Abstract
Transient high-grade bacteremia following invasive procedures carries a risk of infective endocarditis (IE). This is supported by experimental endocarditis. On the other hand, case-control studies showed that IE could be caused by cumulative exposure to low-grade bacteremia occurring during daily activities. However, no experimental demonstration of this latter possibility exists. This study investigated the infectivity in animals of continuous low-grade bacteremia compared to that of brief high-grade bacteremia. Rats with aortic vegetations were inoculated with Streptococcus intermedius, Streptococcus gordonii or Staphylococcus aureus (strains Newman and P8). Animals were challenged with 103 to 106 CFU. Identical bacterial numbers were given by bolus (1 ml in 1 min) or continuous infusion (0.0017 ml/min over 10 h). Bacteremia was 50 to 1,000 times greater after bolus than during continuous inoculation. Streptococcal bolus inoculation of 105 CFU infected 63 to 100% vegetations compared to 30 to 71% infection after continuous infusion (P > 0.05). When increasing the inoculum to 106 CFU, bolus inoculation infected 100% vegetations and continuous infusion 70 to 100% (P > 0.05). S. aureus bolus injection of 103 CFU infected 46 to 57% valves. This was similar to the 53 to 57% infection rates produced by continuous infusion (P > 0.05). Inoculation of 104 CFU of S. aureus infected 80 to 100% vegetations after bolus and 60 to 75% after continuous infusion (P > 0.05). These results show that high-level bacteremia is not required to induce experimental endocarditis and support the hypothesis that cumulative exposure to low-grade bacteremia represents a genuine risk of IE in humans.
INTRODUCTION
Because of its severity, it is generally agreed that infective endocarditis (IE) should be prevented whenever possible. Over the last 50 years, guidelines of IE prophylaxis were edited by numerous international medical societies, which proposed antibiotic prophylaxis in a great number of patients and medico-surgical situations. However, this was without a definite proof of efficacy and without evaluation of its real its cost-benefit ratio (2, 3, 13, 23, 43). In fact, it was suggested that applying antibiotic prophylaxis systematically might be more detrimental than beneficial (36, 38).
Since studying IE prophylaxis in human raised ethical issues and would require too many patients (6), animal models were established to mimic the disease and test antibiotic prophylaxis (7). Moreover, for experimental reliability, animals were inoculated with large bacterial numbers, producing IE in ≥90% untreated individuals (12). Successful antibiotic prophylaxis in such stringent conditions were reinsuring for the medical community. However, although this assumption is likely to be correct, case-control studies and meta-analyses showed that most cases of IE occurred outside the context of dental or other medico-surgical procedures, independently of prophylaxis administration (28, 37, 39, 40). Thus, medico-surgical interventions may only represent one anecdotic event among other factors that should be taken into account in IE development.
Although it is true that dental or other surgical procedures may cause bacteremia (16, 29), routine daily events, such as mastication and tooth brushing, can also result in bacteremia as well (1, 11, 19–21, 29). It was estimated that the cumulative numbers of circulating bacteria (in CFU/ml/year) resulting from either mastication, tooth brushing two times daily or mere dental examination, were up to 5.6 million times, 154,000 times, and 48 times greater than that provoked by a single tooth extraction (14, 32, 42, 43). Thus, cumulative low-grade bacteremia might equal or surpass sporadic procedure-related bacteremia as a risk for IE induction.
Considering all of these arguments, guidelines of IE prophylaxis were recently revisited and greatly simplified, both in the United States and in Europe (15, 43, 44). The resulting simplified guidelines have positive implications, and they identify a great proportion (>90%) of at-risk patients that should not take routine antibiotic prophylaxis, because they might not benefit from it. On the other hand, they are also frustrating, because they do not propose any prevention in these 90% of patients that are at risk of developing IE spontaneously.
To address this issue, we must improve our understanding of disease initiation. Indeed, while there is general agreement that recurrent low-grade bacteremia is likely to promote IE, there is no knowledge of the relative risk of IE represented by such type of bacteremia compared to transient high-grade bacteremia induced by procedures. Moreover, in case of low-grade bacteremia, there is not much knowledge about possible aggravation factors, such as the carriage of particular IE pathogens (e.g., Staphylococcus aureus versus Streptococcus spp.) or concomitant inflammatory or immunological conditions, that might favor bacterial colonization of undamaged endothelia via endothelial expression of integrins (25) or alter microbial clearance from the blood.
The present study addressed the first of these questions by comparing the rates of experimental IE in rats with catheter-induced aortic vegetations after (i) transient high-grade bacteremia, mimicking procedure-induced bacteremia, or (ii) continuous low-grade bacteremia, simulating the cumulative exposure to bacteremia from daily life activities during a long period of time, for instance, 1 year (32). Animals were challenged with either bolus injection (over 1 min) or continuous infusion (over 10 h) with similar bacterial numbers (from 103 to 106 CFU) of viridans streptococci or S. aureus. The infectivity rate of continuous low-grade bacteremia was not different from that of transient high-grade bacteremia. Since the continuous low-grade condition is closer of the common situation in humans, it provides a new model to study endocarditis pathogenesis and novel preventive strategies.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Four well-described bacterial isolates were used, including two types of viridans group streptococci (i.e., Streptococcus intermedius and Streptococcus gordonii) (8, 12) and two types of S. aureus (i.e., methicillin-susceptible S. aureus [MSSA] Newman and methicillin-resistant S. aureus [MRSA] P8) (9, 24). Streptococci were grown at 37°C with 5% CO2 in brain heart infusion broth (Difco/Becton Dickinson, Sparks, MD) and S. aureus at 37°C in tryptic soy broth (Difco). Bacterial stocks were kept at −70°C in liquid medium supplemented with 20% (vol/vol) of glycerol.
Animal studies.
All animal experiments were carried out according to Swiss regulations (authorization, 879.6). For all manipulations, animals were anesthetized with a mixture of ketamine (75 mg/kg) and midazolam (0.5 mg/kg) given intraperitoneally. Catheter-induced sterile aortic vegetations were produced in female Wistar rats as previously described (17). In parallel, an intravenous (i.v.) line was inserted via the jugular vein into the superior vena cava and connected to a programmable infusion pump (Pump 44; Harvard Apparatus, Inc., South Natick, MA) in order to deliver the inocula (10).
Bacterial inocula were prepared from overnight cultures. Microorganisms were recovered by centrifugation, washed, and adjusted to the desired inoculum size in saline. The inoculum size was confirmed by colony counts on blood agar plates. Animals were inoculated 24 h after catheterization, via the infusion pump, by either of two distinct protocols. In protocol 1, they received an i.v. bolus (1 ml in 1 min) containing inoculum sizes capable of infecting 50% and ≥90% of the vegetations. These inoculum sizes were 105 to 106 CFU for viridans streptococci and 103 to 104 CFU for S. aureus (8, 9, 12, 24). In protocol 2, animals received the same total absolute numbers of bacteria as in protocol 1 but progressively delivered at a pace of 0.0017 ml/min over 10 h. During this period, no growth of the original inoculum used for challenge was observed, as checked in preliminary experiments.
To evaluate the concentrations of bacteria in the blood, 1 ml of blood was drawn from selected animals by puncturing the external jugular vein, using aseptic procedures, and collected in heparin-containing tubes. Samples were taken just before inoculation, 1 min and 2 h (for bolus injection) or 2 and 6 h (for continuous infusion) after inoculation onset. The 1-min time point for bolus injection corresponded to the timing of blood cultures in previous human and animal studies (31, 33). The 2- and 6-h time points for continuous infusion were chosen on the basis of numerous pilot experiments, which showed that the continuous-infusion technique yielded stable bacteremia levels over time. Blood was serially diluted in saline and spread onto Columbia blood agar plates, which were incubated for 48 h at 37°C before colony counts. The sensitivity of the method was 1 CFU/ml of blood.
Rats were sacrificed 24 h after the end of inoculation. Quantitative valve and spleen cultures were performed as previously described (8). This method permitted the detection of 2 log10 CFU/g of vegetation and 1 log10 CFU/g of spleen.
Statistical analysis.
The percentages of valve and spleen infection following bolus and continuous infusion were compared by the Fisher exact test. Median bacterial counts in vegetations and spleens in the bolus and continuous infusion groups were compared by the Mann-Whitney rank sum test. Statistical analyses were performed with GraphPad Prism software (version 4.0; GraphPad Software, San Diego, CA). Differences were considered significant when P was < 0.05 by use of two-tailed significance levels.
RESULTS
Bacteremia levels and rates of vegetation and spleen infection for viridans streptococci.
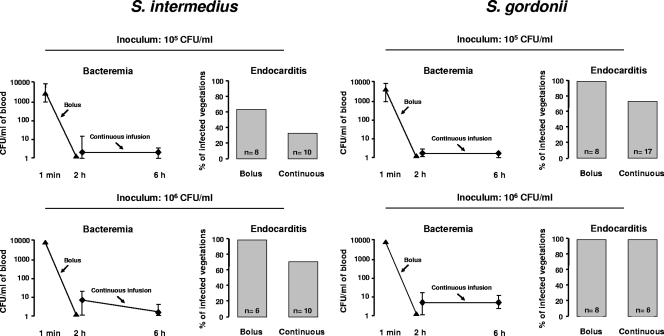
The intensity of bacteremia and the percentage of infected vegetations are shown in Fig. 1. Blood cultures were all negative before bacterial inoculation (baseline). Bolus inoculation of 105 CFU S. intermedius or S. gordonii was followed by bacteremia levels ranging between 5 × 103 and 9 × 103 CFU/ml 1 min after inoculation. In comparison, the median (range) levels of bacteremia during continuous infusion were 1,000 times lower, i.e., 4 (0 to 20) for S. intermedius and 1 (0 to 7) CFU/ml for S. gordonii. Bacteremia levels 1 min after bolus injection of 106 CFU S. intermedius or S. gordonii was 10 times greater than after 105 CFU, i.e., between 2 × 104 and 9 × 104 CFU/ml for both test organisms, and yet negative 2 h later. In comparison, bacteremia levels during continuous infusion had median values of 10 (range, 1 to 24) CFU/ml and 9 (range, 1 to 18) CFU/ml for S. intermedius and S. gordonii, respectively.
Fig. 1.
Level of bacteremia and resulting vegetation infection in rats challenged with 105 and 106 CFU of Streptococcus intermedius or Streptococcus gordonii. Identical inoculum sizes were given by i.v. bolus (1 ml in 1 min) or continuous infusion (0.0017 ml/min over 10 h). The symbols (▴ and ⧫) indicate median and range values.
Figure 1 also depicts the related rates of infection. Bolus injection of 105 CFU resulted in 5 of 8 (63%) and 8 of 8 (100%) infected vegetations for S. intermedius and S. gordonii, respectively, compared to 3 of 10 (30%; P = 0.34) and 12 of 17 (71%; P = 0.14) after continuous infusion. When the inoculum size was increased to 106 CFU, bolus inoculation infected 6 of 6 (100%) and 8 of 8 (100%) vegetations with both strains, respectively, compared to 7 of 10 (70%) and 6 of 6 (100%) infected vegetations after continuous infusion (P > 0.05).
With regard to spleen infection, bolus injection of 105 CFU resulted in 6 of 8 (75%) and 5 of 6 (83%) positive cultures for S. intermedius and S. gordonii, respectively, compared to 2 of 8 (25%; P = 0.13) and 5 of 16 (31%; P = 0.06) after continuous infusion. At the inoculum size of 106 CFU, bolus inoculation of S. intermedius and S. gordonii infected 5 of 5 (100%) and 8 of 8 (100%) spleens, respectively, compared to 4 of 8 (50%) and 6 of 6 (100%) after continuous infusion (P > 0.05).
Bacteremia levels and rates of vegetation and spleen infection for S. aureus.
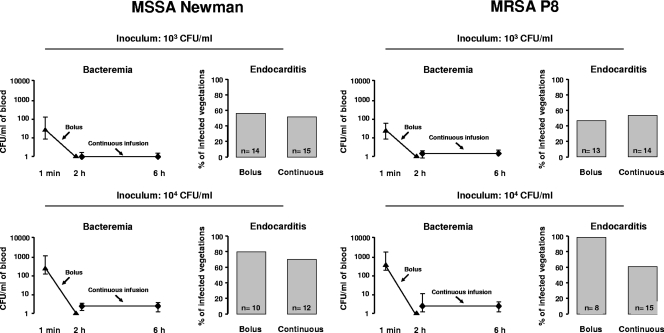
As in the streptococcal experiments, all blood cultures were negative before bacterial inoculation. The intensity of bacteremia and the percentage of infected vegetations are shown in Fig. 2. One minute after bolus injection of 103 CFU, the median CFU/ml of blood values (ranges) were 49 (8 to 140) for MSSA Newman and 56 (10 to 86) for MRSA P8. No circulating bacteria were detected 2 h later. In contrast, the same inoculum injected in continuous infusion produced bacterial concentrations in the blood ranging only between 0 to 2 CFU/ml of blood but lasting for 10 h. Also, as in streptococci, bolus inoculation of 104 CFU produced 10-fold greater levels of bacteremia at 1 min, with medians (ranges) of 473 (120 to 1,110) CFU/ml for MSSA Newman and 510 (240 to 1,400) CFU/ml for MRSA P8. No circulating bacteria were detectable after 2 h. This compares to medians of 2 (0 to 2) CFU/ml for MSSA Newman and 3 (0 to 15) CFU/ml for MRSA P8 during continuous infusion.
Fig. 2.
Level of bacteremia and resulting vegetation infection in rats challenged with 103 and 104 CFU of MSSA Newman or MRSA P8. Identical inoculum sizes were given by i.v. bolus (1 ml in 1 min) or continuous infusion (0.0017 ml/min over 10 h). The symbols (▴ and ⧫) indicate median and range values.
Figure 2 also depicts the corresponding results of valve infections. Bolus injection of 103 CFU resulted in 8 of 14 (57%) and 6 of 13 (46%) of infected valves with MSSA Newman and MRSA P8, respectively. This was very similar to the rate of valve infection produced by continuous infusion, i.e., 8 of 15 (53%) for MSSA Newman and 8 of 14 (57%) for MRSA P8 (P > 0.05 compared to bolus injection), in spite of much lower levels of circulating bacteria. This similarity of infection rates persisted after increasing the inoculum by 10 times (i.e., 104 CFU), in spite of even greater differences (≥100 times) in bacteremia levels. MSSA Newman and MRSA P8 infected 8 of 10 (80%) and 8 of 8 (100%) vegetations after bolus inoculation and 9 of 12 (75%) and 9 of 15 (60%) after continuous infusion, respectively (P > 0.05).
Spleen cultures after bolus injection of 103 CFU were positive in 14 of 14 (100%) and 8 of 13 (62%) animals infected with MSSA Newman and MRSA P8, respectively, which was not significantly different from the rate of spleen infection produced by continuous infusion, i.e., 13 of 15 (87%) for MSSA Newman and 10 of 14 (71%) for MRSA P8 (P > 0.05). After the inoculum of MSSA Newman and MRSA P8 was increased by 10 times (i.e., 104 CFU), spleens were infected in 10 of 10 (100%) and 8 of 8 (100%) animals after bolus infection and 11 of 12 (92%) and 11 of 15 (74%) animals after continuous infusion, respectively (P > 0.05).
Bacterial densities in infected tissues.
The animals were also analyzed for the ability of bacteria to multiply in infected vegetations and spleens after having colonized the tissue. Table 1 shows that, at the time of sacrifice, bolus- and continuous infusion-challenged animals had no significantly different bacterial densities in the vegetations and spleens (P > 0.05). However, in infected vegetations the number of CFU tended to be higher in the bolus groups than in the continuous-infusion groups. This is likely due to the different number of bacteria colonizing the valves early after inoculation. Since at that time the level of circulating bacteremia is much higher after bolus, more bacteria are allowed to colonize and multiply in the vegetations than after continuous infusion.
Table 1.
Bacterial densities in infected vegetations and spleens of animals 24 h after challenge with Streptococcus spp. or S. aureus tested strainsa
| Strain | Inoculum (CFU/ml) | Median (range) log10 CFU/g in: |
|||
|---|---|---|---|---|---|
| Vegetations |
Spleens |
||||
| Bolus | Continuous infusion | Bolus | Continuous infusion | ||
| S. intermedius | 105 | 6.2 (3.5–7.7) | 5.1 (4.7–8.1) | 2.1 (1.7–2.5) | 2.5 (1.7–3.4) |
| 106 | 7.3 (7.1–7.6) | 6.3 (3.4–7.6) | 3.3 (3.0–3.6) | 2.6 (1.8–3.2) | |
| S. gordonii | 105 | 7.6 (4.0–8.1) | 6.2 (3.7–6.9) | 2.9 (2.2–3.6) | 3.1 (2.4–3.2) |
| 106 | 7.8 (6.2–8.2) | 6.4 (6.0–7.9) | 3.3 (2.6–4.6) | 3.0 (2.6–3.4) | |
| MSSA Newman | 103 | 8.5 (3.0–9.4) | 8.3 (5.4–9.3) | 2.5 (1.6–4.5) | 3.2 (1.8–4.6) |
| 104 | 9.4 (5.6–9.4) | 8.8 (5.8–9.4) | 3.9 (2.2–4.9) | 4.5 (1.9–4.8) | |
| MRSA P8 | 103 | 9.2 (4.6–9.4) | 7.1 (6.1–9.3) | 4.5 (1.7–4.9) | 3.9 (1.7–4.7) |
| 104 | 9.3 (5.2–9.6) | 8.6 (4.1–9.6) | 4.6 (2.6–4.8) | 4.5 (1.7–4.7) | |
Differences between values were not statistically different(P > 0.05) as analyzed by the Mann-Whitney test.
DISCUSSION
The results in the present study show that low-grade continuous bacteremia produced infections in experimental IE that were not significantly different from those resulting from transient high-grade bacteremia. This suggests that the most important predictor of valve infection is not the peak level of circulating bacteria but rather the area under the curve over time. Indeed, bacteremia levels of ca. 1,000 to 10,000 CFU of streptococci/ml and 100 to 1,000 CFU of S. aureus/ml were required to infect 50 to 100% of vegetations after bolus infection, whereas 2 to 10 CFU/ml were enough to achieve a comparable infection rate after continuous infusion was maintained for 10 h. This is particularly relevant for the risk of developing streptococcal IE in view of the much greater cumulative exposure (between 50 times and 5 million times) to circulating bacteria during normal activity than after exposure to sporadic dental procedures in humans (14, 32, 42, 43). In practice, this results in a much smaller risk of IE (from 50 times to 5 million times) in a setting of transient bacteremia than in a situation of cumulative exposure.
This supports case-control studies demonstrating that most cases of IE occurred outside the context of procedure-related bacteremia (37, 39, 40). As a result, it helps clarify the debate about antibiotic prophylaxis of IE, which is still ongoing, and fully supports the drastic simplification of the newer guidelines (15, 43, 44).
The case for S. aureus is more intricate. At least two situations should be taken into account, i.e., community-acquired S. aureus bloodstream infection (BSI) and healthcare-associated BSI (4). Community-acquired BSI without a detectable infection focus is often associated with IE. This means that the observed BSI results from already established valve infection. However, it does not provide information on how bacteria accessed the blood and colonized the valve originally. This could occur through repeated bouts of low-grade bacteremia, induced, for instance, by skin breaches of injection of impure material in the case of i.v. drug abuse.
In contrast, healthcare-associated BSI is often related to primary colonization of i.v. devices with S. aureus, followed by secondary infection of the valves. Here again, IE might results from more or less prolonged low-grade staphylococcal discharges from the colonized i.v. device rather that from massive punctual bacterial injection. Thus, also somewhat speculative, both of theses cases resemble more the low-grade continuous infusion scenario than the transient high-grade bolus tested herein.
For both oral streptococci and S. aureus, this model provides a unique system to study more subtly the IE pathogenic features, as well as new prevention measures that apply to cumulative exposure to low bacterial numbers. Regarding pathogenesis, it is noteworthy that both bolus injection and continuous infusion resulted, in particular for S. aureus, in similar infection rates in the spleen. The fact that bacteria accumulated in this lymphoid organ suggests that recirculation might be an issue, at least in the continuous-infusion group. Therefore, measures that might enhance bacterial clearance from the spleen might be useful for protection. For instance, preventing experimental IE by immunization was not unanimously effective in former experiments using bolus inoculation (5, 22, 30, 34, 35, 41). This could be due to the fact that specific antibodies might become overwhelmed in such acute conditions. In the light of the present results, vaccination strategies could thus prove to be much more effective in the more realistic low-gradient infection model, where levels of bacteremia are low. Likewise, anti-aggregant strategies, which also displayed inconsistent results (18, 26, 27), could become effective in this more chronic setting. Indeed, due to a threshold effect, large bacterial numbers could offset the efficacy of antibodies or moderate alterations in bacterial or platelet adherence, which could become fully active with lower numbers of circulating bacteria.
This is probably also true for other measures aimed at decreasing the likelihood of circulating bacteria, such as dental hygiene and drainage of infected foci (15, 43, 44). Such long-lasting interventions (e.g., vaccination or chronic anti-aggregant therapy) are not unrealistic in the context of already-approved preventing measures, such as anti-aggregant prophylaxis of coronary diseases. However, demonstrating their efficacy requires large cohort studies. Therefore, prior demonstration in the present model could be useful. Finally, one interesting observation that modulates somewhat the overall conclusion is the fact that at high inoculum sizes (e.g., 105 CFU for S. intermedius and 104 CFU for S. aureus P8) bolus injection tends to be more infective than continuous infusion. Although the difference was not statistically significant, this finding converged with the observation by Strom et al. (37), who reported a likely causality between procedures and IE only in a small subgroup of patients with invasive dental extraction and high-risk cardiac lesions. Newer guidelines also advise antibiotic prophylaxis in these peculiar at-risk constellations (15, 43, 44). Hence, this concordance further reinforces the improved compatibility of this experimental model with reality.
ACKNOWLEDGMENT
This study was supported by the Swiss National Science Foundation (grant 310030-125325).
Footnotes
Published ahead of print on 14 February 2011.
REFERENCES
- 1. Crasta K., et al. 2009. Bacteremia due to dental flossing. J. Clin. Periodontol. 36:323–332 [DOI] [PubMed] [Google Scholar]
- 2. Dajani A. S., et al. 1997. Prevention of bacterial endocarditis: recommendations by the American Heart Association. Clin. Infect. Dis. 25:1448–1458 [DOI] [PubMed] [Google Scholar]
- 3. Danchin N., Duval X., Leport C. 2005. Prophylaxis of infective endocarditis: French recommendations 2002. Heart 91:715–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. del Rio A., Cervera C., Moreno A., Moreillon P., Miro J. M. 2009. Patients at risk of complications of Staphylococcus aureus bloodstream infection. Clin. Infect. Dis. 48(Suppl. 4):S246–S253 [DOI] [PubMed] [Google Scholar]
- 5. Domanski P. J., et al. 2005. Characterization of a humanized monoclonal antibody recognizing clumping factor A expressed by Staphylococcus aureus. Infect. Immun. 73:5229–5232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Durack D. T. 1975. Chemoprophylaxis of bacterial endocarditis. N. Engl. J. Med. 292:1080–1081(Letter.) [PubMed] [Google Scholar]
- 7. Durack D. T., Petersdorf R. G. 1973. Chemotherapy of experimental streptococcal endocarditis. I. Comparison of commonly recommended prophylactic regimens. J. Clin. Invest. 52:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Entenza J. M., Caldelari I., Glauser M. P., Francioli P., Moreillon P. 1997. Importance of genotypic and phenotypic tolerance in the treatment of experimental endocarditis due to Streptococcus gordonii. J. Infect. Dis. 175:70–76 [DOI] [PubMed] [Google Scholar]
- 9. Entenza J. M., Que Y. A., Vouillamoz J., Glauser M. P., Moreillon P. 2001. Efficacies of moxifloxacin, ciprofloxacin, and vancomycin against experimental endocarditis due to methicillin-resistant Staphylococcus aureus expressing various degrees of ciprofloxacin resistance. Antimicrob. Agents Chemother. 45:3076–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fluckiger U., et al. 1994. Simulation of amoxicillin pharmacokinetics in humans for the prevention of streptococcal endocarditis in rats. Antimicrob. Agents Chemother. 38:2846–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Forner L., Larsen T., Kilian M., Holmstrup P. 2006. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J. Clin. Periodontol. 33:401–407 [DOI] [PubMed] [Google Scholar]
- 12. Glauser M. P., Bernard J. P., Moreillon P., Francioli P. 1983. Successful single-dose amoxicillin prophylaxis against experimental streptococcal endocarditis: evidence for two mechanisms of protection. J. Infect. Dis. 147:568–575 [DOI] [PubMed] [Google Scholar]
- 13. Gould F. K., et al. 2006. Guidelines for the prevention of endocarditis: report of the Working Party of the British Society for Antimicrobial Chemotherapy. J. Antimicrob. Chemother. 57:1035–1042 [DOI] [PubMed] [Google Scholar]
- 14. Guntheroth W. G. 1984. How important are dental procedures as a cause of infective endocarditis? Am. J. Cardiol. 54:797–801 [DOI] [PubMed] [Google Scholar]
- 15. Habib G., et al. 2009. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Eur. Heart J. 30:2369–2413 [DOI] [PubMed] [Google Scholar]
- 16. Heimdahl A., et al. 1990. Detection and quantitation by lysis-filtration of bacteremia after different oral surgical procedures. J. Clin. Microbiol. 28:2205–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heraief E., Glauser M. P., Freedman L. R. 1982. Natural history of aortic valve endocarditis in rats. Infect. Immun. 37:127–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levison M. E., Carrizosa J., Tanphaichitra D., Schick P. K., Rubin W. 1977. Effect of aspirin on thrombogenesis and on production of experimental aortic valvular Streptococcus viridans endocarditis in rabbits. Blood 49:645–650 [PubMed] [Google Scholar]
- 19. Lockhart P. B., et al. 2008. Bacteremia associated with toothbrushing and dental extraction. Circulation 117:3118–3125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lockhart P. B., et al. 2009. Poor oral hygiene as a risk factor for infective endocarditis-related bacteremia. J. Am. Dent. Assoc. 140:1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lucas V. S., Gafan G., Dewhurst S., Roberts G. J. 2008. Prevalence, intensity and nature of bacteraemia after toothbrushing. J. Dent. 36:481–487 [DOI] [PubMed] [Google Scholar]
- 22. McCormick J. K., Tripp T. J., Dunny G. M., Schlievert P. M. 2002. Formation of vegetations during infective endocarditis excludes binding of bacterial-specific host antibodies to Enterococcus faecalis.. J. Infect. Dis. 185:994–997 [DOI] [PubMed] [Google Scholar]
- 23. Moreillon P. 2000. Endocarditis prophylaxis revisited: experimental evidence of efficacy and new Swiss recommendations. Schweiz. Med. Wochenschr. 130:1013–1026 [PubMed] [Google Scholar]
- 24. Moreillon P., et al. 1995. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect. Immun. 63:4738–4743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moreillon P., Que Y. A., Bayer A. S. 2002. Pathogenesis of streptococcal and staphylococcal endocarditis. Infect. Dis. Clin. N. Am. 16:297–318 [DOI] [PubMed] [Google Scholar]
- 26. Nicolau D. P., et al. 1993. Reduction of bacterial titers by low-dose aspirin in experimental aortic valve endocarditis. Infect. Immun. 61:1593–1595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nicolau D. P., Tessier P. R., Nightingale C. H., Quintiliani R. 1996. Influence of ticlopidine on the development of experimental Staphylococcus aureus endocarditis. Int. J. Antimicrob. Agents 7:271–274 [DOI] [PubMed] [Google Scholar]
- 28. Oliver R., Roberts G. J., Hooper L., Worthington H. V. 2008. Antibiotics for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database Syst. Rev. 2008:CD003813. [DOI] [PubMed] [Google Scholar]
- 29. Olsen I. 2008. Update on bacteraemia related to dental procedures. Transfus. Apher. Sci. 39:173–178 [DOI] [PubMed] [Google Scholar]
- 30. Otto M. 2008. Targeted immunotherapy for staphylococcal infections: focus on anti-MSCRAMM antibodies. BioDrugs 22:27–36 [DOI] [PubMed] [Google Scholar]
- 31. Overholser C. D., Moreillon P., Glauser M. P. 1987. Experimental bacterial endocarditis after dental extractions in rats with periodontitis. J. Infect. Dis. 155:107–112 [DOI] [PubMed] [Google Scholar]
- 32. Roberts G. J. 1999. Dentists are innocent! “Everyday” bacteremia is the real culprit: a review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatr. Cardiol. 20:317–325 [DOI] [PubMed] [Google Scholar]
- 33. Roberts G. J., et al. 2006. Duration, prevalence and intensity of bacteraemia after dental extractions in children. Heart 92:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schaffer A. C., Lee J. C. 2008. Vaccination and passive immunization against Staphylococcus aureus. Int. J. Antimicrob. Agents 32(Suppl. 1):S71–S78 [DOI] [PubMed] [Google Scholar]
- 35. Schennings T., Heimdahl A., Coster K., Flock J. I. 1993. Immunization with fibronectin binding protein from Staphylococcus aureus protects against experimental endocarditis in rats. Microb. Pathog. 15:227–236 [DOI] [PubMed] [Google Scholar]
- 36. Seymour R. A., Lowry R., Whitworth J. M., Martin M. V. 2000. Infective endocarditis, dentistry and antibiotic prophylaxis; time for a rethink? Br. Dent. J. 189:610–616 [DOI] [PubMed] [Google Scholar]
- 37. Strom B. L., et al. 1998. Dental and cardiac risk factors for infective endocarditis: a population-based, case-control study. Ann. Intern. Med. 129:761–769 [DOI] [PubMed] [Google Scholar]
- 38. Tzukert A. A., Leviner E., Benoliel R., Katz J. 1986. Analysis of the American Heart Association's recommendations for the prevention of infective endocarditis. Oral Surg. Oral Med. Oral Pathol. 62:276–279 [DOI] [PubMed] [Google Scholar]
- 39. van der Meer J. T., Thompson J., Valkenburg H. A., Michel M. F. 1992. Epidemiology of bacterial endocarditis in The Netherlands. II. Antecedent procedures and use of prophylaxis. Arch. Intern. Med. 152:1869–1873 [DOI] [PubMed] [Google Scholar]
- 40. van der Meer J. T., et al. 1992. Efficacy of antibiotic prophylaxis for prevention of native-valve endocarditis. Lancet 339:135–139 [DOI] [PubMed] [Google Scholar]
- 41. Viscount H. B., Munro C. L., Burnette-Curley D., Peterson D. L., Macrina F. L. 1997. Immunization with FimA protects against Streptococcus parasanguis endocarditis in rats. Infect. Immun. 65:994–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Widmer E., Que Y. A., Entenza J. M., Moreillon P. 2006. New concepts in the pathophysiology of infective endocarditis. Curr. Infect. Dis. Rep. 8:271–279 [DOI] [PubMed] [Google Scholar]
- 43. Wilson W., et al. 2007. Prevention of infective endocarditis: guidelines from the American Heart Association. Circulation 116:1736–1754 [DOI] [PubMed] [Google Scholar]
- 44. Wray D., Ruiz F., Richey R., Stokes T. 2008. Prophylaxis against infective endocarditis for dental procedures: summary of the NICE guideline. Br. Dent. J. 204:555–557 [DOI] [PubMed] [Google Scholar]