiation and adipose functions. These data underscore the profound inflammatory modulation of transcription factor regulation of adipose differentiation and secretory/metabolic functions that affect IR and CVD.
Implications for Humans
Increased CVD risk is found in patients who have chronic, low-grade inflammation and who are also insulin resistant. The mechanisms of adipose inflammation and the related systemic insulin-resistant, atherogenic inflammatory state are complex. Visceral adiposity and its inflammatory dysregulation are strongly implicated in the genesis of this milieu.78 Perhaps anti-inflammatory therapeutic interventions will prove successful in reducing metabolic and vascular complications. In fact, lifestyle changes including exercise, weight loss, and reduction of visceral adiposity are associated with decreased inflammatory markers,79 improved endothelial function, and decreased CVD risk.80 Pharmacological approaches that target adipose inflammation warrant investigation but require specific proof of concept. PPAR-γ agonists, in fact, promote adipocyte differentiation and reduce adipose and systemic inflammation while improving insulin sensitivity.69 However, controversy surrounds their effects on atherosclerotic CVD.81 Modulation of angiotensin and its receptors, already established to reduce CVD,82 provides an alternative strategy for modulation of adipose inflammation and IR but requires evidence of antiinflammatory action in human adipose. High-dose salicylates, long used in inflammatory disorders, inhibit IKKβ83 and may provide significant proof that a targeted antiinflammatory strategy in humans reduces adipose inflammation and IR as well as atherosclerosis. In fact, such proof-of-concept trials are ongoing in clinical research programs sponsored by the National Heart, Lung, and Blood Institute (ClinicalTrials.gov Identifier: NCT00258128 and NCT00624923).
Conclusion
This article reviews recent data regarding mechanisms of adiposity-associated inflammation and IR, which play a pivotal role in the metabolic and cardiovascular consequences of obesity. The initial insult may occur through recruitment of macrophages and innate immune antigen activation of TLRs, which can be perpetuated through secretion of chemokines, retention of macrophages in adipose, and secretion of adipocytokines. This inflammatory milieu induces adipocyte inflammatory cascades, such as the NFκB pathway, via activation of various kinases, and this modulates adipocyte transcription factors, attenuates insulin signaling, and increases pro-inflammatory adipocytokines and FFAs. Inflammatory attenuation of adipocyte differentiation further exacerbates adipose dysfunction. These paracrine and endocrine adipose inflammatory events induce a systemic inflammatory, insulin-resistant, atherogenic state and metabolic dyslipidemia, resulting in type 2 diabetes and CVD. Application of therapeutic strategies that target both adipose inflammation and IR may be required to prevent and treat type 2 diabetes and atherosclerotic CVD in the emerging epidemic of obesity.
Acknowledgments
Financial disclosure: This research was partially funded by a Clinical and Translational Science Award (RFA-RM-06-002) from the National Center for Research Resources, National Institutes of Health (NIH; Bethesda, MD); a Diabetes Endocrinology Research Award (P30 DK-019525) from NIH to the University of Pennsylvania; R01 HL-073278, R01 DK-021224, and P50 HL-083799 from NIH (SCCOR); and a W. W. Smith Charitable Trust award (West Conshohocken, PA; grant H0204), all to MPR.
The 2008 Intersociety Research Workshop: Nutrition and Inflammation: Research Makes the Connection, was supported by grant number U13DK064190 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Footnotes
References
- 1.Goodpaster BH, Krishnaswami S, Harris TB, et al. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch Intern Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 2.Zeyda M, Farmer D, Todoric J, et al. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int J Obes (Lond) 2007;31:1420–1428. doi: 10.1038/sj.ijo.0803632. [DOI] [PubMed] [Google Scholar]
- 3.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341:507–514. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 4.Cani PD, Amar J, Iglesias MA, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 6.Delarue J, Magnan C. Free fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- 7.Chen H. Cellular inflammatory responses: novel insights for obesity and insulin resistance. Pharmacol Res. 2006;53:469–477. doi: 10.1016/j.phrs.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Cousin B, Munoz O, Andre M, et al. A role for preadipocytes as macrophage-like cells. FASEB J. 1999;13:305–312. doi: 10.1096/fasebj.13.2.305. [DOI] [PubMed] [Google Scholar]
- 9.Chung S, Lapoint K, Martinez K, Kennedy A, Boysen Sandberg M, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 10.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of Toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346:739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 11.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, et al. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 12.Kim F, Pham M, Luttrell I, et al. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100:1589–1596. doi: 10.1161/CIRCRESAHA.106.142851. [DOI] [PubMed] [Google Scholar]
- 13.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 14.Anderson PD, Mehta NN, Wolfe ML, et al. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab. 2007;92:2272–2279. doi: 10.1210/jc.2006-2545. [DOI] [PubMed] [Google Scholar]
- 15.Lumeng CN, Deyoung SM, Bodzin JL, Saltiel AR. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56:16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 16.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 17.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouloumie A, Curat CA, Sengenes C, Lolmede K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8:347–354. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 19.Sell H, Eckel J. Monocyte chemotactic protein-1 and its role in insulin resistance. Curr Opin Lipidol. 2007;18:258–262. doi: 10.1097/MOL.0b013e3281338546. [DOI] [PubMed] [Google Scholar]
- 20.Dahlman I, Kaaman M, Olsson T, et al. A unique role of monocyte chemoattractant protein 1 among chemokines in adipose tissue of obese subjects. J Clin Endocrinol Metab. 2005;90:5834–5840. doi: 10.1210/jc.2005-0369. [DOI] [PubMed] [Google Scholar]
- 21.Weisberg SP, Hunter D, Huber R, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matter CM, Handschin C. RANTES (regulated on activation, normal T cell expressed and secreted), inflammation, obesity, and the metabolic syndrome. Circulation. 2007;115:946–948. doi: 10.1161/CIRCULATIONAHA.106.685230. [DOI] [PubMed] [Google Scholar]
- 23.Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 24.Kitamoto S, Egashira K. Anti-monocyte chemoattractant protein-1 gene therapy for cardiovascular diseases. Expert Rev Cardiovasc Ther. 2003;1:393–400. doi: 10.1586/14779072.1.3.393. [DOI] [PubMed] [Google Scholar]
- 25.Veillard NR, Kwak B, Pelli G, et al. Antagonism of RANTES receptors reduces atherosclerotic plaque formation in mice. Circ Res. 2004;94:253–261. doi: 10.1161/01.RES.0000109793.17591.4E. [DOI] [PubMed] [Google Scholar]
- 26.Ludwig A, Weber C. Transmembrane chemokines: versatile “special agents” in vascular inflammation. Thromb Haemost. 2007;97:694–703. [PubMed] [Google Scholar]
- 27.Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- 28.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 29.Lago F, Dieguez C, Gomez-Reino J, Gualillo O. The emerging role of adipokines as mediators of inflammation and immune responses. Cytokine Growth Factor Rev. 2007;18:313–325. doi: 10.1016/j.cytogfr.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PLoS ONE. 2006;1:e31. doi: 10.1371/journal.pone.0000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silswal N, Singh AK, Aruna B, Mukhopadhyay S, Ghosh S, Ehtesham NZ. Human resistin stimulates the pro-inflammatory cytokines TNF-alpha and IL-12 in macrophages by NF-kappaB- dependent pathway. Biochem Biophys Res Commun. 2005;334:1092–1101. doi: 10.1016/j.bbrc.2005.06.202. [DOI] [PubMed] [Google Scholar]
- 32.Rajala MW, Scherer PE. Minireview: the adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 33.Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc Natl Acad Sci U S A. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137–3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 35.Yao-Borengasser A, Varma V, Bodles AM, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moschen AR, Kaser A, Enrich B, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 37.Fukuhara A, Matsuda M, Nishizawa M, et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 38.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–10432. [PubMed] [Google Scholar]
- 39.Yan QW, Yang Q, Mody N, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007;56:2533–2540. doi: 10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Lam KS, Kraegen EW, et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin Chem. 2007;53:34–41. doi: 10.1373/clinchem.2006.075614. [DOI] [PubMed] [Google Scholar]
- 41.Inoue T, Eguchi Y, Matsumoto T, et al. Lipocalin-type prostaglandin D synthase is a powerful biomarker for severity of stable coronary artery disease. Atherosclerosis. 2008 March 16; doi: 10.1016/j.atherosclerosis.2008.03.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 42.Weinberg JM. Lipotoxicity. Kidney Int. 2006;70:1560–1566. doi: 10.1038/sj.ki.5001834. [DOI] [PubMed] [Google Scholar]
- 43.Ye J. Role of insulin in the pathogenesis of free fatty acid-induced insulin resistance in skeletal muscle. Endocr Metab Immune Disord Drug Targets. 2007;7:65–74. doi: 10.2174/187153007780059423. [DOI] [PubMed] [Google Scholar]
- 44.Piro S, Spampinato D, Spadaro L, Oliveri CE, Purrello F, Rabuazzo AM. Direct apoptotic effects of free fatty acids on human endothelial cells. Nutr Metab Cardiovasc Dis. 2008;18:96–104. doi: 10.1016/j.numecd.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 45.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winkler G, Salamon F, Harmos G, et al. Elevated serum tumor necrosis factor-alpha concentrations and bioactivity in Type 2 diabetics and patients with android type obesity. Diabetes Res Clin Pract. 1998;42:169–174. doi: 10.1016/s0168-8227(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 47.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 48.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271:665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 49.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 50.Krogh-Madsen R, Plomgaard P, Moller K, Mittendorfer B, Pedersen BK. Influence of TNF-alpha and IL-6 infusions on insulin sensitivity and expression of IL-18 in humans. Am J Physiol Endocrinol Metab. 2006;291:E108–E114. doi: 10.1152/ajpendo.00471.2005. [DOI] [PubMed] [Google Scholar]
- 51.Plomgaard P, Bouzakri K, Krogh-Madsen R, Mittendorfer B, Zierath JR, Pedersen BK. Tumor necrosis factor-alpha induces skeletal muscle insulin resistance in healthy human subjects via inhibition of Akt substrate 160 phosphorylation. Diabetes. 2005;54:2939–2945. doi: 10.2337/diabetes.54.10.2939. [DOI] [PubMed] [Google Scholar]
- 52.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 53.Gracie JA, Robertson SE, McInnes IB. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 54.Tiret L, Godefroy T, Lubos E, et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112:643–650. doi: 10.1161/CIRCULATIONAHA.104.519702. [DOI] [PubMed] [Google Scholar]
- 55.Esposito K, Pontillo A, Di Palo C, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. JAMA. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 56.Fay WP. Plasminogen activator inhibitor 1, fibrin, and the vascular response to injury. Trends Cardiovasc Med. 2004;14:196–202. doi: 10.1016/j.tcm.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 57.De Taeye B, Smith LH, Vaughan DE. Plasminogen activator inhibitor-1: a common denominator in obesity, diabetes and cardiovascular disease. Curr Opin Pharmacol. 2005;5:149–154. doi: 10.1016/j.coph.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Saltiel AR. New perspectives into the molecular pathogenesis and treatment of type 2 diabetes. Cell. 2001;104:517–529. doi: 10.1016/s0092-8674(01)00239-2. [DOI] [PubMed] [Google Scholar]
- 59.Skurk T, Lee YM, Hauner H. Angiotensin II and its metabolites stimulate PAI-1 protein release from human adipocytes in primary culture. Hypertension. 2001;37:1336–1340. doi: 10.1161/01.hyp.37.5.1336. [DOI] [PubMed] [Google Scholar]
- 60.McCowen KC, Ling PR, Ciccarone A, et al. Sustained endotoxemia leads to marked down-regulation of early steps in the insulin- signaling cascade. Crit Care Med. 2001;29:839–846. doi: 10.1097/00003246-200104000-00032. [DOI] [PubMed] [Google Scholar]
- 61.Qiao LY, Zhande R, Jetton TL, Zhou G, Sun XJ. In vivo phosphorylation of insulin receptor substrate 1 at serine 789 by a novel serine kinase in insulin-resistant rodents. J Biol Chem. 2002;277:26530–26539. doi: 10.1074/jbc.M201494200. [DOI] [PubMed] [Google Scholar]
- 62.Shoelson SE, Lee J, Yuan M. Inflammation and the IKK beta/I kappa B/NF-kappa B axis in obesity- and diet-induced insulin resistance. Int J Obes Relat Metab Disord. 2003;27 suppl 3:S49–S52. doi: 10.1038/sj.ijo.0802501. [DOI] [PubMed] [Google Scholar]
- 63.Hirosumi J, Tuncman G, Chang L, et al. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 64.Carlson CJ, Koterski S, Sciotti RJ, Poccard GB, Rondinone CM. Enhanced basal activation of mitogen-activated protein kinases in adipocytes from type 2 diabetes: potential role of p38 in the down- regulation of GLUT4 expression. Diabetes. 2003;52:634–641. doi: 10.2337/diabetes.52.3.634. [DOI] [PubMed] [Google Scholar]
- 65.Bashan N, Dorfman K, Tarnovscki T, et al. Mitogen-activated protein kinases, inhibitory-kappaB kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology. 2007;148:2955–2962. doi: 10.1210/en.2006-1369. [DOI] [PubMed] [Google Scholar]
- 66.Kim JA, Yeh DC, Ver M, et al. Phosphorylation of Ser24 in the pleckstrin homology domain of insulin receptor substrate-1 by Mouse Pelle-like kinase/interleukin-1 receptor-associated kinase: cross-talk between inflammatory signaling and insulin signaling that may contribute to insulin resistance. J Biol Chem. 2005;280:23173–23183. doi: 10.1074/jbc.M501439200. [DOI] [PubMed] [Google Scholar]
- 67.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 69.Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 70.Suzawa M, Takada I, Yanagisawa J, et al. Cytokines suppress adi- pogenesis and PPAR-gamma function through the TAK1/TAB1/NIK cascade. Nat Cell Biol. 2003;5:224–230. doi: 10.1038/ncb942. [DOI] [PubMed] [Google Scholar]
- 71.Menghini R, Marchetti V, Cardellini M, et al. Phosphorylation of GATA2 by Akt increases adipose tissue differentiation and reduces adipose tissue-related inflammation: a novel pathway linking obesity to atherosclerosis. Circulation. 2005;111:1946–1953. doi: 10.1161/01.CIR.0000161814.02942.B2. [DOI] [PubMed] [Google Scholar]
- 72.Nakae J, Kitamura T, Kitamura Y, Biggs WH, III, Arden KC, Accili D. The forkhead transcription factor Fox01 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- 73.Zhang X, Gan L, Pan H, et al. Phosphorylation of serine 256 suppresses transactivation by FKHR (FOX01) by multiple mechanisms: direct and indirect effects on nuclear/cytoplasmic shuttling and DNA binding. J Biol Chem. 2002;277:45276–45284. doi: 10.1074/jbc.M208063200. [DOI] [PubMed] [Google Scholar]
- 74.Jing E, Gesta S, Kahn CR. SIRT2 regulates adipocyte differentiation through Fox01 acetylation/deacetylation. Cell Metab. 2007;6:105–114. doi: 10.1016/j.cmet.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tseng YH, He TC. Bone morphogenetic proteins and adipocyte differentiation. Cellsci Rev. 2007;3:342–360. [Google Scholar]
- 76.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 77.Eguchi J, Yan QW, Schones DE, et al. Interferon regulatory factors are transcriptional regulators of adipogenesis. Cell Metab. 2008;7:86–94. doi: 10.1016/j.cmet.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haffner SM. Abdominal adiposity and cardiometabolic risk: do we have all the answers? Am J Med. 2007;120:S10–S17. doi: 10.1016/j.amjmed.2007.06.006. discussion S16–S17. [DOI] [PubMed] [Google Scholar]
- 79.Troseid M, Lappegard KT, Claudi T, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25:349–355. doi: 10.1016/j.ehj.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 80.Ziccardi P, Nappo F, Giugliano G, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial function in obese women after weight loss over one year. Circulation. 2002;105:804–809. doi: 10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 81.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocar- dial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 82.Stojiljkovic L, Behnia R. Role of renin angiotensin system inhibitors in cardiovascular and renal protection: a lesson from clinical trials. Curr Pharm Des. 2007;13:1335–1345. doi: 10.2174/138161207780618768. [DOI] [PubMed] [Google Scholar]
- 83.Hundal RS, Petersen KF, Mayerson AB, et al. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–1326. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]