Abstract
Infections of avian pathogenic Escherichia coli (APEC) result in annual multimillion-dollar losses to the poultry industry. Despite this, little is known about the mechanisms by which APEC survives and grows in the bloodstream. Thus, the aim of this study was to identify molecular mechanisms enabling APEC to survive and grow in this critical host environment. To do so, we compared the transcriptome of APEC O1 during growth in Luria-Bertani broth and chicken serum. Several categories of genes, predicted to contribute to adaptation and growth in the avian host, were identified. These included several known virulence genes and genes involved in adaptive metabolism, protein transport, biosynthesis pathways, stress resistance, and virulence regulation. Several genes with unknown function, which were localized to pathogenicity islands or APEC O1's large virulence plasmid, pAPEC-O1-ColBM, were also identified, suggesting that they too contribute to survival in serum. The significantly upregulated genes dnaK, dnaJ, phoP, and ybtA were subsequently subjected to mutational analysis to confirm their role in conferring a competitive advantage during infection. This genome-wide analysis provides novel insight into processes that are important to the pathogenesis of APEC O1.
INTRODUCTION
Avian colibacillosis is one of the most significant infectious diseases in the poultry industry, resulting in annual multimillion-dollar losses due to mortality, lost production, and condemnations (5). Avian pathogenic Escherichia coli (APEC) infections take many forms, with one of the more common being septicemia (5). In order to cause septicemia, APEC must evade a multitude of host defense mechanisms in the bloodstream, including the bactericidal effects of phagocytosis, serum complement, and antimicrobial peptides (38). Indeed, serum resistance has been shown to be an important virulence trait of APEC (37). Previous studies have shown that many bacterial traits contribute to E. coli's serum resistance, including production of a capsule, a smooth lipopolysaccharide (LPS) layer, and certain outer membrane proteins (OMPs) (37, 55), including OmpA, TraT, and Iss (11, 40, 42, 59). OmpA is a major protein in E. coli's outer membrane, TraT is encoded by conjugative F plasmids, and Iss is homologous to the Bor protein of bacteriophage λ, which is typically encoded by large virulence plasmids in APEC (29). Such OMPs may contribute to APEC's serum resistance by restricting C3 deposition on the bacterial surface (43), blockage of the membrane attack complex (31), or some other as yet unknown mechanism.
However, merely resisting the bactericidal effects of serum is not sufficient to allow APEC to proliferate in serum; APEC must also adjust its metabolism to suit the nutrients available in serum. For example, the concentration of free iron in animal serum is extremely low because iron is sequestered by host compounds like transferrin. Since iron is necessary for a series of bacterial functions, APEC has developed several strategies to acquire iron in such iron-limiting environments. At least five iron uptake and assimilation systems have been found in APEC, and several have had their roles in APEC pathogenesis confirmed in animal models of disease (27, 36, 48). However, the uniqueness of serum as a niche for APEC growth likely extends far beyond iron limitation. In an effort to increase our understanding of APEC's ability to cause septicemia, we performed a genome-wide transcriptional analysis of APEC O1 during its growth in chicken serum.
MATERIALS AND METHODS
Bacterial strains, plasmids, oligonucleotide primers, and growth conditions.
APEC O1, a sequenced O1:K1:H7 reference strain that shares strong similarities with human extraintestinal pathogenic E. coli (ExPEC) genomes (27), was used in these studies. All other E. coli strains, plasmids, and oligonucleotide primers used in this study are listed in Table 1. Bacterial cultures were grown at 37°C in Luria-Bertani (LB) broth or chicken serum (Sigma). When necessary, antibiotics were added at the following concentrations: ampicillin (Ap), 50 μg ml−1; kanamycin (Kn), 50 μg ml−1; and chloramphenicol (Cm), 25 μg ml−1. For growth kinetics, overnight cultures of E. coli strains were diluted 1:100 in LB medium and incubated at 37°C with shaking at 160 rpm until an optical density (OD) at 600 nm (OD600) of 1.0 (Eppendorf biophotometer) was attained. These cultures were again diluted 1:100 into fresh LB or chicken serum to give parallel cultures representing the test conditions and controls and then incubated at 37°C until the stationary phase was reached. Growth was monitored by measuring the OD600 and confirmed by obtaining viable counts.
Table 1.
Bacterial strains, plasmids, and primers used in this study
| Strain, plasmid, or primer target | Description or sequencea | Reference |
|---|---|---|
| Strains | ||
| APEC O1 | O1:K1:H7; fyuA sitA chuA irp2 iroN ireA tsh iucD fimC iss ompA vat traT; contains four plasmids, including pAPEC-O1-ColBM | 27 |
| JW1116-1 | E. coli BW25141 derivative, ΔphoP790::kan | 4 |
| JW0013-4 | E. coli BW25141 derivative, ΔdnaK734::kan | 4 |
| JW0014-1 | E. coli BW25141 derivative, ΔdnaJ735::kan | 4 |
| APEC O1 MdnaK | APEC O1 derivative, ΔdnaK | This study |
| APEC O1 MdnaJ | APEC O1 derivative, ΔdnaJ | This study |
| APEC O1 MphoP | APEC O1 derivative, ΔphoP | This study |
| APEC O1 MybtA | APEC O1 derivative, ΔybtA | This study |
| Plasmids | ||
| pKD46 | Apr; expresses λ red recombinase | 14 |
| pKD3 | cat gene, template plasmid | 14 |
| Targets of primers for gene mutagenesis | ||
| phoP | This study | |
| Forward | CCAGTCAGGCTGGATCATCT | |
| Reverse | GAAAGAGCTGACTCGCGGTA | |
| dnaK | This study | |
| Forward | CTTGGCTGCGATTCATTCTT | |
| Reverse | AATATCGCCGAAAACGTCAC | |
| dnaJ | This study | |
| Forward | GGTCTGAACGAAGATGAAATCC | |
| Reverse | TAGAGAAAAGCCCCGAGTGATA | |
| ybtA | This study | |
| Forward | ATACCCGCATTGGTCTAAGCCACAGGGAGATAACCAGGTCATGGTGTAGGCTGGAGCTGCTTCGA | |
| Reverse | GGCCTCTGTCAGGGAGGAGTTTAGGGGGGCGCGACCCCGGCATATGAATATCCTCCTTAG | |
| Targets of primers for real-time PCR | ||
| chuA | 34 | |
| Forward | CAGATAACAAAAGGAGCAAGGC | |
| Reverse | CTGACGATAATACTCACCGCC | |
| sitA | 34 | |
| Forward | TGTCGCCAGATAATGCTCTG | |
| Reverse | TAAGTATCGGCATTGCGTTG | |
| ybtA | This study | |
| Forward | CCGATTCGAGAGCATTACCC | |
| Reverse | CAACAGCAGTGGGAGTCGAT | |
| fyuA | This study | |
| Forward | CAAACTCCCCAGAGTCTTGC | |
| Reverse | CATCGACATACAGGGTGACG | |
| APECO1_2945 | This study | |
| Forward | AGCCATGTCTCGTTACAGGAAT | |
| Reverse | GGAGAGGGCAGATTTTTCACTA | |
| APECO1_2950 | This study | |
| Forward | CAGGGATACGATGTTTTTCACC | |
| Reverse | TGCAACATTACGTCGATCTCTT | |
| dnaK | This study | |
| Forward | GAAGCTAACGCCGAAGCTGA | |
| Reverse | TCAACCTGCTTACGGGTGCT | |
| yfaE | This study | |
| Forward | CACAATGTGGCGGTTGAGTA | |
| Reverse | AGCTAACGGTTCGGCAATC | |
| glpA | This study | |
| Forward | GGATCACCGCATTAATCAGC | |
| Reverse | AAAGAGGTGGTGCCAATCAG | |
| lldR | This study | |
| Forward | GGACGTTCGTTTTCATCTGG | |
| Reverse | TGCTTCACAGAGGATTGCAG | |
| glpF | This study | |
| Forward | AGGATCAGCCCCATCAGAAT | |
| Reverse | GTTGATCTGGCTGGCACTTT | |
| tus | 50 | |
| Forward | CGATAACCTTTCGCAAGCAGCGTT | |
| Reverse | GGCAAATGACGATGCACCCATTCA |
The underlined portions of the primer sequences are identical to the flanking regions of the target genes.
RNA isolation, cDNA synthesis, labeling, microarray, and hybridization.
RNA from APEC O1, cultured in both LB and chicken serum, was extracted using an RNeasy minikit (Qiagen) with a 1-h on-column DNase digestion (Qiagen) according to the manufacturer's instructions. Four biological replicates were used for each culture condition. The concentration of RNA was determined using a spectrophotometer (ND-1000; NanoDrop). Five micrograms of total RNA was used for labeling, and cDNA was synthesized and labeled with Fairplay III microarray labeling kit (Agilent Technologies-Stratagene Products) according to the recommendations of the manufacturer and labeled with the Cy3 or Cy5 monoreactive dye pack (GE Healthcare). Labeled cDNA was purified and quantified according to the manufacturer's instructions. The 8 × 15,000 (15K) DNA high-density microarray of APEC O1 was designed by Oxford Gene Technology (Oxford OX5 1PF, United Kingdom) and validated by the University of Birmingham E. coli Centre (UBEC) (United Kingdom). During validation, three 60-mer probes per predicted gene were designed for the 5,042 open reading frames (ORFs) in the chromosome and 4 plasmids of APEC O1. For each of the designed probes, a mismatch probe (containing 3 mismatches per 60-mer probe at positions 10, 25, and 40) was also generated. These mismatch probes and the perfect-match probes were placed on an array (4 × 44k) in triplicate. This array was hybridized with genomic DNA and a pool of mRNA representing conditions in which as many genes as practicable would be induced (derived from an equimolar pool of total RNA from E. coli grown in morpholinepropanesulfonic acid (MOPS) minimal medium at 30°C mid-log phase, 37°C for mid-log phase, and 37°C for stationary phase). The results were processed to select the best-performing probe for each gene. This derived and optimized probe set of 5,063 probes was printed in triplicate by Agilent Technologies on the 8 × 15K array used in this study. The probes are also arranged randomly by design. For each of four biological replicates, equal quantities of Cy5- and Cy3-labeled cDNA from APEC O1, cultured in chicken serum or LB, were added to hybridization solution, and hybridization was performed using the Gene Expression hybridization kit (Agilent Technologies).
Data and statistical analysis.
Slides were scanned with a GenePix 4000B scanner (Axon Instruments), and the fluorescence intensities were collected with ImaGene software (BioDiscovery, El Segundo, CA). Median signal intensities for each spot were background corrected and log transformed. The LOWESS procedure was used to correct the intensity-dependent dye bias for each 2-color array (15). Linear models (51) were then fitted to the normalized data for each gene. The moderated t test was applied using the R software package program limma to detect the genes differentially expressed between APEC O1 grown in chicken serum and that grown in LB (51). The set of P values was converted to q values for false discovery rate control using the R package program q value developed by Storey et al. (54). Along with q values, estimates of fold change were computed.
Real-time qPCR.
Real-time quantitative reverse transcription-PCR (RT-PCR) (qPCR) was used to validate the expression levels for selected genes. Total RNA from APEC O1, cultured in LB and serum, was extracted as described above. Treatment of total RNA with DNase was performed, followed by subjection to purification using the RNeasy kit. RNA samples without reverse transcription served as PCR templates to confirm that they were free of contaminating DNA. One microgram of total RNA was reverse transcribed in triplicate using random hexamers and Moloney murine leukemia virus (MMLV) reverse transcriptase (Promega). Primer pairs used are listed in Table 1, and real-time PCR was performed as previously described (34). Melting-curve analyses were performed after each reaction to ensure amplification specificity. Differences (n-fold) in transcripts were calculated using the relative comparison method, and amplification efficacies of each primer set were verified as described by Schmittgen et al. (48a). RNA levels were normalized using the housekeeping gene tus as a control (50).
Mutant generation and in vitro and in vivo virulence assays.
Deletion of selected genes was achieved using the method of Datsenko and Wanner (14). The Kn resistance cassette, flanked by 5′ and 3′ sequences of the phoP, dnaK, or dnaJ gene, was amplified from genomic DNA of the mutant strains of E. coli BW25141 (Table 1) (4), while the Cm resistance cassette, flanked by 5′ and 3′ sequences of ybtA, was amplified from plasmid pKD3 (14). The primers used for mutagenesis are listed in Table 1. The Kn or Cm cassette was introduced into APEC O1 by homologous recombination using λ Red recombinase. For both in vitro and in vivo competition assays, cultures of the mutant and wild-type strains were mixed at a ratio of 1:1. For in vitro competition, the bacteria were incubated in LB broth for 4 h at 37°C and then plated on medium with or without antibiotics (Kn or Cm). For in vivo competition, 10 1-day-old chickens were infected with approximately 107 CFU of the mixed culture by the intratracheal route. At 24 h postinfection, birds were euthanatized by CO2 inhalation, lungs were collected, weighed, and homogenized, and dilutions of the homogenates were plated on medium with or without antibiotics for selection of mutants or total bacteria, respectively. A competitive index (CI) was calculated for each mutant by dividing the output ratio (mutant/wild type) by the input ratio (mutant/wild type). Values greater than 1 indicated that the mutant outcompeted the wild type, whereas values less than 1 indicated that the wild type outcompeted the mutant.
Microarray data accession number.
Microarray data are available at the National Center for Biotechnology Information Gene Expression Omnibus database (http://www.ncbi.nlm.nih.gov/geo) under accession number GSE25478.
RESULTS
APEC O1 rapidly adapts to chicken serum.
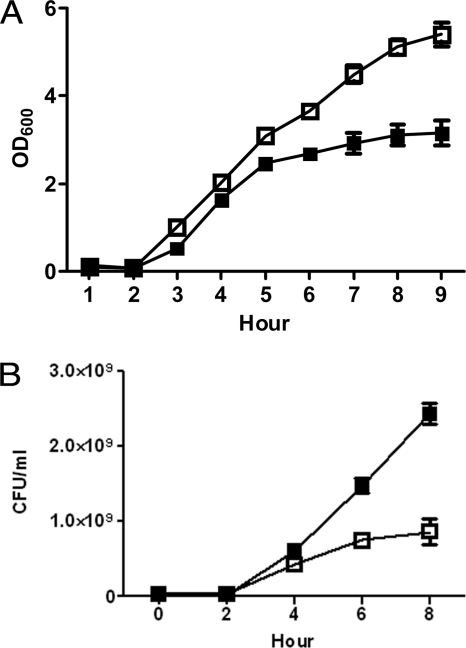
In an effort to understand the mechanisms that enable APEC to survive in the bloodstream, APEC O1 was grown in 100% chicken serum. Initial experiments were performed to assess the growth of APEC O1 in chicken serum and LB by measuring the optical density (OD600). At the beginning, the OD values of APEC O1 grown in serum were only slightly lower than those of APEC O1 grown in LB. However, after 5 h of incubation, the OD values differed to some extent, reaching a maximum level of difference (OD in LB, 5.39; OD in serum, 3.13) after 8 h of incubation (Fig. 1 a). The growth of APEC O1 in serum and that in LB were also compared using viable counts (Fig. 1b). After 2 h of incubation, APEC O1 grown in serum lagged behind APEC grown in LB (CFU in LB, 4.1 × 107/ml; CFU in serum, 2.2 × 107/ml). Four hours after inoculation, viable counts of APEC in serum were higher than those in LB (CFU in LB, 4.3 × 108/ml; CFU in serum, 6.2 × 108/ml), with differences of nearly 2-fold reached at 6 and 8 h of incubation, although their OD values were lower. These results suggested that APEC O1 rapidly adapts to serum.
Fig. 1.
Growth of APEC O1 in LB and chicken serum. Optical density (OD600) (A) or CFU counts (B) were used to assess the growth of APEC O1 in culture medium LB (□) and chicken serum (▪). APEC O1 grew faster in serum than in LB, suggesting that the APEC O1 can rapidly adapt to chicken serum.
Transcriptome alteration of APEC O1.
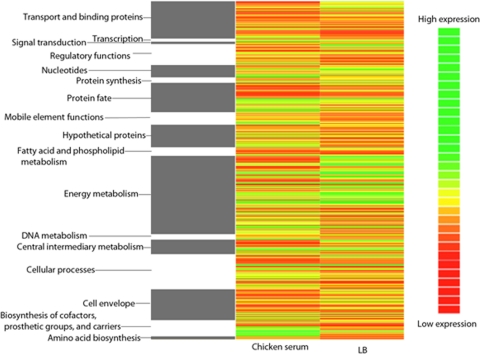
To further understand the mechanisms by which APEC adapts to serum, we used a DNA microarray to compare the transcriptome of APEC O1 following growth in LB and chicken serum. Care was used in handling the serum so as not to inactivate its complement components to ensure that our analysis would identify genes which enable complement resistance, a trait that shows strong correlation with APEC virulence (61). The expression level of each ORF in the APEC O1 genome was determined in serum relative to that for growth in LB. Genes with at least a 1.5-fold change and a q value of <0.10 (the false discovery rate was controlled at 10%) were considered significantly differentially expressed. Overall, 311 genes were found to be significantly upregulated, while 299 genes were significantly downregulated. The heat map in Fig. 2 presents an overview of the differentially regulated genes within each functional category in the serum and LB. Growth in serum caused upregulation of most genes involved in biosynthesis of cofactors, prosthetic groups, and carriers while also leading to downregulation of genes involved in energy metabolism (Fig. 2). Thirty-nine of the upregulated genes were localized to genomic islands of APEC O1 that are not present in the E. coli K-12 genome (Table 2) (27). In addition, many genes contained within three plasmids of APEC O1, one of which is considered to be cryptic (17), were significantly upregulated under these same conditions (Table 3). Fifty-six genes found within APEC O1's large virulence plasmid, pAPEC-O1-ColBM, were significantly upregulated in chicken serum (Table 3), suggesting that this virulence plasmid is important in APEC O1's adaptation to chicken serum.
Fig. 2.
Heat map visualizing regulated genes in APEC O1 grown in chicken serum compared to growth in LB. Genes found to be significantly regulated are indicated by either green (upregulation) or red (downregulation). Significantly differentially expressed genes of APEC O1 during growth in serum compared to that in LB are listed in columns 2 and 3. The genes are sorted by functional categories (column 1) according to annotation by the J. Craig Venter Institute.
Table 2.
Significantly upregulated genes of APEC O1 localized in genomic islands that are not present in E. coli K-12
| Gene locus | Gene name | Function of product | Genomic island no.a | Fold change in expression | P value | q value |
|---|---|---|---|---|---|---|
| APECO1_274 | sitA | ABC Mn2+/Fe2+ transporter | 9 | 10.8375 | 3.49E-07 | 4.40E-05 |
| APECO1_273 | sitB | ABC Mn2+/Fe2+ transporter | 9 | 8.816332 | 6.27E-08 | 2.99E-05 |
| APECO1_272 | sitC | ABC Mn2+/Fe2+ transporter | 9 | 8.502317 | 9.67E-07 | 6.60E-05 |
| APECO1_271 | sitD | ABC Mn2+/Fe2+ transporter | 9 | 7.292451 | 1.90E-06 | 0.000101 |
| APECO1_246 | Hypothetical protein | 9 | 3.891812 | 2.06E-05 | 0.000508 | |
| APECO1_275 | Hypothetical protein | 10 | 1.671198 | 0.000918 | 0.006928 | |
| APECO1_277 | ycgE | Transcriptional regulator | 10 | 1.606007 | 0.005808 | 0.021801 |
| APECO1_391 | Hypothetical protein | 11 | 1.702988 | 0.000305 | 0.003246 | |
| APECO1_1020 | gp7 | Hypothetical protein | 15 | 1.61494 | 0.011325 | 0.034143 |
| APECO1_1036 | Hypothetical protein | 15 | 1.546323 | 0.005074 | 0.019965 | |
| APECO1_1057 | Putative AraC type regulator | 16 | 11.80515 | 1.25E-06 | 7.55E-05 | |
| APECO1_1063 | fyuA | Yersiniabactin receptor protein | 16 | 2.80218 | 5.62E-06 | 0.000215 |
| APECO1_1067 | Transporter | 16 | 2.306758 | 1.58E-05 | 0.000429 | |
| APECO1_1092 | Hypothetical protein | 16 | 2.086762 | 4.67E-05 | 0.000899 | |
| APECO1_1058 | irp2 | Yersiniabactin biosynthetic protein | 16 | 1.997221 | 0.000811 | 0.006389 |
| APECO1_1059 | irp1 | Yersiniabactin biosynthetic protein | 16 | 1.959211 | 9.01E-05 | 0.001376 |
| APECO1_1052 | Salicylate synthase Irp9 | 16 | 1.663941 | 0.000526 | 0.004743 | |
| APECO1_1105 | yeeX | COG2926S;hypothetical protein | 16 | 1.659641 | 0.003874 | 0.016885 |
| APECO1_39122 | Hypothetical protein | 22 | 2.031662 | 0.000108 | 0.001554 | |
| APECO1_3897 | Hypothetical protein | 22 | 1.72608 | 0.00025 | 0.002821 | |
| APECO1_3497 | ireA | Iron-regulated element | 25 | 3.5312 | 1.35E-05 | 0.000379 |
| APECO1_3530 | tia | Tia invasion determinant | 25 | 1.734973 | 0.000748 | 0.006046 |
| APECO1_3385 | fepC | Enterobactin transport | 28 | 3.664637 | 2.16E-05 | 0.000518 |
| APECO1_3389 | Putative phosphosugar isomerases | 28 | 2.734085 | 6.79E-06 | 0.000246 | |
| APECO1_3386 | Protein of ABC transporter family | 28 | 2.188546 | 0.000239 | 0.002726 | |
| APECO1_3388 | Protein of ABC transporter family | 28 | 2.076194 | 6.10E-05 | 0.00105 | |
| APECO1_2947 | chuT | Outer membrane binding protein | 32 | 10.76088 | 2.51E-07 | 4.36E-05 |
| APECO1_2948 | chuA | Heme/hemoglobin receptor | 32 | 10.12316 | 7.87E-08 | 3.05E-05 |
| APECO1_2946 | chuW | n III oxidase | 32 | 9.730668 | 2.17E-06 | 0.000107 |
| APECO1_2945 | Hypothetical protein | 32 | 8.480435 | 4.11E-07 | 4.40E-05 | |
| APECO1_2944 | Hypothetical protein | 32 | 7.263724 | 3.14E-07 | 4.40E-05 | |
| APECO1_2949 | chuS | Heme/hemoglobin transport | 32 | 5.863794 | 2.89E-07 | 4.36E-05 |
| APECO1_2950 | yhiF | Transcriptional regulator | 32 | 3.361944 | 1.28E-05 | 0.000372 |
| APECO1_2306 | Insertion element | 40 | 4.556443 | 5.59E-07 | 5.07E-05 | |
| APECO1_2257 | insB | COG1662L;IS1 InsB protein | 40 | 1.896451 | 0.000155 | 0.001939 |
| APECO1_2262 | insA | COG3677L;IS1 InsA protein | 40 | 1.803386 | 0.000129 | 0.001727 |
| APECO1_2256 | insA | COG3677L;IS1 InsA protein | 40 | 1.752979 | 0.000752 | 0.006046 |
| APECO1_2279 | Putative hemolysin activator protein | 40 | 1.606824 | 0.014772 | 0.040738 | |
| APECO1_2024 | Hypothetical protein | 43 | 1.636221 | 0.002654 | 0.013566 |
Genomic island no. according to Johnson et al., 2007 (27).
Table 3.
Significantly upregulated genes from plasmids of APEC O1 cultured in chicken serum compared to results with LB
| Gene/locus name | Function or description of product | Fold change in expression | P value | q value |
|---|---|---|---|---|
| pAPEC-O1-ColBM_143 | Putative integrase | 36.16894 | 6.60E-08 | 2.99E-05 |
| pAPEC-O1-ColBM_142 | Hypothetical protein | 23.16964 | 5.49E-08 | 2.99E-05 |
| pAPEC-O1-ColBM_138 | Salmochelin system component | 14.35178 | 8.22E-07 | 5.93E-05 |
| pAPEC-O1-ColBM_135 | Hypothetical protein | 14.04616 | 9.74E-07 | 6.60E-05 |
| insA | Hypothetical protein | 11.40321 | 2.23E-07 | 4.36E-05 |
| iroB | Salmochelin system component | 9.474809 | 1.56E-06 | 8.50E-05 |
| sitA | ABC Mn2+/Fe2+ transporter | 8.833535 | 2.68E-07 | 4.36E-05 |
| iucC | Aerobactin siderophore system | 8.818279 | 4.08E-07 | 4.40E-05 |
| sitC | ABC Mn2+/Fe2+ transporter | 8.212306 | 3.61E-07 | 4.40E-05 |
| sitB | ABC Mn2+/Fe2+ transporter | 7.501994 | 9.74E-08 | 3.31E-05 |
| pAPEC-O1-ColBM_136 | Hypothetical protein | 7.247297 | 2.00E-06 | 0.000103 |
| crcB | Hypothetical protein | 6.613707 | 4.21E-07 | 4.40E-05 |
| iucB | Aerobactin siderophore system | 6.417662 | 6.95E-07 | 5.72E-05 |
| iucA | Aerobactin siderophore system | 6.05921 | 1.13E-06 | 7.29E-05 |
| iroC | Salmochelin system component | 5.455652 | 3.12E-06 | 0.000143 |
| iucD | Aerobactin siderophore system | 5.194098 | 1.44E-06 | 8.12E-05 |
| pAPEC-O1-ColBM_126 | IS2 transposase subunit | 5.168253 | 5.12E-07 | 4.97E-05 |
| pAPEC-O1-ColBM_149 | S2 transposase | 5.075606 | 5.02E-07 | 4.97E-05 |
| shiF | Putative transport protein | 5.048586 | 1.10E-05 | 0.000347 |
| pAPEC-O1-ColBM_94 | Hypothetical protein | 4.373617 | 9.96E-07 | 6.60E-05 |
| pAPEC-O1-ColBM_150 | Hypothetical protein | 4.110728 | 7.65E-06 | 0.000263 |
| pAPEC-O1-ColBM_125 | Transposase for IS2 | 4.094815 | 4.45E-06 | 0.000189 |
| iroD | Salmochelin system component | 3.924875 | 8.79E-06 | 0.000295 |
| iroN | Salmochelin system component | 3.179762 | 0.000814 | 0.006392 |
| pAPEC-O1-ColBM_132 | Hypothetical protein | 3.123875 | 2.44E-05 | 0.000565 |
| pAPEC-O1-ColBM_95 | IS2 transposase | 2.866879 | 0.000107 | 0.001554 |
| cvaA | Hypothetical protein | 2.553727 | 1.11E-05 | 0.000347 |
| pAPEC-O1-ColBM_1 | IS1 InsA protein | 2.526474 | 8.48E-06 | 0.000288 |
| eitC | Putative iron transport system | 2.42768 | 2.12E-05 | 0.000517 |
| pAPEC-O1-ColBM_74 | Transposase for IS1 | 2.024255 | 0.000388 | 0.003795 |
| sitD | ABC iron transport system | 1.882365 | 0.00037 | 0.003676 |
| pAPEC-O1-ColBM_133 | Hypothetical protein | 1.870467 | 0.001961 | 0.011098 |
| cvaB | Assembly verified for accuracy | 1.860303 | 0.004612 | 0.018642 |
| eitB | Putative iron transport system | 1.8456 | 0.000255 | 0.002854 |
| relE | Cytotoxic translational repressor | 1.805647 | 0.002744 | 0.01381 |
| pAPEC-O1-ColBM_69 | Hypothetical protein | 1.799338 | 0.003073 | 0.014697 |
| etsA | Putative transport system | 1.779548 | 0.000281 | 0.003057 |
| eitD | Putative iron transport system | 1.772026 | 0.000131 | 0.00173 |
| insB | Hypothetical protein | 1.767588 | 0.000153 | 0.001919 |
| insL | Transposase | 1.757236 | 0.001065 | 0.007573 |
| sopA | Plasmid partitioning protein | 1.745449 | 0.000263 | 0.002926 |
| traY | Conjugal transfer protein | 1.729107 | 0.00036 | 0.003622 |
| pAPEC-O1-ColBM_167 | Hypothetical protein | 1.727454 | 0.000894 | 0.006819 |
| eitA | Putative iron transport system | 1.718027 | 0.003175 | 0.015078 |
| iutA | Aerobactin siderophore system | 1.671313 | 0.001793 | 0.010636 |
| insB | Hypothetical protein | 1.66898 | 0.000938 | 0.006979 |
| pAPEC-O1-ColBM_160 | Hypothetical protein | 1.666459 | 0.000377 | 0.003727 |
| etsB | Putative transport system | 1.659028 | 0.000583 | 0.005023 |
| relE | Cytotoxin translational repressor | 1.647127 | 0.009351 | 0.029787 |
| pAPEC-O1-ColBM_185 | Hypothetical protein | 1.639247 | 0.005399 | 0.020854 |
| eno | Putative enolase | 1.608608 | 0.001787 | 0.010636 |
| etsC | Putative transport system | 1.599998 | 0.001159 | 0.00799 |
| pAPEC-O1-ColBM_187 | Hypothetical protein | 1.585905 | 0.00394 | 0.017012 |
| pAPEC-O1-ColBM_175 | Hypothetical protein | 1.582197 | 0.000443 | 0.00416 |
| traQ | Pilin chaperone protein | 1.53625 | 0.016134 | 0.043008 |
| pAPEC-O1-ColBM_4 | Hypothetical protein | 1.500467 | 0.000909 | 0.00692 |
| pAPEC-O1-R_91 | Hypothetical protein | 1.742547 | 0.004732 | 0.01896 |
| pAPEC-O1-Cryp2_19 | Hypothetical protein | 1.649976 | 0.000361 | 0.000362 |
| pAPEC-O1-R_194 | Hypothetical protein | 1.526988 | 0.001155 | 0.033743 |
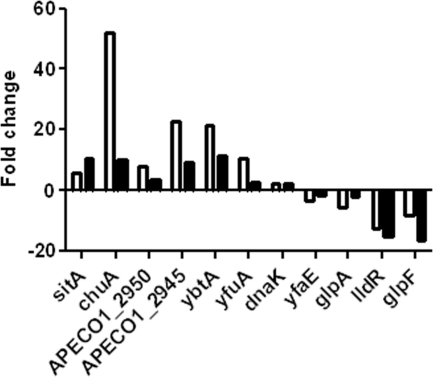
To confirm the results from the microarray experiments, real-time quantitative reverse transcription-PCR was performed on the 11 genes listed in Table 1, in which the expression of 7 of these genes was upregulated and that of the other 4 was downregulated, as evidenced by microarray results. The qPCR results were consistent with the results obtained from the microarray experiments (Fig. 3). However, for most of these genes, the qPCR results showed fold changes greater than those seen with the corresponding microarray results. Therefore, results of the qPCR analysis provided evidence that the transcriptional data obtained from microarray experiments were reliable, indicating that the rejection level applied to the microarray data was adequate.
Fig. 3.
Comparison of gene regulation analyzed by microarray (filled) or real-time quantitative RT-PCR (open). Real-time quantitative RT-PCR was used to validate the expression level for 11 selected genes, including 6 upregulated genes and 4 downregulated genes revealed by microarray analysis.
Virulence traits.
Several traits are known to contribute to APEC virulence. The expression of several of these “known” loci was affected by exposure to chicken serum. We found that the genes involved in iron acquisition were among the genes most significantly upregulated. Among the top 20 most significantly upregulated genes, 13 were involved in iron uptake. The yersiniabactin system (46) was the most significantly upregulated iron acquisition system in APEC O1, with the ybtA gene upregulated more than 11-fold in serum compared to results in LB. Other significantly upregulated systems included the genes of the aerobactin (56), enterobactin (60), and sit operons (63), plus the chu genes, which are required for hemin utilization (25). Also, the expression of some genes involved in ferrisiderophore complexes, such as fhuA and fep, was also significantly upregulated.
Stress response to serum.
In addition to the failure to detect upregulation of “known” complement resistance mechanisms, one of the most interesting findings in this study was that several categories of genes involved in stress resistance were upregulated during incubation in serum. The genes uspG and uspA, which contribute to universal stress resistance (32), were significantly upregulated when APEC O1 was grown in serum. Exposure to serum also resulted in upregulation of such acid resistance genes as hdeA, hdeB, gadA, gadB, and gadX (19), although the pH value of the serum used in this study was 8.20.
Several genes associated with oxidative stress in APEC O1 were upregulated during serum growth. sodA, which encodes an E. coli superoxide dismutase, was upregulated more than 1.5-fold after exposure to serum (44). Also, the gene katE, a well-known gene that participates in the antioxidant defense mechanism against H2O2-induced stress in E. coli (44), was significantly upregulated in serum. In addition to genes participating in H2O2 resistance, msrA and dps were identified as being significantly upregulated. They are predicted to protect cells and DNA from oxidative damage (49).
Also, several genes contributing to heat shock protection were significantly upregulated, though APEC O1 was cultured in serum at 37°C. The genes of the rpoH regulon, including dnaK, dnaJ, hchA, ibpA, clpB, and htpG (20), were upregulated when APEC O1 was cultured in serum at 37°C. To determine if heat shock genes contribute to APEC virulence, we deleted dnaK and dnaJ from APEC O1 and performed in vitro and in vivo competition assays using the mutants and the wild type. Since rpoH is an essential gene for E. coli, it was not possible to test its contribution to virulence by deletion analysis. The wild type significantly outcompeted the two heat shock mutants for growth in day-old chickens (Table 4), despite the fact that the mutants grew only slightly slower than the wild type in vitro at 37°C, confirming that the heat shock genes of the rpoH regulon are involved in APEC virulence.
Table 4.
In vitro and in vivo competition index of selected mutants
| Gene | Function of product | Fold change in expression | P value | q value | CIa,b |
|
|---|---|---|---|---|---|---|
| In vitro | In vivo | |||||
| dnaK | Chaperone protein | 7.17442 | 0.001626 | 0.033028 | 0.88 (0.23–0.46) | 0.43 (0.08–0.11) |
| dnaJ | Chaperone protein | 4.08406 | 0.001597 | 0.032868 | 0.91 (0.19–0.33) | 0.50 (0.13–0.25) |
| phoP | Response regulator in TCSc | 3.690768 | 0.009174 | 0.065915 | 0.76 (0.29–0.46) | 0.23 (0–0.35) |
| ybtA | Regulator | 7.828241 | 1.06E-05 | 0.00089 | 1.03 (0.24–0.68) | 0.35 (0–0.28) |
CI, competitive index. Values > 1 indicate that the mutant outcompeted the wild type; values < 1 indicate that the wild type outcompeted the mutant.
Values are the mean CI values obtained from 5 to 10 chicks each, along with upper and lower confidence limits. Values in bold are significant from 1 (P < 0.05).
TCS, two-component signal transduction system.
Adaptive metabolic shift during growth in chicken serum.
The growth of APEC O1 in serum resulted in a substantial transcriptional response in genes involved in metabolism, reflecting a need for rapid adaptation to the nutritional conditions encountered by APEC in serum. Among these changes was an alteration in APEC O1's preference for nutrient utilization. Increased expression of a gluconate binding protein (YhgH) and APECO1_3052, which encodes a gluconate transport-associated protein, was also observed. Significant inhibition was observed in glycerol metabolic genes, which were downregulated by 10- to 25-fold. Also, cultivation in serum resulted in inhibition of genes involved in catabolism of amino sugars, N-acetyglucosamine (NAG) and N-acetylneuraminic (sialic) acid; C4-dicarboxylates, fumarate and succinate; and the amino acids, serine, tryptophan, and aspartate.
Several genes involved in central metabolism were significantly upregulated after APEC O1 was transferred from LB to serum. There was an increase in the expression of three central metabolic enzymes that are involved in the pentose phosphate pathway (PP pathway). The gene gcd, which encodes a glucose dehydrogenase and is used in the oxidative PP pathway, was upregulated 2.18-fold. In addition, two genes, talA and tktB, encoding two nonoxidative pentose phosphate pathway enzymes (53), were upregulated 2.16- and 2.19-fold, respectively. The transaldolase (TalA) converts sedoheptulose-7-phosphate and glyceraldehyde-3-phosphate to erythrose-4-phosphate and fructose-6-phosphate, while the transketolase catalyzes the reverse reaction, the conversion of sedoheptulose-7-P and glyceraldehyde-3-P to the aldose d-ribose-5-P and ketose d-xylulose-5-P.
Growth in serum influences transport.
In addition to differential regulation of iron transport systems (described in the virulence section), exposure of APEC O1 to serum also changed the transcription of a number of genes encoding other transport systems. The manganese transport protein MntH (47) was significantly upregulated more than 3-fold, and the copper transporter YbaR was upregulated 2.8-fold. These results suggest that in addition to being a low-iron environment, serum is a low-manganese and -copper environment and metal ion limitation presents a significant obstacle to APEC infection. Interestingly, eight genes encoding a type II secretion system (T2SS), including gspC, gspD, gspE, gspF, gspH, gspI, gspK, and gspL, were significantly downregulated when APEC O1 was cultured in serum compared to results in LB (12).
Regulators.
Several regulatory genes, including regulators of unknown function, showed altered expression in serum. We found that a putative regulator encoded by the Yersinia high-pathogenicity island (HPI) (27) was significantly upregulated in serum. This gene (APECO1_1057) is localized immediately upstream of the gene irp2 of the HPI and is identical to the gene ybtA of HPI. A mutant generated by nonpolar deletion of this gene was shown to lead to APEC attenuation using the in vivo competition assay (Table 4).
PhoP/PhoQ is a known two-component signal transduction system. It responds to antimicrobial peptides and a low Mg2+ concentration and is involved in bacterial virulence. Several genes belonging to the phoP regulon, including yrbL, slyB, gadA, hdeA, hdeB, gadX, and ompT (39), were significantly upregulated in serum. However, its importance in extraintestinal pathogenic E. coli (ExPEC) virulence has never been demonstrated. We deleted phoP from APEC O1 and tested the mutant in the in vivo competition assay (Table 4). The phoP mutant was attenuated compared to the wild type, further suggesting that PhoP plays a role in APEC virulence.
Expression of two two-component signal transduction systems (TCSs) was reduced in the microarray analysis. The sensor histidine kinase gene of TCS, encoding PmrA/PmrB, was found to be significantly downregulated. TCS PmrA/PmrB mediates Salmonella resistance to stress of Fe(III), Al(III), and antimicrobial peptides by modifying LPS expression (41). The response regulator NarP of NarQ/NarP was also downregulated. This TCS activates gene expression in response to nitrate and is especially sensitive to low concentrations of nitrate (58).
DISCUSSION
The ability to survive and grow in chicken serum appears to be an important virulence determinant for APEC strains and may play a major role in the pathogenesis of avian colibacillosis (37). Here, we describe the first pan-genome microarray analysis of the global transcriptional response of APEC to chicken serum using the first APEC genome-specific microarray. Previous studies of APEC using transcriptional analysis compared expression of 152 virulence genes of APEC in vivo (62) and sought to identify the consequences of a pst mutation (13). Our study provides insight into the global gene regulation and the interplay required for adaptation to serum, a condition for growth that was used to mimic the conditions that APEC encounters during host infection.
The microarray results presented here are internally consistent and often are compatible with those in previous studies. All iron uptake systems were significantly upregulated, which is consistent with the knowledge that serum is a low-iron environment (10). Also, genes within the same iron uptake operons (i.e., the enterobactin, sit, and chu operons) were uniformly upregulated, suggesting that there is coordinated regulation of iron acquisition genes within an operon. Several iron uptake systems, such as sit, chu, aerobactin, and iro, have been associated with APEC virulence in previous studies (8, 36, 48, 52), and our results confirm their importance in APEC pathogenesis. Also, the contribution of APEC O1's large pathogenicity island (PAI)-containing plasmid, pAPEC-O1-ColBM, to virulence was demonstrated here. Fifty-six genes that are mostly localized to the conserved regions of APEC's plasmid PAIs (26, 28) were significantly upregulated. While the biological functions of some of these upregulated plasmid genes are known and include iron uptake, adhesion, plasmid replication, and stability, many were of unknown function. This strong representation of “genes of unknown function” among those upregulated under host-like conditions underscores how much there is to learn about E. coli's complement resistance and virulence and how little we know about the contributions of plasmids to the pathogenesis of disease. Further studies to delineate the functions of these genes certainly seem justified.
Since colonization is a first step in bacterial infections, it is notable that none of APEC O1's known fimbrial gene clusters were upregulated. That is, of the 9 fimbrial gene clusters predicted to occur in APEC O1 (3, 27), all were either not significantly changed or mostly downregulated. In addition, the expression of the autotransporter adhesin AatA, which was previously described as being upregulated in serum (35), did not significantly change. However, the nonfimbrial adhesin gene tia, which was previously localized to PAI IAPEC-O1 in APEC O1, was upregulated significantly (30). This gene is identical to ETEC tia and shares significant homology with hraI from porcine enterotoxigenic E. coli (ETEC) and limited homology with the ail locus from Yersinia spp. (18). The encoded 25-kDa Tia OMP plays a role in ETEC's adherence of epithelial cells; however, the role of tia in APEC pathogenesis has yet to be determined. A recent study suggested that APEC contains adhesins that have not yet been identified (2), and Tia may be one such candidate.
In addition to these findings, our microarray data present provocative contrasts to previous reports on the mechanisms involved in E. coli's complement resistance (11, 40, 42, 59). A number of bacterial cell surface structures, including capsule, LPS, and OMPs, have been previously associated with resistance of E. coli to serum. In contrast, neither genes involved with K1 capsular synthesis nor LPS synthesis genes were upregulated in APEC O1 when it was grown in serum. Similarly, three OMP genes, ompA, iss, and traT, thought to contribute to E. coli's serum resistance (11, 40, 42, 59), were not upregulated in APEC O1 when grown in serum. At this time, it is not known whether the conditions used (incubation at 37°C and pH 8.2) are responsible for this observation. If not, this may suggest that some virulence genes may be constitutively expressed in vivo and in vitro.
One of the most provocative findings in this study is the identification of the role that adaptation to stress plays in serum resistance. Several genes involved in resistance to general, acid, oxidative, and heat stress were upregulated in serum, confirming that serum is a stressful environment for APEC. During growth in serum, expression of the superoxidase protein, SodA, and the H2O2 scavenger protein, KatE, was upregulated. This suggests that APEC differentially regulates expression of these systems to cope with oxidative stress encountered during growth in serum. Superoxide (O2−) is generated when E. coli produces ATP via the respiratory chain, and SodA, which exists in the bacterial cytoplasm, can convert O2− to H2O2. APEC cells may also be exposed to H2O2 produced by phagocytes during infection. Since H2O2 can produce cell damage, it must be removed or detoxified. Thus, such mechanisms, which confer the ability to resist oxidative stress, are associated with bacterial virulence (21, 23).
Besides the upregulation of oxidative resistance genes, five heat shock genes, all belonging to the rpoH regulon, were significantly upregulated when APEC O1 was grown in serum at 37°C. The product of rpoH, σ32, is the major transcriptional activator of the heat shock genes. However, the bacterial heat shock response is not limited to changes in temperature alone and is regarded as a general stress response (20). Our data showing that deletion of several heat shock proteins led to attenuation of APEC O1 in chickens confirm that these genes contribute to APEC virulence. Thus, upregulation of the rpoH regulon may be necessary for APEC to resist general stress during infection or to adapt to the chicken's body temperature of 42°C. Many previous studies have demonstrated a potential correlation between pathogenesis and the heat shock response (20). However, in most cases the genes encoding these proteins are not part of the heat shock regulon, and the induction of these genes at increased temperature is mediated by other processes or is regulated only indirectly by the heat shock response (20). Here we have provided direct evidence that heat shock proteins contribute to APEC virulence.
We also found that some genes encoding metabolic enzymes are differentially expressed, which signifies metabolic adaptation for APEC grown in chicken serum. Our results demonstrated that gluconate is a preferred sugar and PP pathway may be an important route of carbon flux during APEC O1's growth in serum. Similar findings were reported for uropathogenic E. coli (UPEC) growing in human urine (1) and E. coli K-12 colonizing the mouse intestine (9). Notably, several genes involved in glycerol, amino sugars, or C4-dicarboxylate catabolism were significantly downregulated, while the corresponding genes were upregulated in UPEC (1) and E. coli K-12 (9).
In Gram-negative bacteria, T2SS is one of five protein secretion systems that export bacterial proteins from within the cell to the periplasmic space or the extracellular milieu or into host cells. A complete set of T2SS genes includes gspCDEFGHIJKLMO, are common among the proteobacteria. Type II secretion has been associated with virulence in many bacterial pathogens (12). The downregulation of T2SS in serum suggests this system may play a limited role in APEC virulence or that its importance is restricted to some particular site of infection or pathogenic process, which occurs somewhere other than in serum.
Iron is necessary for various cellular functions (57). Thus, the fact that the concentration of free iron is exceedingly low in host tissues necessitates that APEC acquire iron inside the host (57). However, excess iron is also toxic to bacterial cells. Consequently, acquisition of iron by bacteria is under the tight control of the master negative regulator Fur (57). The elegant regulation of iron acquisition in host niches also requires three other types of activators, including TCSs, extracytoplasmic function (ECF) sigma factors, and AraC-like regulators of the synthesis of siderophores and their uptake systems (22, 57). Our microarray results revealed that an AraC-like regulator, YbtA, encoded by APEC O1's HPI, was significantly upregulated during growth in serum. In Yersinia, this regulator induces the yersiniabactin biosynthesis operon irp21, ybtUTE, the ybtPQXS operon, and the Fe-yersiniabactin receptor FyuA (17). The HPI has been shown to be involved in the virulence of Yersinia pestis, Y. enterocolitica, and Y. pseudotuberculosis (7) and is widely distributed among APEC strains (16). However, its role in APEC pathogenesis has never been clearly demonstrated. Deletion of ybtA in this study led to APEC attenuation in chickens, providing evidence that this HPI contributes to APEC O1 virulence.
TCSs are widely employed by bacteria for sensing and responding to the environment (33). Over 32 TCSs have been found in nonpathogenic E. coli that are also found in pathogenic E. coli (45). Several TCSs have been associated with the virulence of pathogenic E. coli, and a previous study demonstrated that the TCS BarA/UvrY contributes to APEC pathogenesis (24). Our microarray data showed that several target genes of TCS PhoP/PhoQ were significantly upregulated during APEC O1's growth in serum. Furthermore, the phoP deletion mutant was outcompeted by the APEC wild type, suggesting that this gene contributes to APEC virulence. The PhoQ/PhoP TCS senses external Mg2+ and antimicrobial peptide concentrations and controls adaptation to the in vivo environment. Although it is a major regulator of virulence in the enteric pathogen Salmonella enterica serovar Typhimurium (6) and its regulon in E. coli K-12 was extensively studied (39), a link between this signal transduction system and the virulence of pathogenic E. coli has not been established. To our knowledge, this is the first report linking this important signal transduction system with ExPEC virulence.
ACKNOWLEDGMENT
This work was supported by the USDA NRICGP Microbial Functional Genomics program (grant no. 2008-3560418805).
Footnotes
Published ahead of print on 28 February 2011.
REFERENCES
- 1. Alteri C. J., Smith S. N., Mobley H. L. 2009. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathog. 5:e1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Amabile de Campos T., et al. 2005. Adhesion properties, fimbrial expression and PCR detection of adhesin-related genes of avian Escherichia coli strains. Vet. Microbiol. 106:275–285 [DOI] [PubMed] [Google Scholar]
- 3. Antao E. M., et al. 2009. Signature-tagged mutagenesis in a chicken infection model leads to the identification of a novel avian pathogenic Escherichia coli fimbrial adhesin. PLoS One 4:e7796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes J., Nolan L. K., Vaillancourt J. P. 2008. Colibacillosis, p. 691–732 Diseases of poultry, 12th ed. Blackwell Publishing, Hoboken, NJ [Google Scholar]
- 6. Beuzon C. R., Unsworth K. E., Holden D. W. 2001. In vivo genetic analysis indicates that PhoP-PhoQ and the Salmonella pathogenicity island 2 type III secretion system contribute independently to Salmonella enterica serovar Typhimurium virulence. Infect. Immun. 69:7254–7261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Burrows T. W., Jackson S. 1956. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br. J. Exp. Pathol. 37:577–583 [PMC free article] [PubMed] [Google Scholar]
- 8. Caza M., Lepine F., Milot S., Dozois C. M. 2008. Specific roles of the iroBCDEN genes in virulence of an avian pathogenic Escherichia coli O78 strain and in production of salmochelins. Infect. Immun. 76:3539–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang D. E., et al. 2004. Carbon nutrition of Escherichia coli in the mouse intestine. Proc. Natl. Acad. Sci. U. S. A. 101:7427–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Che P., Xu J., Shi H., Ma Y. 1995. Quantitative determination of serum iron in human blood by high-performance capillary electrophoresis. J. Chromatogr. B Biomed. Appl. 669:45–51 [DOI] [PubMed] [Google Scholar]
- 11. Chuba P., Palchaudhuri S., Leon M. 1986. Contributions of traT and iss genes to the serum resistance phenotype of plasmid colV2-K94. FEMS Microbiol. Lett. 37:135–140 [Google Scholar]
- 12. Cianciotto N. P. 2005. Type II secretion: a protein secretion system for all seasons. Trends Microbiol. 13:581–588 [DOI] [PubMed] [Google Scholar]
- 13. Crepin S., et al. 2008. Genome-wide transcriptional response of an avian pathogenic Escherichia coli (APEC) pst mutant. BMC Genomics 9:568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dudoit S., Yang Y. H., Callow M. J., Speed T. P. 2002. Statistical methods for identifying differentially expressed genes in replicated cDNA microarray experiments. Stat. Sin. 12:111–139 [Google Scholar]
- 16. Ewers C., Janssen T., Kiessling S., Philipp H. C., Wieler L. H. 2004. Molecular epidemiology of avian pathogenic Escherichia coli (APEC) isolated from colisepticemia in poultry. Vet. Microbiol. 104:91–101 [DOI] [PubMed] [Google Scholar]
- 17. Fetherston J. D., Bearden S. W., Perry R. D. 1996. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol. Microbiol. 22:315–325 [DOI] [PubMed] [Google Scholar]
- 18. Fleckenstein J. M., Kopecko D. J., Warren R. L., Elsinghorst E. A. 1996. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 64:2256–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foster J. W. 2004. Escherichia coli acid resistance: tales of an amateur acidophile. Nat. Rev. Microbiol. 2:898–907 [DOI] [PubMed] [Google Scholar]
- 20. Gophna U., Ron E. Z. 2003. Virulence and the heat shock response. Int. J. Med. Microbiol. 292:453–461 [DOI] [PubMed] [Google Scholar]
- 21. Halsey T. A., Vazquez-Torres A., Gravdahl D. J., Fang F. C., Libby S. J. 2004. The ferritin-like Dps protein is required for Salmonella enterica serovar Typhimurium oxidative stress resistance and virulence. Infect. Immun. 72:1155–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hantke K., Braun V. 1998. Control of bacterial iron transport by regulatory proteins, p. 11–44 In silver S., Walden W. (ed.), Metal ions in gene regulation. Chapman and Hall, New York, NY [Google Scholar]
- 23. Hebrard M., Viala J. P., Meresse S., Barras F., Aussel L. 2009. Redundant hydrogen peroxide scavengers contribute to Salmonella virulence and oxidative stress resistance. J. Bacteriol. 191:4605–4614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herren C. D., et al. 2006. The BarA-UvrY two-component system regulates virulence in avian pathogenic Escherichia coli O78:K80:H9. Infect. Immun. 74:4900–4909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoffmann H., Hornef M. W., Schubert S., Roggenkamp A. 2001. Distribution of the outer membrane haem receptor protein ChuA in environmental and human isolates of Escherichia coli. Int. J. Med. Microbiol. 291:227–230 [DOI] [PubMed] [Google Scholar]
- 26. Johnson T. J., Johnson S. J., Nolan L. K. 2006. Complete DNA sequence of a ColBM plasmid from avian pathogenic Escherichia coli suggests that it evolved from closely related ColV virulence plasmids. J. Bacteriol. 188:5975–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson T. J., et al. 2007. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J. Bacteriol. 189:3228–3236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Johnson T. J., Siek K. E., Johnson S. J., Nolan L. K. 2006. DNA sequence of a ColV plasmid and prevalence of selected plasmid-encoded virulence genes among avian Escherichia coli strains. J. Bacteriol. 188:745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Johnson T. J., Wannemuehler Y. M., Nolan L. K. 2008. Evolution of the iss gene in Escherichia coli. Appl. Environ. Microbiol. 74:2360–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kariyawasam S., Johnson T. J., Nolan L. K. 2006. The pap operon of avian pathogenic Escherichia coli strain O1:K1 is located on a novel pathogenicity island. Infect. Immun. 74:744–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kottom T. J., et al. 1997. Further characterization of a complement-sensitive mutant of a virulent avian Escherichia coli isolate. Avian Dis. 41:817–823 [PubMed] [Google Scholar]
- 32. Kvint K., Nachin L., Diez A., Nystrom T. 2003. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 6:140–145 [DOI] [PubMed] [Google Scholar]
- 33. Laub M. T., Goulian M. 2007. Specificity in two-component signal transduction pathways. Annu. Rev. Genet. 41:121–145 [DOI] [PubMed] [Google Scholar]
- 34. Li G., et al. 2008. Characterization of a yjjQ mutant of avian pathogenic Escherichia coli (APEC). Microbiology 154:1082–1093 [DOI] [PubMed] [Google Scholar]
- 35. Li G., et al. 2010. AatA is a novel autotransporter and virulence factor of avian pathogenic Escherichia coli. Infect. Immun. 78:898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li G., Laturnus C., Ewers C., Wieler L. H. 2005. Identification of genes required for avian Escherichia coli septicemia by signature-tagged mutagenesis. Infect. Immun. 73:2818–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mellata M., et al. 2003. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect. Immun. 71:536–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mellata M., et al. 2003. Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infect. Immun. 71:494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monsieurs P., et al. 2005. Comparison of the PhoPQ regulon in Escherichia coli and Salmonella typhimurium. J. Mol. Evol. 60:462–474 [DOI] [PubMed] [Google Scholar]
- 40. Nemeth J., Muckle C. A., Lo R. Y. 1991. Serum resistance and the traT gene in bovine mastitis-causing Escherichia coli. Vet. Microbiol. 28:343–351 [DOI] [PubMed] [Google Scholar]
- 41. Nishino K., et al. 2006. Identification of the lipopolysaccharide modifications controlled by the Salmonella PmrA/PmrB system mediating resistance to Fe(III) and Al(III). Mol. Microbiol. 61:645–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nolan L. K., et al. 2002. Complement resistance, as determined by viable count and flow cytometric methods, and its association with the presence of iss and the virulence of avian Escherichia coli. Avian Dis. 46:386–392 [DOI] [PubMed] [Google Scholar]
- 43. Nolan L. K., Wooley R. E., Cooper R. K. 1992. Transposon mutagenesis used to study the role of complement resistance in the virulence of an avian Escherichia coli isolate. Avian Dis. 36:398–402 [PubMed] [Google Scholar]
- 44. Ohtsu I., et al. 2010. The L-cysteine/L-cystine shuttle system provides reducing equivalents to the periplasm in Escherichia coli. J. Biol. Chem. 285:17479–17487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Oshima T., et al. 2002. Transcriptome analysis of all two-component regulatory system mutants of Escherichia coli K-12. Mol. Microbiol. 46:281–291 [DOI] [PubMed] [Google Scholar]
- 46. Pelludat C., Rakin A., Jacobi C. A., Schubert S., Heesemann J. 1998. The yersiniabactin biosynthetic gene cluster of Yersinia enterocolitica: organization and siderophore-dependent regulation. J. Bacteriol. 180:538–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Runyen-Janecky L., Dazenski E., Hawkins S., Warner L. 2006. Role and regulation of the Shigella flexneri sit and MntH systems. Infect. Immun. 74:4666–4672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sabri M., et al. 2008. Contribution of the SitABCD, MntH, and FeoB metal transporters to the virulence of avian pathogenic Escherichia coli O78 strain chi7122. Infect. Immun. 76:601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48a. Schmittgen T. D., et al. 2000. Quantitative reverse transcription-polymerase chain reaction to study mRNA decay: comparison of endpoint and real-time methods. Anal. Biochem. 285:194–204 [DOI] [PubMed] [Google Scholar]
- 49. Schnetz K. 2008. Fine-tuned growth phase control of dps, encoding a DNA protection protein, by FIS and H-NS. Mol. Microbiol. 68:1345–1347 [DOI] [PubMed] [Google Scholar]
- 50. Skyberg J. A., Johnson T. J., Nolan L. K. 2008. Mutational and transcriptional analyses of an avian pathogenic Escherichia coli ColV plasmid. BMC Microbiol. 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smyth G. K. 2004. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3:Article 3 [DOI] [PubMed] [Google Scholar]
- 52. Sorsa L. J., Dufke S., Heesemann J., Shubert S. 2003. Characterization of an iroBCDEN gene cluster on a transmissible plasmid of uropathogenic Escherichia coli: evidence for horizontal transfer of a chromosomal virulence factor. Infect. Immun. 71:3285–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sprenger G. A. 1995. Genetics of pentose-phosphate pathway enzymes of Escherichia coli K-12. Arch. Microbiol. 164:324–330 [DOI] [PubMed] [Google Scholar]
- 54. Storey J. D., Taylor J. E., Siegmund D. 2004. Strong control, conservative point estimation and simultaneous conservative consistency of false discovery rates: a unified approach, p. 187–205 J. R. Stat. Soc. 66: 187–205 [Google Scholar]
- 55. Suerbaum S., Friedrich S., Leying H., Opferkuch W. 1994. Expression of capsular polysaccharide determines serum resistance in Escherichia coli K92. Zentralbl. Bakteriol. 281:146–157 [DOI] [PubMed] [Google Scholar]
- 56. Vokes S. A., Reeves S. A., Torres A. G., Payne S. M. 1999. The aerobactin iron transport system genes in Shigella flexneri are present within a pathogenicity island. Mol. Microbiol. 33:63–73 [DOI] [PubMed] [Google Scholar]
- 57. Wandersman C., Delepelaire P. 2004. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 58:611–647 [DOI] [PubMed] [Google Scholar]
- 58. Wang H., Tseng C. P., Gunsalus R. P. 1999. The napF and narG nitrate reductase operons in Escherichia coli are differentially expressed in response to submicromolar concentrations of nitrate but not nitrite. J. Bacteriol. 181:5303–5308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weiser J., Gotschlich E. 1991. Outer membrane protein A (OmpA) contributes to serum resistance and pathogenicity of Escherichia coli K-1. Infect. Immun. 59:2252–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Williams P. H., Carbonetti N. H. 1986. Iron, siderophores, and the pursuit of virulence: independence of the aerobactin and enterochelin iron uptake systems in Escherichia coli. Infect. Immun. 51:942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wooley R. E., Spears K. R., Brown J., Nolan L. K., Fletcher O. J. 1992. Relationship of complement resistance and selected virulence factors in pathogenic avian Escherichia coli. Avian Dis. 36:679–684 [PubMed] [Google Scholar]
- 62. Zhao L., et al. 2009. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E. coli in a murine urinary tract infection model and a chicken challenge model. Microbiology 155:1634–1644 [DOI] [PubMed] [Google Scholar]
- 63. Zhou D., Hardt W. D., Galan J. E. 1999. Salmonella typhimurium encodes a putative iron transport system within the centisome 63 pathogenicity island. Infect. Immun. 67:1974–1981 [DOI] [PMC free article] [PubMed] [Google Scholar]