Abstract
Background
Niacin has multiple lipoprotein effects that may provide cardiovascular benefit when added to statin monotherapy.
Methods
In this randomized, placebo-controlled trial (n = 75) of magnetic resonance imaging of carotid atherosclerosis, we performed a secondary comparison of combination niacin-statin (simvastatin 20 mg/Niacin-ER 2G [S20/N]) to monotherapy with moderate (20 mg [S20]) and high-dose (80 mg [S80]) simvastatin on lipids, apolipoproteins (apo), low density lipoprotein (LDL) and high density lipoprotein (HDL) particle subclasses, and inflammatory markers.
Results
At baseline, average age was 71, 72% were male, 62.5% used statins, and average LDL-cholesterol was 111 mg/dL. At 12 months, S20/N, compared to S80, significantly reduced apoB (−36.6% vs −11.9%; P = .05) and lipoprotein(a) (−18% vs +3.5%; P = .001) and had at least an equivalent effect on LDL-cholesterol (−39.3% vs −24.3%; P = .24). The combination reduced the proportion of subjects with atherogenic LDL pattern-B (50% to 11.5%) compared to S80 (56% to 56%) (P = .01). Despite increases in plasma free fatty acids (+62.4%; F = 5.65, P = .005 vs S20 and S80), plasma triglycerides (−29.4%; F = 6.88, P = .002 vs S20 and S80), and very-low-density lipoprotein (−44.2%; F = 7.94, P < .001 vs S20 and S80), levels were reduced by S20/N. S20/N increased HDL-cholesterol levels (+18.1%) as compared to S20 (0%) and S80 (+5.9%) (P < .001 vs both statin arms), largely due to an increase in HDL particle size (+4.6%; P = .01 vs both statin arms).
Conclusions
We demonstrate that full-dose niacin/moderate-dose simvastatin combination has sustained benefits on atherogenic apoB lipoproteins, at least comparable to high-dose simvastatin, while also raising HDL-cholesterol. Results of large clinical trials will inform whether niacin-statin combinations reduce cardiovascular disease events.
Although statins lower low density lipoprotein-cholesterol (LDL-C) and reduce atherosclerotic cardiovascular disease (CVD),1,2 they do not meaningfully raise high density lipoprotein-cholesterol (HDL-C), a major determinant of CVD risk.1,3 The failure of the HDL-raising drug, torcetrapib, a cholesterol ester transport inhibitor, has highlighted the challenges in HDL therapeutics.4 In this context, and given the recent identification of a niacin receptor,5–7 there has been a renewed interest in niacin for treatment and prevention of CVD.
Niacin has multiple lipoprotein effects that may provide benefit beyond statins.8 Niacin raises HDL-C, lowers plasma triglycerides, and alters composition and size of apolipoprotein (apo) B lipoproteins and HDL particles. Niacin reduces small-dense LDL particles, which when compared to average-sized LDL particles are more atherogenic and predict CVD9 independent of LDL-C.10,11 Niacin also increases large HDL particles, which are considered more cardioprotective than smaller particles.12–14
Combinations of niacin and statins retard coronary atherosclerosis15 and slow carotid atherosclerosis progression,16,17 although benefit on CVD beyond statins has not been established. There are no long-term data comparing niacin/statin combinations to high-dose statins, the contemporary standard for treating patients at high risk for CVD.2 We compared the 12-month effects of a niacin-statin combination (Niaspan 2G/simvastatin 20 mg) to moderate-dose (20 mg) and high-dose (80 mg) simvastatin on plasma lipoproteins, LDL and HDL particle subclasses, and inflammatory markers. We chose a high dose of niacin to maximize LDL-C lowering18,19 while also raising HDL-C.
Methods
Participants
Patients with carotid atherosclerosis at the Hospital of the University of Pennsylvania and the Philadelphia VA Medical Center (both in Philadelphia, PA) were invited to participate between May 2002 and June 2005. Enrollment criteria included age 18 to 90 years, LDL-C >100 or LDL-C >80 mg/dL if HDL-C was also <40 mg/dL, blood pressure <170/90, negative pregnancy test (women), and carotid disease (>30% stenosis at ultrasonography). Exclusion criteria included recent (3 months) stroke, transient ischemic attack, myocardial infarction, unstable angina or critical limb ischemia; contraindications to magnetic resonance imaging; history of adverse events on statins or niacin; poorly controlled diabetes (HbA1c >8%); history of myositis or abnormal liver function tests; active infection or malignancy; or the need for combination lipid-lowering therapy. The protocol was approved by the Institutional Review Boards at Penn and Philadelphia VA Medical Center. All participants provided written, informed consent. Invitations were sent to >1,000 eligible patients; after telephone screening, 169 subjects underwent screening, and 87 participants were enrolled.
Trial design
This trial was designed to explore the effect of a niacin-statin combination on carotid atherosclerosis at magnetic resonance imaging (analysis ongoing). Changes in lipoprotein and inflammatory parameters, the focus of this article, were secondary outcomes. Based on published short-term studies,19,20 our trial sample had adequate power to detect moderate differences in HDL-C and apoB lipoprotein levels between treatments. Participants were randomized, in a double-masked, placebo-controlled manner, to 1 of 3 treatment arms: simvastatin 20 mg/placebo (S20), simvastatin 80 mg/placebo (S80), or simvastatin 20 mg/Niacin-ER 2G (S20/N). Simvastatin tablets were provided by Merck Pharmaceuticals (Whitehouse Station, NJ). Naicin-ER (Niaspan) tablets and matching placebo were provided by KOS pharmaceuticals. Clinical history, physical examination, medication use, and adverse effects were recorded at randomization and at 1, 3, 6, and 12 months. American Heart Association's Step 2 Dietary counseling was provided. Blood testing for hematology, chemistry, and lipid analyses was performed in stored plasma (−80C). All statins were discontinued at randomization and replaced by study simvastatin. Niaspan dosing (night-time) was titrated from 500 to 2,000 mg over months 1 to 3 and maintained for the remainder of the study. When 2,000 mg was not achieved, the highest tolerated dose was maintained and recorded. A priori, participants who did not complete at least 6 months of the study were excluded from efficacy analysis. Overall, 87 subjects were randomized, 75 returned at 6 months, and 70 completed the 12-month trial.
Measurement of lipoproteins, lipoprotein subclasses, and inflammatory markers
Fasting lipids were analyzed in Penn's Centers for Disease Control and Prevention-certified lipid laboratory. The LDL and very-low-density lipoprotein (VLDL)–cholesterol were separated by ultracentrifugation, and total cholesterol, LDL-C, HDL-C, and triglycerides (TGs) were measured enzymatically on a Hitachi autoanalyzer (Roche Diagnostics, Branford, CT) using Sigma reagents. ApoB, apoAI, lipoprotein(a), high-sensitivity C-reactive protein, and free fatty acids (FFAs) were measured using Wako reagents (Wako Chemicals USA Inc, Richmond, VA).21 Nuclear magnetic resonance (NMR) spectroscopy was performed at LipoScience, Inc (Raleigh, NC), as described.22,23 Large LDL were particles measuring 21.3 to 23.0 nm; medium LDL, 19.8 to 21.2 nm; small LDL, 18.3–21.2 nm; large HDL (HDL2), 8.8 to 13 nm; medium HDL, 8.2 to 8.8 nm; and small HDL, 7.3 to 8.2 nm. Pattern B LDL particles were defined as ≤20.5 nm. Nuclear magnetic resonance particle subclass estimation correlates with traditional approaches.9–11,24 Levels of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule (VCAM), E-selectin, matrix metalloproteinase-9 (MMP-9), and myeloperoxidase (MPO) were measured using multiplex ELISAs (Linco Research, Inc, St. Charles, MO) on a Luminex IS100 (Luminex, Austin, TX). The intra- and interassay coefficients of variation were ICAM-1, 5.5%, 12.4%; VCAM-1, 4.6%, 4.3%; E-selectin, 4.1%, 11.6%; MMP-9, 8.66%, 20.4%; and MPO, 8.7%, 10.9%, respectively.
Statistical analysis
Efficacy analyses included data from all 75 randomized participants who completed 6 months of treatment. Subjects with missing data at 12 months (n = 5) were excluded from 12 months analyses. Data are reported as mean ± SEM, or median and interquartile range (25th, 75th percentile) and as proportions for categorical variables. Continuous variables with nonnormal distributions were log-transformed for modeling. Differences between treatment groups were evaluated by analysis of variance (ANOVA); when significant differences were found, post hoc Scheffe-corrected t tests were used for pairwise comparisons. Secondary analysis adjusted for baseline LDL-C and statin use. For discreet variables, group differences were assessed using a χ2 test. Statistical analyses were performed using Stata 9.0 software (Stata Corp, College Station, TX). All values were for 2-tailed comparisons.
This work was supported by a Clinical and Translational Science Award (RFA-RM-06-002) and a General Clinical Research Center (M01 RR00040) from the NCRR/NIH to the University of Pennsylvania (Philadelphia, PA), by a Dana Brain Imaging and Immuno-imaging award from The Dana Foundation (New York, NY) (to M. P. R.), and by investigator-initiated grant support from Merck Research Laboratories (Whitehouse Station, NJ) and KOS Pharmaceuticals (now Abbot, Abbott Park, IL). M. P. R. is also supported by HL RO1-073278 and HL P50-083799.
Results
Baseline characteristics (Table I) were broadly similar across groups, but race/ethnicity (more African Americans in S20/N group) and apoB levels (higher in S20/N) did differ at baseline. Twelve subjects withdrew before 6 months because of flushing (n = 5), personal reasons (n = 5), and accidental trauma (n = 2). Adverse events in the remaining 75 subjects are summarized in Table II. Overall, 2 subjects in S80 and 1 in S20/N discontinued. At 12 months in the S20/N group, 17 participants were taking 2,000 mg of niacin daily; 1 participant, 1,500 mg; 3 participants, 1,000 mg; and 1 participant, 500 mg.
Table I.
Baseline characteristics*
| S20 (N = 25) | S80 (N = 24) | S20/N (N = 26) | |
|---|---|---|---|
| Age | 71 (66–76) | 72.5 (65–76.5) | 70.5 (60–80) |
| Male sex | 17 (68) | 19 (79) | 18 (69) |
| Race† | |||
| White | 21 (84) | 23 (96) | 16 (61) |
| African American | 4 (16) | 1 (4) | 9 (35) |
| Hispanic | 0 | 0 | 1 (4) |
| Body mass index (kg/m2) | 26.4 (25–30.7) | 28.2 (25.5–33.8) | 27 (23.8–31.7) |
| Hypertension | 18 (72) | 17 (70.8) | 18 (69.2) |
| Diabetes | 3 (12) | 3 (13) | 8 (30.8) |
| Statin use | 18 (75) | 15 (62.5) | 13 (50) |
| Blood glucose (mg/dL) | 85 (79–97) | 89 (81–102) | 88.5 (81–98) |
| Total cholesterol (mg/dL) | 178.5 (159.5–187.5) | 191 (163–215) | 199.5 (174–223) |
| TGs (mg/dL) | 127.5 (87–178) | 132 (108–159) | 117.5 (94–193) |
| LDL-C (mg/dL) | 102 (96.5–119.5) | 107 (89–133) | 123.5 (104–143) |
| ApoB (mg/dL)† | 82.5 (73–94.5) | 88 (76–99) | 97 (81–112) |
| Non-HDL cholesterol (mg/dL) | 133.5 (122–145.5) | 137 (119–158) | 151.5 (119–173) |
| HDL-C (mg/dL) | 42.5 (37–54) | 41 (34–49) | 45 (35–57) |
| ApoAI (mg/dL) | 118.5 (108–130.5) | 116 (106–137) | 128 (100–150) |
| ApoAII (mg/dL) | 30.5 (27–38.5) | 32 (28–34) | 32.5 (27–41) |
Values are reported as medians (interquartile range) for continuous variables or number (percentage) for categorical variable. All laboratory parameters were measured in fasting blood samples.
P < .05 for between-treatment group comparisons for these variables.
Table II.
Adverse events
| Event | S20 (n = 25) |
Action taken |
S80 (n = 24) |
Action taken | S20/N (n = 26) |
Action taken |
|---|---|---|---|---|---|---|
| Flushing, n | 0 | – | 2 | Decreased dose of study medications (n = 1) | 8 | Decreased dose of study medications (n = 2) |
| Muscle cramps, n | 2 | None (transient) | 2 | Discontinued study medications (n = 1) | 0 | – |
| Nausea or emesis, n | 1 | None (transient) | 0 | – | 2 | Discontinued study medications (n = 1) |
| Acute coronary syndrome, n | 0 | – | 1 | None | 0 | – |
| Elevated transaminases (<3× upper limit of normal), n | 1 | None (transient) | 1 | None (transient) | 0 | – |
Adverse events and actions taken in participants who completed ≥6 months (n = 75) by treatment groups.
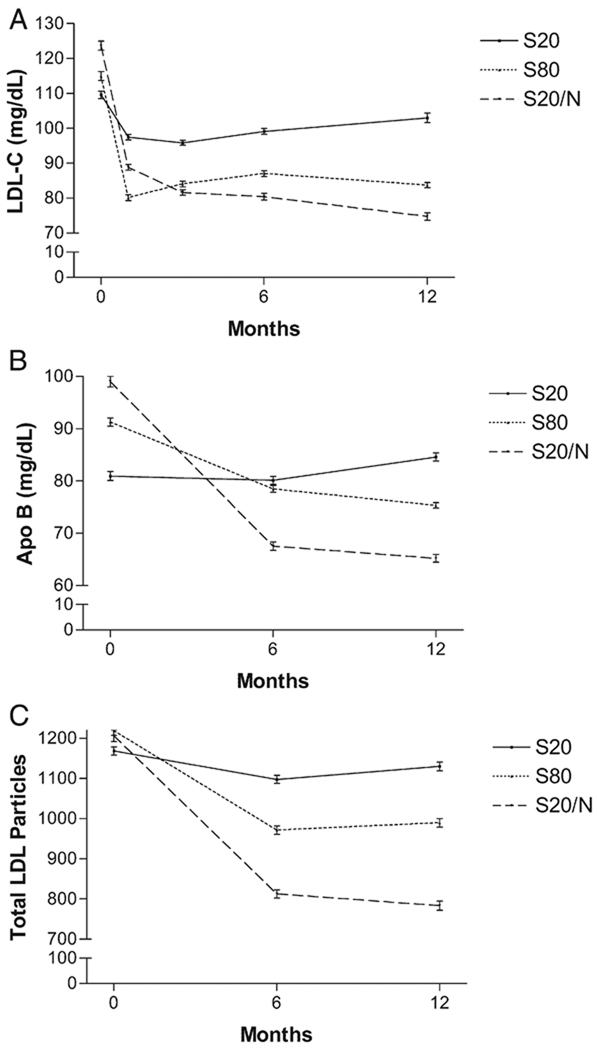
In the S20 group, there was no significant reduction in LDL-C or apoB (Table III, Figure 1) as expected (prerandomization statin use 75%). In contrast, both S80 and S20/N reduced levels of LDL-C (by 24.3% and 39.3%, respectively) and apoB (by 11.9% and 36.6%) at 12 months (Table III, Figure 1). There were trends toward greater effects on all apoB lipoproteins with S20/N compared to S80 (Table III). Notably, S20/N had a statistically greater effect than S80 on Lp(a) (P = .001) and total apo B (P = .05) (Figure 1). The effects of treatments on the number of LDL particles measured by NMR (at 12 months; −7.6%, −20.7%, and −37.9% for S20, S80, and S20/N, respectively) (Table III, Figure 1) were similar to effects on LDL-C. There was a significant reduction (ANOVA, F = 8.8, P < .001) in the number of LDL particles with both S80 (−27.8%) and S20/N (−47.4%); S20/N tended to have a greater effect than S80 (P = .05). S20/N reduced the proportion of subjects with atherogenic LDL pattern B (from 50% to 11.5%) compared to S20 (from 42% to 37.5%) or S80 (from 56% to 56%) (χ2 test, P = .01). The effects on apoB lipoproteins (P = .04) and LDL particles (P = .05) were similar after adjusting for prerandomization differences in LDL-C levels and statin use.
Table III.
Low-density lipoprotein and apoB-related data
| Lipoprotein | Pre* | 6 mo | 12 mo | F (P)† | Pairwise P | |
|---|---|---|---|---|---|---|
| LDL-C (mg/dL) | ||||||
| S20 | 102 | 101 (−1) | 99 (−2.5) | 9.53 (<.001) | S20 vs S80 | .04 |
| S80 | 107 | 89 (−16.8) | 81 (−24.3) | S20 vs S20/N | <.001 | |
| S20/N | 123 | 77 (−37.3) | 75 (−39.3) | S80 vs S20/N | .24 | |
| Triglycerides (mg/dL) | ||||||
| S20 | 127 | 126 (−1.2) | 116 (−9) | 6.88 (.002) | S20 vs S80 | .24 |
| S80 | 132 | 135 (2.3) | 114 (−13.3) | S20 vs S20/N | .002 | |
| S20/N | 117 | 83 (−29.4) | 83 (−29.4) | S80 vs S20/N | .14 | |
| Non-HDL cholesterol (mg/dL) | ||||||
| S20 | 133 | 122 (−8.6) | 133 (−0.4) | 13.17 (<.001) | S20 vs S80 | .005 |
| S80 | 137 | 110 (−19.7) | 103 (−24.8) | S20 vs S20/N | <.001 | |
| S20/N | 151 | 96 (−36.3) | 92 (−39.3) | S80 vs S20/N | .23 | |
| Lipoprotein (a) (mg/dL) | ||||||
| S20 | 19 | 23 (17.1) | 35 (83.9) | 10.31 (<.001) | S20 vs S80 | 1.0 |
| S80 | 29 | 27 (−6.9) | 30 (3.5) | S20 vs S20/N | .001 | |
| S20/N | 28 | 23 (−19.4) | 23 (−18) | S80 vs S20/N | .001 | |
| ApoB (mg/dL) | ||||||
| S20 | 82 | 80 (−2.4) | 84 (1.8) | 15.35 (<.001) | S20 vs S80 | .01 |
| S80 | 88 | 79(−10.2) | 77 (−11.9) | S20 vs S20/N | <.001 | |
| S20/N | 97 | 63 (−34.5) | 61 (−36.6) | S80 vs S20/N | .05 | |
| NMR LDL particle size (nm) | ||||||
| S20 | 20.85 | 20.6 (−1.2) | 20.7 (−0.7) | 3.41 (.04) | S20 vs S80 | .91 |
| S80 | 20.50 | 20.50 (0.0) | 20.35 (−0.7) | S20 vs S20/N | .06 | |
| S20/N | 20.50 | 21.00 (2.4) | 20.90 (2) | S80 vs S20/N | .13 | |
| NMR LDL particle number | ||||||
| S20 | 1203 | 1086 (−10) | 1112 (−8) | 10.40 (<.001) | S20 vs S80 | .09 |
| S80 | 1211 | 935 (−22.8) | 960 (−20.7) | S20 vs S20/N | <.001 | |
| S20/N | 1169 | 758 (−35.2) | 725 (−37.9) | S80 vs S20/N | .06 | |
Values are medians (percent change from baseline).
F statistic and global P value for ANOVA of differences between treatments in baseline to 12 month change in lipoprotein variables.
Figure 1.
Effects of S20, S80, and S20/N on LDL-C (A), apoB (B), and total number of LDL Particles (LDLp) (C). There were significant between-treatment effects at 12 months on LDL-C, apoB, and LDLp (ANOVA, P < .001 for all). S20/N had greater effects than S20 on LDL-C (P < .001), apoB (P < .001), and LDLp (P < .001) and greater effects than S80 on apoB (P = .05).
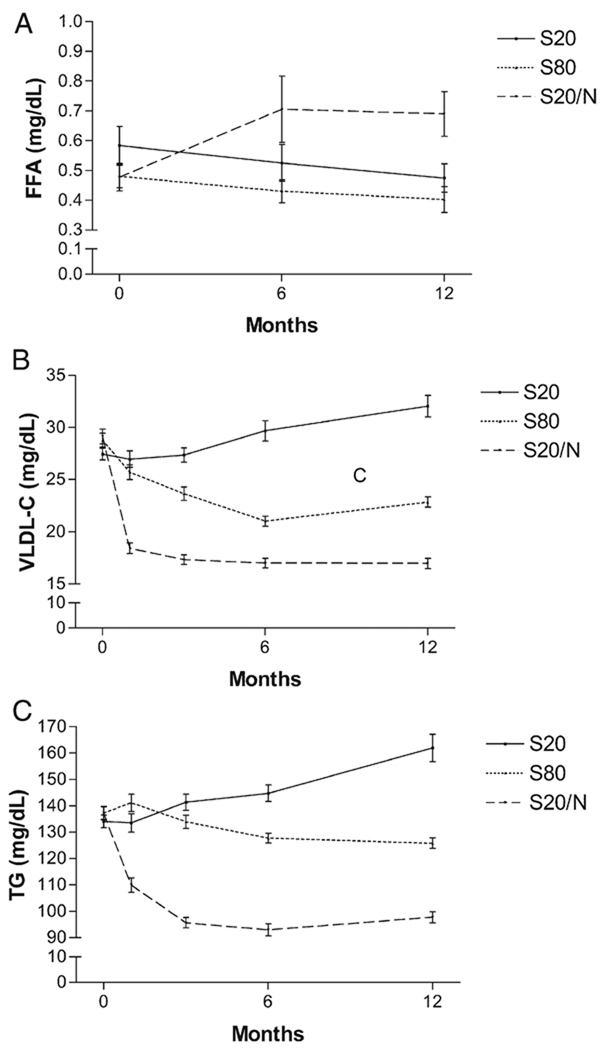
There were statistically significant increases in FFAs with S20/N at 6 and 12 months (P = .01 vs S20, P = .04 vs S80), whereas S20 and S80 had no effect (Figure 2). Despite increases in FFAs, plasma TG and VLDL fell on S20/N (−29.4% and −44.2%, respectively) (Figure 2, Table III).
Figure 2.
Effects of S20, S80, and S20/N on FFA (A), VLDL (B), and TG (C). Despite increases in FFA by S20/N (12 months, +62.4%, F = 5.65, P = .005 vs S20 and S80), TG (12 months, −29.4%, F = 6.88, P = .002 vs S20 and S80), and VLDL (12 months, −44.2%, F = 7.94, P < .001 vs S20 and S80), levels were reduced by S20/N.
At 12 months, S20/N (+18.1%) but not S20 (0%) or S80 (+5.9%) increased HDL-C significantly (P < .001 vs S20, P = .001 vs S80) (Table IV, Figure 2). S20/N had no effect on plasma apoAI (Figure 2) or apoAII levels (Table IV). Consistent with a selective increase in HDL-C, NMR analysis showed that S20/N (+4.6%), but not S20 or S80, increased HDL particle size (P = .01, S20/N vs S20 and S80) but had no effect on HDL particle number (Table IV). Concordantly, S20/N increased large HDL (82% at 12 months; P = .003 and .05 for S20/N vs S20 and S80, respectively). Of measured inflammatory markers, there was no difference in treatment effects at 6 or 12 months (Table V).
Table IV.
High-density lipoprotein–related data
| Lipoprotein | Pre* | 6 mo | 12 mo | F (P)† | Pairwise P | |
|---|---|---|---|---|---|---|
| HDL-C (mg/dL) | ||||||
| S20 | 42 | 46 (8) | 45 (6) | 12.12 (<.001) | S20 vs S80 | .76 |
| S80 | 41 | 42 (2.4) | 41 (0) | S20 vs S20/N | <.001 | |
| S20/N | 47 | 55 (17) | 56 (18) | S80 vs S20/N | .001 | |
| ApoAI (mg/dL) | ||||||
| S20 | 118 | 122 (3.4) | 125 (5.5) | 0.09 (.91) | ||
| S80 | 116 | 128 (10.3) | 116 (0.0) | |||
| S20/N | 128 | 129 (1.2) | 134 (5.1) | |||
| ApoAII (mg/dL) | ||||||
| S20 | 30 | 32 (4.9) | 33 (6.2) | 2.04 (.14) | ||
| S80 | 32 | 33 (3.1) | 29 (−7.8) | |||
| S20/N | 32 | 30 (−7.7) | 31 (−4.6) | |||
| Average HDL size (nm) | ||||||
| S20 | 8.9 | 8.8 (−1.1) | 8.8 (−1.1) | 6.27 (.003) | S20 vs S80 | 1.0 |
| S80 | 8.7 | 8.7 (0.0) | 8.7 (−0.6) | S20 vs S20/N | .01 | |
| S20/N | 8.8 | 9.2 (4.6) | 9.2 (4.6) | S80 vs S20/N | .01 | |
| HDL particles number (µmol/L) | ||||||
| S20 | 30.8 | 29.9 (−2.8) | 31.1 (1) | 0.02 (.99) | ||
| S80 | 28.9 | 29.5 (2.1) | 30.9 (6.8) | |||
| S20/N | 27.6 | 28.1 (1.8) | 28.2 (2.2) | |||
Values are medians (percent change from baseline).
F statistic and global P value for ANOVA of differences between treatments in baseline to 12 month change in lipoprotein variables.
Table V.
Inflammatory markers*
| Marker | Pre | 6 mo | 12 mo | F† |
|---|---|---|---|---|
| High sensitivity C-reactive proteins (mg/L) | ||||
| S20 | 1.9 | 2.0 (6.6) | 2.0 (7.7) | 1.07 |
| S80 | 1.9 | 1.3 (−33.5) | 1.1 (−38.8) | |
| S20/N | 1.6 | 2.0 (27.4) | 1.3 (−16.4) | |
| ICAM (ng/mL) | ||||
| S20 | 0.167 | 0.181 (8.37) | 0.175 (4.66) | 0.99 |
| S80 | 0.152 | 0.180 (18.47) | 0.179 (17.32) | |
| S20/N | 0.166 | 0.161 (−2.80) | 0.187 (12.93) | |
| VCAM (ng/mL) | ||||
| S20 | 1.32 | 1.35 (2.64) | 1.31 (−0.70) | 0.17 |
| S80 | 1.36 | 1.41 (3.71) | 1.36 (−0.57) | |
| S20/N | 1.39 | 1.43 (3.29) | 1.45 (5.01) | |
| MMP-9 (ng/mL) | ||||
| S20 | 40.26 | 30.57 (−24.06) | 37.14 (−7.77) | 0.130 |
| S80 | 45.68 | 39.80 (−12.88) | 44.74 (−2.06) | |
| S20/N | 33.60 | 33.52 (−0.24) | 31.52 (−6.20) | |
| MPO (ng/mL) | ||||
| S20 | 5.84 | 6.50 (11.32) | 5.57 (−4.68) | 1.39 |
| S80 | 13.89 | 4.80 (−65.46) | 5.92 (−57.38) | |
| S20/N | 7.17 | 8.38 (16.92) | 6.29 (−12.28) | |
| E-selectin (pg/mL) | ||||
| S20 | 35.06 | 34.18 (−2.51) | 39.32 (12.15) | 2.13 |
| S80 | 34.43 | 35.78 (3.92) | 34.67 (0.70) | |
| S20/N | 31.85 | 33.53 (5.27) | 33.68 (5.75) | |
ICAM, Intercellular adhesion molecule; VCAM, vascular cell adhesion molecule; MMP-9, matrix metalloproteinase-9; MPO, myeloperoxidase.
Values are medians (percent change from baseline).
F statistic for ANOVA of differences between treatments in baseline to 12-month change in lipoprotein variables. P values are not shown because no ANOVA global P values reached statistical significance.
Discussion
Combination high-dose Niaspan (2G daily) with moderate-dose simvastatin (20 mg) lowered apoB lipoproteins to a greater extent than moderate-dose (20 mg) and even high-dose (80 mg) simvastatin monotherapy, while also raising HDL-C. The prevalence of the LDL pattern B profile was reduced by almost 80% by S20/N, whereas high-dose simvastatin had no effect on this atherogenic phenotype. The S20/N combination increased HDL-C levels, but not apoAI levels or HDL particle number, consistent with a shift toward larger atheroprotective HDL-2 particles.15,16 These findings support the concept that niacin/statin combinations, when targeted to maximize LDL-C lowering as well as increase HDL-C, might have equivalent or greater CVD benefit than high dose statin alone.
Relative to statin monotherapy, short-term niacin studies (3–6 months) demonstrate favorable effects on Lp (a), TG, and LDL-C as well as raising HDL-C, although LDL-C lowering required higher doses of niacin.19,20,25,26 Small studies of lipoprotein subclasses revealed reductions in small-dense LDL with niacin15,27–30 even beyond statins.29 In the 3-year HDL-Atherosclerosis Treatment Study (HATS) trial (n = 160), Brown et al15 demonstrated regression of coronary plaques and sustained effects on apoB lipoproteins and HDL-C with a niacin (2.4G daily)-statin compared to placebo; however, a statin monotherpay arm was not included, confounding interpretation. Taylor et al16,17 reported that adding niacin (1G daily) to statins retarded carotid atherosclerosis coincident with increased HDL-C, lower TGs, but without change in LDL-C.
Ours is the first trial to compare a niacin 2G/moderate-dose statin combination to both moderate- and high-dose statins, the contemporary standard for treating patients at high risk of CVD.1,2 This combination provided at least a comparable degree LDL-C lowering as high-dose statins as well as sustained benefits on apoB lipoproteins, Lp(a), lipoprotein particles, and HDL-C. Small trials suggest greater effects of niacin/statin combinations, compared to statin alone, on subclinical atherosclerosis.16,17,31 Whether such effects translate into reduced clinical events awaits the results of phase III trials (eg, NCT00120289 and NCT00461630).
In the Investigation of Lipid Level Management to Understand its Impact on Atherosclerotic Events (ILLUMINATE) trial,4 a combination of atorvastatin and torcetrapib compared to atorvastatin alone, increased CVD events despite marked increases in HDL-C. Although increased CVD may have been related to off-target effects of torcetrapib,32 torcetrapib did not reduce carotid or coronary atherosclerosis progression in imaging trials.33–35 These results have questioned the efficacy of cholesterol ester transport inhibition in humans and, more generally, provide a cautionary note in targeting HDL-C elevation for CVD.36 In this context, there is renewed interest in niacin particularly because of promising imaging studies16,17,31 and its proven CVD benefit as a monotherapy.37
Despite identification of a G-protein–coupled receptor for niacin,5,7 the specific mechanism(s) of HDL-C raising and apoB lipoprotein lowering remains uncertain. Suppression of adipose FFA release is one mechanism proposed to reduce hepatic FFA flux and thus lower TG and VLDL production.5 We found, however, that chronic extended-release niacin increased plasma FFA levels. This may be due to a diurnal rebound in which high midmorning FFA levels follow an overnight suppression with niacin. It has been suggested that, unlike short acting niacin, extended release niacin may cause a limited rebound in adipocyte FFA release.38 Our data, however, suggest at least a modest rebound in FFA. Vega et al39 also demonstrated small increases in FFA with long-acting niacin. Despite increased FFAs, we observed reductions in both plasma TG and VLDL, suggesting that modulation of adipocyte FFA flux is unlikely to account for niacin effects on apoB lipoproteins. Adipose-independent effects on hepatic lipoproteins appear likely.40
Despite elevation in HDL-C (~18%) with S20/N, we failed to detect a significant increase in apoAI levels (~+5%) or HDL particle number (~+2%). Our findings differ from several previous studies that demonstrated significant 10% to 16% increases in apoAI with niacin/statin combinations.20,41,42 This might relate to our older study sample, which carries a substantial burden of comorbidities and atherosclerosis. It is also important to note that HDL particles are heterogeneous and vary in size, maturity, and apoAI composition,43,44 which may contribute to differences in apoAI and HDL-C responses to niacin. An important limitation of our study is the difference between treatment groups in some baseline parameters, including statin use and LDL-C. Although these differences were not statistically significant, the subsequent changes in apoB-containing lipoproteins may have been affected. Our results, however, were consistent across additional analysis that adjusted for differences in prerandomization statin use and LDL-C.
In conclusion, our study is the first to report incremental and sustained benefit of a full-dose niacin/moderate-dose statin combination on atherogenic apoB lipoproteins that is at least comparable to high-dose statins, while also raising HDL-C. Ongoing atherosclerosis imaging studies and outcome trials will reveal whether and how much this strategy improves atherosclerotic CVD. The mechanism of niacin lowering of apoB lipoproteins is uncertain but appears unlikely to be mediated by suppression of adipose FFA flux to liver.
Footnotes
NIH clinical trial number: NCT00307307.
Disclosures
The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the article and its final contents.
References
- 1.Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 3.Gordon DJ, Probstfield JL, Garrison RJ, et al. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation. 1989;79:8–15. doi: 10.1161/01.cir.79.1.8. [DOI] [PubMed] [Google Scholar]
- 4.Barter PJ, Caulfield M, Eriksson M, et al. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007 doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 5.Tunaru S, Kero J, Schaub A, et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat Med. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- 6.Tunaru S, Lattig J, Kero J, et al. Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G) Mol Pharmacol. 2005;68:1271–1280. doi: 10.1124/mol.105.015750. [DOI] [PubMed] [Google Scholar]
- 7.Wise A, Foord SM, Fraser NJ, et al. Molecular identification of high and low affinity receptors for nicotinic acid. J Biol Chem. 2003;278:9869–9874. doi: 10.1074/jbc.M210695200. [DOI] [PubMed] [Google Scholar]
- 8.Dunbar RL, Rader DJ. Current drug options for raising HDL cholesterol. Curr Treat Options Cardiovasc Med. 2005;7:15–23. doi: 10.1007/s11936-005-0002-6. [DOI] [PubMed] [Google Scholar]
- 9.Kuller L, Arnold A, Tracy R, et al. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2002;22:1175–1180. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 10.El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49:547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 11.Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113:1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 12.Griffin BA, Skinner ER, Maughan RJ. Plasma high density lipoprotein subfractions in subjects with different coronary risk indices as assessed by plasma lipoprotein concentrations. Atherosclerosis. 1988;70:165–169. doi: 10.1016/0021-9150(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 13.Cheung MC, Brown BG, Wolf AC, et al. Altered particle size distribution of apolipoprotein A-I-containing lipoproteins in subjects with coronary artery disease. J Lipid Res. 1991;32:383–394. [PubMed] [Google Scholar]
- 14.Asztalos BF, Collins D, Cupples LA, et al. Value of high-density lipoprotein (HDL) subpopulations in predicting recurrent cardiovascular events in the Veterans Affairs HDL Intervention Trial. Arterioscler Thromb Vasc Biol. 2005;25:2185–2191. doi: 10.1161/01.ATV.0000183727.90611.4f. [DOI] [PubMed] [Google Scholar]
- 15.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 16.Taylor AJ, Sullenberger LE, Lee HJ, et al. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation. 2004;110:3512–3517. doi: 10.1161/01.CIR.0000148955.19792.8D. [DOI] [PubMed] [Google Scholar]
- 17.Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin. 2006;22:2243–2250. doi: 10.1185/030079906x148508. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe ML, Vartanian SF, Ross JL, et al. Safety and effectiveness of Niaspan when added sequentially to a statin for treatment of dyslipidemia. Am J Cardiol. 2001;87:476–479. doi: 10.1016/s0002-9149(00)01410-7. A7. [DOI] [PubMed] [Google Scholar]
- 19.Hunninghake DB, McGovern ME, Koren M, et al. A dose-ranging study of a new, once-daily, dual-component drug product containing niacin extended-release and lovastatin. Clin Cardiol. 2003;26:112–118. doi: 10.1002/clc.4960260304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bays HE, Dujovne CA, McGovern ME, et al. Comparison of oncedaily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]) Am J Cardiol. 2003;91:667–672. doi: 10.1016/s0002-9149(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 21.Reilly MP, Wolfe ML, Localio AR, et al. C-reactive protein and coronary artery calcification. The Study of Inherited Risk of Coronary Atherosclerosis (SIRCA) Arterioscler Thromb Vasc Biol. 2003 doi: 10.1161/01.ATV.0000092327.60858.4A. [DOI] [PubMed] [Google Scholar]
- 22.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Szapary PO, Bloedon LT, Samaha FF, et al. Effects of pioglitazone on lipoproteins, inflammatory markers, and adipokines in nondiabetic patients with metabolic syndrome. Arterioscler Thromb Vasc Biol. 2006;26:182–188. doi: 10.1161/01.ATV.0000195790.24531.4f. [DOI] [PubMed] [Google Scholar]
- 24.Gardner CD, Fortmann SP, Krauss RM. Association of small low-density lipoprotein particles with the incidence of coronary artery disease in men and women. JAMA. 1996;276:875–881. [PubMed] [Google Scholar]
- 25.Jacobson TA, Chin MM, Fromell GJ, et al. Fluvastatin with and without niacin for hypercholesterolemia. Am J Cardiol. 1994;74:149–154. doi: 10.1016/0002-9149(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 26.Guyton JR, Capuzzi DM. Treatment of hyperlipidemia with combined niacin-statin regimens. Am J Cardiol. 1998;82:82U–84U. doi: 10.1016/s0002-9149(98)00955-2. [DOI] [PubMed] [Google Scholar]
- 27.McKenney JM, McCormick LS, Schaefer EJ, et al. Effect of niacin and atorvastatin on lipoprotein subclasses in patients with atherogenic dyslipidemia. Am J Cardiol. 2001;88:270–274. doi: 10.1016/s0002-9149(01)01639-3. [DOI] [PubMed] [Google Scholar]
- 28.Morgan JM, Capuzzi DM, Baksh RI, et al. Effects of extended-release niacin on lipoprotein subclass distribution. Am J Cardiol. 2003;91:1432–1436. doi: 10.1016/s0002-9149(03)00394-1. [DOI] [PubMed] [Google Scholar]
- 29.Bays HE, McGovern ME. Once-daily niacin extended release/lovastatin combination tablet has more favorable effects on lipoprotein particle size and subclass distribution than atorvastatin and simvastatin. Prev Cardiol. 2003;6:179–188. doi: 10.1111/j.1520-037x.2003.03142.x. [DOI] [PubMed] [Google Scholar]
- 30.Morgan JM, Carey CM, Lincoff A, et al. The effects of niacin on lipoprotein subclass distribution. Prev Cardiol. 2004;7:182–187. doi: 10.1111/j.1520-037x.2004.3129.x. [quiz 188] [DOI] [PubMed] [Google Scholar]
- 31.Zhao XQ, Yuan C, Hatsukami TS, et al. Effects of prolonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI: a case-control study. Arterioscler Thromb Vasc Biol. 2001;21:1623–1629. doi: 10.1161/hq1001.098463. [DOI] [PubMed] [Google Scholar]
- 32.Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27:257–260. doi: 10.1161/01.ATV.0000256728.60226.77. [DOI] [PubMed] [Google Scholar]
- 33.Nissen SE, Tardif JC, Nicholls SJ, et al. Effect of torcetrapib on the progression of coronary atherosclerosis. N Engl J Med. 2007;356:1304–1316. doi: 10.1056/NEJMoa070635. [DOI] [PubMed] [Google Scholar]
- 34.Kastelein JJ, van Leuven SI, Burgess L, et al. Effect of torcetrapib on carotid atherosclerosis in familial hypercholesterolemia. N Engl J Med. 2007;356:1620–1630. doi: 10.1056/NEJMoa071359. [DOI] [PubMed] [Google Scholar]
- 35.Bots ML, Visseren FL, Evans GW, et al. Torcetrapib and carotid intima-media thickness in mixed dyslipidaemia (RADIANCE 2 study): a randomised, double-blind trial. Lancet. 2007;370:153–160. doi: 10.1016/S0140-6736(07)61088-5. [DOI] [PubMed] [Google Scholar]
- 36.Cuchel M, Rader DJ. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation. 2006;113:2548–2555. doi: 10.1161/CIRCULATIONAHA.104.475715. [DOI] [PubMed] [Google Scholar]
- 37.Berge KG, Canner PL. Coronary drug project: experience with niacin. Coronary Drug Project Research Group. Eur J Clin Pharmacol. 1991;40 Suppl 1:S49–S51. [PubMed] [Google Scholar]
- 38.Carlson LA, Havel RJ, Ekelund LG, et al. Effect of nicotinic acid on the turnover rate and oxidation of the free fatty acids of plasma in man during exercise. Metabolism. 1963;12:837–845. [PubMed] [Google Scholar]
- 39.Vega GL, Cater NB, Meguro S, et al. Influence of extended-release nicotinic acid on nonesterified fatty acid flux in the metabolic syndrome with atherogenic dyslipidemia. Am J Cardiol. 2005;95:1309–1313. doi: 10.1016/j.amjcard.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 40.Ganji SH, Tavintharan S, Zhu D, et al. Niacin noncompetitively inhibits DGAT2 but not DGAT1 activity in HepG2 cells. J Lipid Res. 2004;45:1835–1845. doi: 10.1194/jlr.M300403-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Capuzzi DM, Morgan JM, Weiss RJ, et al. Beneficial effects of rosuvastatin alone and in combination with extended-release niacin in patients with a combined hyperlipidemia and low high-density lipoprotein cholesterol levels. Am J Cardiol. 2003;91:1304–1310. doi: 10.1016/s0002-9149(03)00318-7. [DOI] [PubMed] [Google Scholar]
- 42.Lamon-Fava S, Diffenderfer MR, Barrett PH, et al. Extended-release niacin alters the metabolism of plasma apolipoprotein (Apo) A–I and ApoB-containing lipoproteins. Arterioscler Thromb Vasc Biol. 2008;28:1672–1678. doi: 10.1161/ATVBAHA.108.164541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barter PJ, Rye KA. Relationship between the concentration and antiatherogenic activity of high-density lipoproteins. Curr Opin Lipidol. 2006;17:399–403. doi: 10.1097/01.mol.0000236365.40969.af. [DOI] [PubMed] [Google Scholar]
- 44.Asztalos BF, Schaefer EJ. High-density lipoprotein subpopulations in pathologic conditions. Am J Cardiol. 2003;91:12E–17E. doi: 10.1016/s0002-9149(02)03383-0. [DOI] [PubMed] [Google Scholar]